Abstract
Both the carotid ultrasound and coronary artery calcium (CAC) score quantify subclinical atherosclerosis and are associated with cardiovascular disease and events. This study investigated the association between CAC score and carotid plaque quantity and composition. Adult participants (n = 43) without history of cardiovascular disease were recruited to undergo a carotid ultrasound. Maximum plaque height (MPH), total plaque area (TPA), carotid intima-media thickness (CIMT), and plaque score were measured. Grayscale pixel distribution analysis of ultrasound images determined plaque tissue composition. Participants then underwent CT to determine CAC score, which were also categorized as absent (0), mild (1–99), moderate (100–399), and severe (400+). Spearman correlation coefficients between carotid variables and CAC scores were computed. The mean age of participants was 63 ± 11 years. CIMT, TPA, MPH, and plaque score were significantly associated with CAC score (ρ = 0.60, p < 0.0001; ρ = 0.54, p = 0.0002; ρ = 0.38, p = 0.01; and ρ = 0.49, p = 0.001). Echogenic composition features %Calcium and %Fibrous tissue were not correlated to a clinically relevant extent. There was a significant difference in the TPA, MPH, and plaque scores of those with a severe CAC score category compared to lesser categories. While carotid plaque burden was associated with CAC score, plaque composition was not. Though CAC score reliably measures calcification, carotid ultrasound gives information on both plaque burden and composition. Carotid ultrasound with assessment of plaque features used in conjunction with traditional risk factors may be an alternative or additive to CAC scoring and could improve the prediction of cardiovascular events in the intermediate risk population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of enhanced screening tools for coronary artery disease is paramount for the prediction and prevention of major adverse cardiovascular events (MACE). Current screening tools are moderately useful for predicting events for those who fall in the high- and low-risk categories by the Framingham Risk Score (FRS). The intermediate-risk population, however, is at the highest risk of being misclassified by existing tools1. Optimizing the screening protocol for this population may reduce both healthcare costs and the incidence of MACE. Carotid artery ultrasound is a promising risk stratification tool that addresses these goals. Further, plaque composition analysis from carotid ultrasound imaging has been underutilized and could improve the prediction of events making carotid ultrasound a valuable tool in this population [1,2,3].
The CAC score is a sensitive and reliable tool for predicting the presence of coronary artery disease (CAD) and long-term risk of MACE [4, 5]. CAC scoring uses non-contrast cardiac-gated computed tomography (CT) to quantify calcified lesions of the coronary arteries according to the Agatston method. A CAC score of > 400 is associated with a > 20% risk of 10-year MACE [5]. Studies consistently show that the CAC score has a greater predictive value than risk factor formulas including the FRS [4, 6,7,8]. When considered in conjunction with the FRS, its performance is marginally improved [8]. It is generally accepted as a useful risk stratification tool and relevant in deciding treatment courses for adults with subclinical atherosclerosis. However, CT may underestimate the burden of non-calcified lesions and is rarely used for long-term repeated follow-up [9]. Plaque with a greater proportion of low-density tissue is more likely to rupture and cause MACE [10,11,12,13,14]. Thus, CAC scores have been reported to underestimate risk in certain populations, including females and Black patients [15, 16]. Hence, investigation into the alternative tools to maximize the value of tests is warranted. Additionally, CT is expensive and has limited accessibility, especially in the outpatient setting, necessitating the development of risk stratification tools that are readily available and cost-effective.
Previously we and others have established the relationship between carotid plaque assessment by ultrasound and CAD and MACE in at-risk patients [17, 18]. This relationship needs further examination in the low to intermediate risk population. The strengths of carotid ultrasound include its relative low-cost, speed, simplicity of performance, and ease of interpretation. An abbreviated carotid ultrasound can offer quantification of plaque burden including measures such as total plaque area (TPA), maximum plaque height (MPH), carotid intimal-medial thickness (CIMT), and plaque score. More recently, there has been widespread interest in the utility of plaque composition analysis as a method to predict plaque rupture, a precursor to most cardiovascular events [19]. Plaque composition analysis has been used by our lab to predict 5-year MACE and stenosis severity on angiography in the high-risk population [20]. The proportion of calcium, fibrous, lipid, and bloody tissue may provide similar insights in the intermediate risk population as well. The absence of carotid plaque also has a good negative predictive value for the absence of CAD [2, 21].
CAC score was found to be a similar predictor of stroke and a better predictor of cardiovascular events and CAD than carotid plaque score in the Multi-Ethnic Study of Atherosclerosis [22]. Another study found that carotid ultrasound was more sensitive for detecting asymptomatic atherosclerosis than CAC scoring in a cohort of a similar age to the sample studied here [23]. The American Heart Association listed carotid ultrasound and CAC scoring at the same level of treatment effect (IIa, Benefit > > Risk) in their guidelines [24]. In this pilot study, we sought to clarify the association between carotid ultrasound measures and the CAC score. We hypothesized that carotid plaque burden (TPA, MPH, plaque score, and CIMT) is associated with CAC score in the low to intermediate risk population. Additional comparisons of carotid plaque %Calcium and %Fibrous tissue were also assessed.
Materials and methods
Study design
This study was approved by the Queen’s Health Sciences Research Ethics Board. We performed a cross-sectional, 2-centre study assessing the association between carotid ultrasound and CAC scoring for an assessment of subclinical atherosclerosis in stable outpatients. We enrolled adults with no history of significant coronary artery disease and cardiovascular events who were referred for stress echocardiography for cardiovascular risk stratification. Patients were recruited from the Kingston Health Sciences Centre and Kingston Heart Clinic. At Kingston Health Sciences Centre, this sub-study ran concurrently under an ongoing parent project, the CIRCE Study (Combining Intraplaque Neovascularization with Risk Stratification by Carotid Stress Echo). Briefly, the CIRCE study investigates the additive value of an abbreviated carotid ultrasound applied to patients receiving stress echo for the prediction of events and the need for angiography. The present sub-study enrolled a subset of CIRCE participants to undergo cardiac CT for calcium scoring within 6-months of their carotid ultrasound and stress echo. This window was chosen based on studies which found that significant increases in CAC scores can be found at time points > 6 months [25].We selected a purposeful population with a ranging extent of plaque in the carotid arteries based on their carotid ultrasound. We also included eligible data sets from the Kingston Heart Clinic database of adults who had received both carotid ultrasound and CAC scoring assessment within 6-months prior to the commencement of the CIRCE Study a part of their standard of care.
CIRCE Inclusion Criteria: (1) Males or females aged ≥ 18 years; (2) Referred for an assessment of ischemia and risk stratification; (3); and the ability and willingness to give informed written consent. CIRCE Exclusion Criteria: (1) Emergency procedure, or active acute coronary syndrome (active chest pain, ischemic electrocardiogram changes, or cardiac enzyme elevation); (2) Referral for viability, pulmonary hypertension, or valve assessment; (3) Referral outside of the normal working hours; (4) History of significant CAD: percutaneous coronary intervention [PCI], coronary artery bypass graft [CABG], or coronary angioplasty; (5) History of stroke or myocardial infarction (MI); (6) Known or documented hypersensitivity or allergy to perflutren (DEFINITY® contrast agent); (7) Known or documented allergy to Polyethylene Glycol (Peg) a component of DEFINITY®; (8) History of carotid surgery (endarterectomy or stenting); (9) Any serious medical condition or complication from the stress test that according to the investigator could interfere with the carotid scan or optimal care; (10) Currently pregnant or breastfeeding; and (11) Previous enrolment into the study. Additional Sub-Study Exclusion Criteria: (1) Incomplete CIRCE study images; (2) Any contraindication for receiving a cardiac CT; and (3) Weight > 675lb, abdominal width > 70 cm, or other physical inhibition from using the CT.
Carotid ultrasound protocol and interpretation
A focused carotid ultrasound protocol was carried out for all participants at the time of their stress echo. The sensitivity, and intra- and inter-rater reliability of this protocol has been validated by our lab to be 0.86 and 0.99 respectively [26]. Carotid ultrasounds were performed by cardiac sonographers trained in the vascular scan and supervised by a research delegate. An example of the images captured for each participant is presented in Fig. 1. The abbreviated scan included a cross-sectional sweep of the common carotid artery up to the bifurcation into the internal and external carotid arteries to screen for lesions. Longitudinal still images of the common carotid, bulb, and internal carotid arteries were obtained for plaque measuring and composition analyses. Care was taken to ensure that any focal protrusions were captured at their maximal height. This process was repeated for both the left and right sides of each participant. For all participants, plaque height, plaque area, and CIMT were collected manually using calipers on EchoPAC software (v. 113, GE healthcare) on longitudinal images by an experienced reader. Regular quality check of measurements by a secondary reader took place throughout data collection and analysis. Plaques are defined as focal protrusions > 1.5 mm in height or 50% greater than the adjacent CIMT. Where multiple plaques are present, all plaque areas were summed to define the TPA. MPH was recorded as the greatest single plaque height measurement between both sides taken at a perpendicular angle to the associated adventitia. The mean CIMT between the measurements for the left and right sides was considered as the CIMT for analysis. For the plaque score, carotid arteries were scored according to the Rotterdam method. The result of the stress echo was also recorded, and inconclusive tests were categorized as negative.
Carotid composition analysis
Carotid plaque composition was determined using pixel distribution analysis. An example of composition analysis is shown in Fig. 2. B-mode DICOM images were exported and uploaded to Intelliplaque © (Ferko Liblik Inc.). Images were normalized so that the lumen had a greyscale median value of 0 and the far wall adventitia had a value of 190. Ranges established and validated by histological analysis by Lal et al. in 2002 have been widely used, they are as follows: 0–4 (Blood), 8–26 (Fat), 41–76 (Muscle), 112–196 (Fibrous), and 211–255 (Calcium) [27]. The total proportion of analyzed pixels that fall within each category between both sides for a patient was recorded and used in data analysis. For those with no plaque detected all values were 0.
Example of plaque size and composition analysis. Blue and orange calipers describe the plaque height, and the green and yellow outlines measure plaque areas (left). Intelliplaque composition analysis shows colour-coded pixels to describe the makeup of each lesion based on the greyscale median ranges by Lal et al. Red = Blood, Yellow = Fat, Green = Muscle, Purple = Fibrous, and Blue = Calcium (right)
CAC score interpretation
CAC scoring and ultrasound image analysis was performed at Queen’s University by experienced readers. The absolute Agatston score was determined by an experienced cardiac CT reader who was blinded to the participant’s stress echo and carotid ultrasound results. An example of one slice of the CT imaging and pixel attenuation is shown in Fig. 3. Raw scores were categorized to be Absent (0), Mild (1–99), Moderate (100–399), or Severe (400+) [28]. CAC scores were then compared to published reference values according to each participant’s age, sex, and race to define their CAC percentile (https://www.mesa-nhlbi.org/CACReference.aspx) [29]. CAC percentiles are useful for providing context to overall risk based on a patient’s demographics, and appear frequently in guidelines [30].
Statistical analysis
We ran paired analyses to determine the Spearman correlation coefficient (ρ) for burden measures TPA, MPH, plaque score, and CIMT to CAC score. Similar analyses were carried out for all composition parameters, including %Calcium, and %Fibrous. The same variables were compared to CAC categories (absent, mild, moderate, and severe) using the Kruskal-Wallis ANOVA test and post-hoc Dunn’s test where relevant. These factors were also compared to CAC score age- and sex-based percentiles. Correlation coefficients that are 0.70–0.99 were considered very strong, 0.50–0.69 were substantial, 0.30–0.49 were fair, 0.10–0.29 were low, and 0.01–0.09 were negligible [31]. A correlation of > 0.60 is commonly considered to be clinically relevant, and a p-value < 0.05 was considered statistically significant [31].
Results
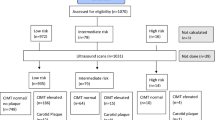
A total of 21 participants were enrolled from Kingston Health Sciences Centre June 2022 to May 2023. An additional 22 participant datasets were identified from the Kingston Heart Clinic database from 2012 to 2022. B-mode carotid ultrasound scans took an average of 5-minutes to complete. The enrollment process is detailed in Fig. 4.
The baseline characteristics of participants with complete ultrasound and CT imaging (n = 43) are described in Table 1. The mean age of participants was 62.8 ± 11.3 years old, and 53.5% were female. Carotid plaque was found in 90% of participants, and 67% of participants had a non-zero CAC score (Table 2). CAC scores ranged from 0 to 3059.
The correlations found between carotid plaque features and CAC scores are summarized in Table 3. CIMT showed the strongest correlation to CAC score at ρ = 0.60 (p < 0.0001). TPA, MPH, and plaque score were also significantly associated with CAC score (ρ = 0.54, p = 0.0002; ρ = 0.38, p = 0.01; and ρ = 0.49, p = 0.001). The TPA, MPH, and plaque scores of those with severe CAC scores are significantly different than those with CAC scores of 0.
Plaque composition features were not associated with the CAC score (Table 3). %Fibrous tissue was mildly correlated (ρ = 0.19, p = 0.006), but this association is unlikely to impact clinical decision-making. The primary feature of interest, %Calcium of carotid plaques, had no clear relationship with coronary calcium within this sample.
We repeated the analysis with the same plaque burden and composition measures compared to CAC percentiles (Table 4). We found similar correlation trends to the raw CAC score. The association with plaque score was strongest (ρ = 0.52, p = 0.0002), and associations with composition features did not reach significance. Plaque composition features did not hold significant relationships with CAC percentiles (Table 4).
Discussion
In this pilot study we used carotid ultrasound and cardiac CT imaging to examine the relationship between carotid plaque burden and composition and coronary calcified plaque burden. Carotid plaque burden features appear to show moderate correlations with CAC score in adults with no history of significant cardiovascular disease. The strongest correlation found in this study was between CIMT and CAC score, consistent with findings in prior studies. The relationship between elevated CIMT and CAC scores > 0 has been well documented [32,33,34]. Comparisons to measures of plaque burden are not as well documented, however, this study suggests that TPA and MPH also correlate with CAC score. The correlations of the measures of plaque burden and CAC score ranged from 0.38 to 0.60, which are generally considered to indicate a moderately strong relationship [31]. This modest correlation suggests that ultrasound of the carotid arteries and calcium scoring of the coronary arteries should not be used to infer one another in most cases. Instead, these findings provide some initial evidence that carotid ultrasound may complement other assessments to contribute to a full picture of a patient’s risk. The association between CIMT and CAC percentile was notably lower than CIMT and raw CAC score, potentially in part due to CIMT’s strong association with age. As CAC percentiles compare scores to those of the same age, we do not expect CAC percentile to increase over time linearly as CIMT typically does.
Our study found that the strongest correlations were between CAC score and CIMT. Literature has shown that CIMT has less predictive value for clinical outcomes compared to TPA and MPH [35, 36]. While elevated CIMT and carotid plaques are distinct, it is well established that elevated CIMT is strongly associated with the development of carotid plaque across ages, sexes, and time course [37]. They are often understood as different stages of atherogenesis [38, 39]. The fact that CAC score aligns best with CIMT may be indicative of a gap in risk assessment. Unlike CIMT and plaque score, TPA and MPH are direct measures of the amount of plaque in the carotid arteries. As TPA has a greater predictive accuracy for MACE than CIMT, this may suggest that plaque identification and composition analysis will be a better predictor of events than CAC score, and further research is required to confirm this. Vulnerable plaques can be present in the absence of clinically significant coronary stenosis [40]. While angina and ischemic symptoms are credited to coronary stenosis, acute coronary syndromes are most often caused by spontaneous plaque rupture which is not entirely associated with the degree of stenosis [40]. Since CAC score is primarily used to predict coronary plaque burden, there remains a role for other assessments of plaque vulnerability. Carotid composition analysis can provide useful insight in terms of mortality and cardiovascular outcomes [10, 41,42,43]. For example, carotid calcium was found to be associated with a lower probability of survival than coronary calcium [44]. Ultrasound-based assessments also carry additional advantages including shorter duration of assessment, lower cost, portability, and serial assessment capabilities [26, 41]. This study has demonstrated that consideration of several carotid features together is possible and may improve accuracy of event prediction. When considered alongside FRS, it contributes to the body of work that suggests that low and intermediate risk patients may be reclassified by CAC scoring and carotid assessment [45,46,47].
Our results did not support the hypothesis that echogenic plaque composition features are correlated to CAC score. In fact, the incidence of calcium deposits in carotid plaques was low, with only 7 (16%) participants with a carotid %Calcium > 0. These findings suggest that the deposition of calcium is far greater in the coronary system than the carotid system. Atherosclerosis is generally thought of as a systemic disease, but carotid and coronary arteries do not develop plaque in a phenotypically identical manner, especially prior to the advanced lesion stage [48, 49]. Other works have assigned this discrepancy in part to the difference in pressure and augmentation index, as well as the inherent distinction with which atherosclerosis impacts different vascular beds through variances in inflammatory pathways [48]. Coronary arteries are predisposed to calcium deposition due to their tortuousness, bifurcations, and expression of pro-calcific processes activated by mechanical stimulus from pulsatile pressure in the coronary system [49]. Greater coronary calcium deposition was also found in a study of a high-risk cohort using Agatston scoring across both coronary and carotid sites [48]. A meta-analysis concluded that while plaque builds in both the coronary and carotid systems simultaneously, plaque calcification, lipid-rich necrotic core, and intraplaque hemorrhage as detected by MRI imaging did not share a strong link [34]. However, they reported an r of 0.61 comparing carotid calcification and CAC scores > 400 [34]. This describes a complex relationship between plaque composition variables but confirms that those with severe CAC scores are more likely to have calcified plaques throughout the body, which is supported by our findings [20, 48]. Taken together, our results support findings that suggest calcium in separate vascular beds in those with less than advanced disease are not predictive of one another, and carotid assessment may reveal otherwise undetected vulnerabilities.
The differences in plaque morphology and development have been attributed to several factors and pathways [49, 50]. The most widely reported factors dictating development include redox signaling pathways, vascular tone and endothelial function, wall shear stress, and vessel circumference [49]. For example, detectable plaque tends to develop first in large-diameter vessels, and only in smaller distal vessels with more advanced disease and aging [51]. Wall shear stress exerts a great frictional force on large vessels at points of curvature and bifurcation. Interestingly, in human carotid arteries at the common carotid bifurcation, low wall shear stress promotes vulnerable lesion development, whereas plaque develops slower and with a less vulnerable phenotype in areas of high shear stress [51, 52]. Some studies do, however, discuss how high shear stress exerted on plaque can trigger inflammatory processes that can increase the vulnerability of a plaque [53]. High shear stress is present in areas of bifurcation or tortuous vessels, triggering vascular smooth muscle cells to switch to a more fibrous phenotype which may contribute to the coronary bed appearing more calcified than carotid arteries. This in part explains why the lipid-rich core and fibrous cap features are expected to appear differently between beds. Altogether, carotid arteries will most likely show detectable plaque sooner than coronary arteries, and these plaques are more likely to be larger and exhibit a thin fibrous cap, neovascularization, and a lipid core as signs of vulnerability. Coronary arteries, on the other hand, tend to develop more diffuse plaque, vascular remodeling, and calcification [40]. The association found within our sample between CIMT and CAC score is consistent with these theories, as both CIMT and calcification processes increase as a result of vascular smooth muscle remodelling [54, 55].
While this pilot study did not support a large enough sample to adequately power subgroup analyses, future analyses should examine differences in correlations between sexes, statin use history, and smoking history. Females tend to have smaller and more echogenic plaques than males [56, 57]. It is well-acknowledged throughout the literature that cardiovascular risk assessment of females must consider the physiological differences and disproportionate late diagnosis of cardiovascular disease. Screening tests and risk calculators that do not account for females specifically lead to elevated morbidity and mortality for this population. Secondly, statin use is associated with a more stable clinical presentation of both coronary and carotid plaques including greater calcium content and smaller plaque burden [12, 58]. Cigarette smoking has a nonlinear relationship with plaque morphology but increasing pack years is associated with echogenicity and markers of vulnerability [59]. Smoking appears to have a similarly complex relationship with CAC score, with both an association to greater CAC score and high-risk coronary plaque in low CAC scoring patients having been reported [60, 61]. Considerations for factors that are known to impact atherosclerosis development may lend further insight of how each screening tool should be applied when considering invasive testing and medical therapies.
To conclude, CAC scoring is a strong indicator of clinically significant CAD but does not directly assess the presence of vulnerable plaques. The use of traditional risk scores and CAC scoring alone may underestimate some patients’ risk of acute coronary and cerebrovascular syndromes. Our results in consideration with related literature point towards carotid ultrasound not replacing CAC scoring, but instead contributing to a more holistic picture of a patient’s likelihood of developing CAD and MACE. For example, in low-risk patients with a CAC score of 0, carotid plaque presence was independently associated with the development of CAD and MACE over 10 years [62]. Our results show that carotid plaque burden modestly correlates with CAC score, indicating that plaque does develop throughout the body at similar rates. They also emphasize that the composition of plaques in the carotid arteries is not predictive of the calcium content in coronary vessels, thus plaque composition should be considered as an additive risk stratification tool that may be helpful for the detection of vulnerable lesions throughout the body.
Limitations
A small and majority Caucasian sample limits the applicability of these results for immediate incorporation into practice. Since this study was designed and funded as a pilot study, a sufficiently powered sample size was not the priority at this stage. The design also prioritized enrolling a majority sample with detectable carotid plaque, thus random sampling was not employed for this study to maximize the value of limited CT use and funding. As a result, the sample is expected to have a higher proportion of plaque present than the general population of interest. This design was also unable to accommodate the administration of both imaging procedures at the same study visit, and thus there is potential for some minor remodelling of the coronary or carotid arteries to have taken place between image captures. Finally, the limited duration of this study did not allow us to collect outcomes data for sensitivity analysis. Future work should aim to follow-up over 10 years to investigate if one method has greater sensitivity and specificity for MACE. This study does, however, confirm that further investigation into the comparison of carotid and coronary atherosclerosis screening is warranted.
Data availability
No datasets were generated or analysed during the current study.
References
Kanwar M, Rosman HS, Fozo PK et al (2007) Usefulness of Carotid Ultrasound to improve the ability of stress testing to Predict Coronary Artery Disease. Am J Cardiol 99:1196–1200. https://doi.org/10.1016/j.amjcard.2006.12.028
Johri AM, Chitty DW, Matangi M et al (2013) Can Carotid Bulb Plaque Assessment Rule out Significant Coronary Artery Disease? A comparison of Plaque quantification by two- and three-Dimensional Ultrasound. J Am Soc Echocardiogr 26:86–95. https://doi.org/10.1016/j.echo.2012.09.005
Medical Advisory Secretariat (2010) Stress echocardiography for the diagnosis of coronary artery disease: an evidence-based analysis. Ontario Health Assessment Services, Toronto, Ont
Hecht HS (2015) Coronary artery calcium scanning. JACC Cardiovasc Imaging 8:579–596. https://doi.org/10.1016/j.jcmg.2015.02.006
Liew G, Chow C, Van Pelt N et al (2017) Cardiac Society of Australia and New Zealand position Statement: coronary artery calcium scoring. Heart Lung Circ 26:1239–1251. https://doi.org/10.1016/j.hlc.2017.05.130
Detrano R, Bild DE, Liu K et al (2008) Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med
Hecht HS, Narula J (2012) Coronary artery calcium scanning in asymptomatic patients with diabetes mellitus: a paradigm shift: coronary calcium in diabetes mellitus. J Diabetes 4:342–350. https://doi.org/10.1111/j.17530407.2012.00212.x
Huang W, Lim LMH, Aurangzeb AS et al (2021) Performance of the coronary calcium score in an outpatient chest pain clinic and strategies for risk stratification. Clin Cardiol 44:267–275. https://doi.org/10.1002/clc.23539
Kral BG, Becker LC, Vaidya D et al (2014) Noncalcified coronary plaque volumes in healthy people with a family history of early onset coronary artery disease. Circ Cardiovasc Imaging 7:446–453. https://doi.org/10.1161/CIRCIMAGING.113.000980
Honda O, Sugiyama S, Kugiyama K et al (2004) Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J Am Coll Cardiol 43:1177–1184. https://doi.org/10.1016/j.jacc.2003.09.063
Pucci A, Sheiban I, Formato L et al (2007) In vivo coronary plaque histology in patients with stable and acute coronary syndromes. Atherosclerosis 194:189–195. https://doi.org/10.1016/j.atherosclerosis.2006.07.026
Mujaj B, Bos D, Selwaness M et al (2018) Statin use is associated with carotid plaque composition: the Rotterdam Study. Int J Cardiol 260:213–218. https://doi.org/10.1016/j.ijcard.2018.02.111
Spanos K, Tzorbatzoglou I, Lazari P et al (2018) Carotid artery plaque echomorphology and its association with histopathologic characteristics. J Vasc Surg 68:1772–1780. https://doi.org/10.1016/j.jvs.2018.01.068
Urbak L, Sandholt B, Graebe M et al (2019) Statin Naïve patients with myocardial infarction have more echolucent plaques compared to long-term statin treated chronic atherosclerotic patients – A 3D Ultrasound Study. Eur J Vasc Endovasc Surg 58:e750. https://doi.org/10.1016/j.ejvs.2019.09.303
Shaw LJ, Min JK, Nasir K et al (2018) Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J 39:3727–3735. https://doi.org/10.1093/eurheartj/ehy534
Madu C, Kvasic J, Niazi K, Makaryus J, ACC/AHA Coronary artery calcium score guidelines may underestimate coronary artery disease in African-American patients (2017) J Am Coll Cardiol 69:1827. https://doi.org/10.1016/S0735-1097(17)35216-6
Mantella LE, Colledanchise KN, Feinstein SB et al Carotid intraplaque neovascularization predicts coronary artery disease and cardiovascular events. 9
Johri AM, Behl P, Hétu M-F et al (2016) Carotid Ultrasound Maximum Plaque Height-A sensitive imaging biomarker for the Assessment of Significant Coronary Artery Disease. Echocardiography 33:281–289. https://doi.org/10.1111/echo.13007
Huang R, DeMarco JK, Ota H et al (2021) Prognostic value of Intraplaque Neovascularization detected by carotid contrast-enhanced Ultrasound in patients undergoing stress Echocardiography. J Am Soc Echocardiogr 34:614–624. https://doi.org/10.1016/j.echo.2020.12.016
Herr JE, Hétu M-F, Li TY et al (2019) Presence of Calcium-Like Tissue Composition in Carotid Plaque is indicative of significant coronary artery disease in high-risk patients. J Am Soc Echocardiogr 32:633–642. https://doi.org/10.1016/j.echo.2019.01.001
Colledanchise KN, Mantella LE, Bullen M et al (2020) Combined femoral and carotid plaque Burden identifies obstructive coronary artery disease in women. J Am Soc Echocardiogr 33:90–100. https://doi.org/10.1016/j.echo.2019.07.024
Gepner AD, Young R, Delaney JA et al (2017) Comparison of carotid plaque score and coronary artery calcium score for Predicting Cardiovascular Disease events: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 6:e005179. https://doi.org/10.1161/JAHA.116.005179
Schroeder B, Francis G, Leipsic J et al (2013) Early atherosclerosis detection in asymptomatic patients: a comparison of Carotid Ultrasound, coronary artery calcium score, and Coronary Computed Tomography Angiography. Can J Cardiol 29:1687–1694. https://doi.org/10.1016/j.cjca.2013.10.003
Writing Committee Members, Greenland P, Alpert JS et al (2010) 2010 ACCF/AHA Guideline for Assessment of Cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice guidelines. Circulation 122:2748–2764. https://doi.org/10.1161/CIR.0b013e3182051bab
Lee W, Yoon YE, Kwon O et al (2019) Evaluation of coronary artery calcium progression in asymptomatic individuals with an initial score of Zero. Korean Circ J 49:448. https://doi.org/10.4070/kcj.2018.0318
Johri AM, Calnan CM, Matangi MF et al (2016) Focused vascular ultrasound for the Assessment of atherosclerosis: a proof-of-Concept Study. J Am Soc Echocardiogr 29:842–849. https://doi.org/10.1016/j.echo.2016.05.003
Lal BK, Hobson RW, Pappas PJ et al (2002) Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg 35:1210–1217. https://doi.org/10.1067/mva.2002.122888
Budoff MJ, Nasir K, McClelland RL et al (2009) Coronary calcium predicts events better with Absolute Calcium scores Than Age-Sex-Race/Ethnicity Percentiles. J Am Coll Cardiol 53:345–352. https://doi.org/10.1016/j.jacc.2008.07.072
McClelland RL, Chung H, Detrano R et al (2006) Distribution of coronary artery calcium by race, gender, and age: results from the multi-ethnic study of atherosclerosis (MESA). Circulation 113:30–37. https://doi.org/10.1161/CIRCULATIONAHA.105.580696
Osei AD, Mirbolouk M, Dardari Z et al (2022) A simple Approach to the identification of Guideline-based coronary artery calcium score percentiles (from the multi-ethnic study of atherosclerosis). Am J Cardiol 179:18–21. https://doi.org/10.1016/j.amjcard.2022.06.006
Chan YH Biostatistics 104: Correlational Analysis
Cohen GI, Aboufakher R, Bess R et al (2013) Relationship between carotid disease on Ultrasound and Coronary Disease on CT Angiography. JACC Cardiovasc Imaging 6:1160–1167. https://doi.org/10.1016/j.jcmg.2013.06.007
Polak JF, Szklo M, Kronmal RA et al (2013) The Value of Carotid Artery Plaque and Intima-Media thickness for Incident Cardiovascular Disease: the Multi‐ethnic study of atherosclerosis. J Am Heart Assoc 2:e000087. https://doi.org/10.1161/JAHA.113.000087
Bytyçi I, Shenouda R, Wester P, Henein MY (2021) Carotid atherosclerosis in Predicting Coronary Artery Disease: a systematic review and Meta-analysis. Arterioscler Thromb Vasc Biol 41. https://doi.org/10.1161/ATVBAHA.120.315747
Inaba Y, Chen JA, Bergmann SR (2012) Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 220:128–133. https://doi.org/10.1016/j.atherosclerosis.2011.06.044
Naqvi TZ, Lee M-S (2014) Carotid Intima-Media Thickness and Plaque in Cardiovascular Risk Assessment. JACC Cardiovasc Imaging 7:1025–1038. https://doi.org/10.1016/j.jcmg.2013.11.014
Tschiderer L, Klingenschmid G, Seekircher L, Willeit P (2020) Carotid intima-media thickness predicts carotid plaque development: Meta‐analysis of seven studies involving 9341 participants. Eur J Clin Invest 50:e13217. https://doi.org/10.1111/eci.13217
Rundek T, Gardener H, Della-Morte D et al (2015) The relationship between carotid intima-media thickness and carotid plaque in the Northern Manhattan Study. Atherosclerosis 241:364–370. https://doi.org/10.1016/j.atherosclerosis.2015.05.027
Eigenbrodt ML, Bursac Z, Tracy RE et al (2008) B-mode ultrasound common carotid artery intima-media thickness and external diameter: cross-sectional and longitudinal associations with carotid atherosclerosis in a large population sample. Cardiovasc Ultrasound 6:10. https://doi.org/10.1186/1476-7120-6-10
Stone GW, Maehara A, Lansky AJ et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235. https://doi.org/10.1056/NEJMoa1002358
Johri AM, Herr JE, Li TY et al (2017) Novel ultrasound methods to investigate carotid artery plaque vulnerability. J Am Soc Echocardiogr 30:139–148. https://doi.org/10.1016/j.echo.2016.11.003
Deyama J, Nakamura T, Takishima I et al (2013) Contrast-enhanced Ultrasound Imaging of Carotid Plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J 77:1499–1507. https://doi.org/10.1253/circj.CJ-12-1529
Kwee RM, van Oostenbrugge RJ, Prins MH et al (2010) Symptomatic patients with mild and moderate carotid stenosis: plaque features at MRI and Association with Cardiovascular Risk factors and statin use. Stroke 41:1389–1393. https://doi.org/10.1161/STROKEAHA.109.575670
Allison MA, Hsi S, Wassel CL et al (2012) Calcified atherosclerosis in different vascular beds and the risk of Mortality. Arterioscler Thromb Vasc Biol 32:140–146. https://doi.org/10.1161/ATVBAHA.111.235234
Lau K-K, Chan Y-H, Yiu K-H et al (2008) Incremental predictive value of vascular assessments combined with the Framingham risk score for prediction of coronary events in subjects of low–intermediate risk. Postgrad Med J 84:153–157. https://doi.org/10.1136/pgmj.2007.064089
Eleid MF, Lester SJ, Wiedenbeck TL et al (2010) Carotid Ultrasound identifies high risk subclinical atherosclerosis in adults with low Framingham Risk scores. J Am Soc Echocardiogr 23:802–808. https://doi.org/10.1016/j.echo.2010.06.003
Yano Y, O’Donnell CJ, Kuller L et al (2017) Association of Coronary Artery Calcium Score vs Age with Cardiovascular Risk in older adults: an analysis of Pooled Population-Based studies. JAMA Cardiol 2:986. https://doi.org/10.1001/jamacardio.2017.2498
Shenouda R, Vancheri S, Maria Bassi E et al (2021) The relationship between carotid and coronary calcification in patients with coronary artery disease. Clin Physiol Funct Imaging 41:271–280. https://doi.org/10.1111/cpf.12694
Sigala F, Oikonomou E, Antonopoulos AS et al (2018) Coronary versus carotid artery plaques. Similarities and differences regarding biomarkers morphology and prognosis. Curr Opin Pharmacol 39:9–18. https://doi.org/10.1016/j.coph.2017.11.010
Jashari F, Ibrahimi P, Nicoll R et al (2013) Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis 227:193–200. https://doi.org/10.1016/j.atherosclerosis.2012.11.008
Aboyans V, Lacroix P, Criqui MH (2007) Large and small vessels atherosclerosis: similarities and differences. Prog Cardiovasc Dis 50:112–125. https://doi.org/10.1016/j.pcad.2007.04.001
Chatzizisis YS, Toutouzas K, Giannopoulos AA et al (2017) Association of global and local low endothelial shear stress with high-risk plaque using intracoronary 3D optical coherence tomography: introduction of ‘shear stress score’. Eur Heart J - Cardiovasc Imaging 18:888–897. https://doi.org/10.1093/ehjci/jew134
Wang Y, Qiu J, Luo S et al (2016) High shear stress induces atherosclerotic vulnerable plaque formation through angiogenesis. Regen Biomater 3:257–267. https://doi.org/10.1093/rb/rbw021
Zaid M, Fujiyoshi A, Kadota A et al (2017) Coronary artery calcium and carotid artery Intima Media Thickness and Plaque: clinical use in need of clarification. J Atheroscler Thromb 24:227–239. https://doi.org/10.5551/jat.RV16005
Towler DA (2008) Vascular calcification: a perspective on an Imminent Disease Epidemic. IBMS BoneKEy 5:41–58. https://doi.org/10.1138/20080298
Joakimsen O, Bønaa KH, Stensland-Bugge E, Jacobsen BK (1999) Age and sex differences in the distribution and Ultrasound morphology of Carotid atherosclerosis: the Tromsø Study. Arterioscler Thromb Vasc Biol 19:3007–3013. https://doi.org/10.1161/01.ATV.19.12.3007
Nicholls SJ, Wolski K, Sipahi I et al (2007) Rate of progression of coronary atherosclerotic plaque in women. J Am Coll Cardiol 49:1546–1551. https://doi.org/10.1016/j.jacc.2006.12.039
Kadohira T, Mintz GS, Souza CF et al (2017) Impact of chronic statin therapy on clinical presentation and underlying lesion morphology in patients undergoing percutaneous intervention: an ADAPT-DES IVUS substudy. Coron Artery Dis 28:218–224. https://doi.org/10.1097/MCA.0000000000000480
Yang D, Iyer S, Gardener H et al (2015) Cigarette smoking and carotid plaque echodensity in the Northern Manhattan Study. Cerebrovasc Dis 40:136–143. https://doi.org/10.1159/000434761
Senoner T, Plank F, Langer C et al (2021) Smoking and obesity predict high-risk plaque by coronary CTA in low coronary artery calcium score (CACS). J Cardiovasc Comput Tomogr 15:499–505. https://doi.org/10.1016/j.jcct.2021.04.003
Trab T, Attar R, Jensen SE et al (2021) Coronary artery calcium in patients with schizophrenia. BMC Psychiatry 21:422. https://doi.org/10.1186/s12888-021-03412-x
Mehta A, Rigdon J, Tattersall MC et al (2021) Association of Carotid Artery Plaque with Cardiovascular events and incident coronary artery calcium in individuals with absent coronary calcification: the MESA. Circ Cardiovasc Imaging 14:e011701. https://doi.org/10.1161/CIRCIMAGING.120.011701
Author information
Authors and Affiliations
Contributions
Conceptualization: GK, MH, RP, AJ; Methodology: GK, MH, RP, AJ; Formal analysis and investigation: GK, MH; Writing - original draft preparation: GK; Writing - review and editing: GK, LM, MJB, RP, AJ; Funding acquisition: MH, AJ; Software: DL; Supervision: AJ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kersche, G., Liblik, D., Hétu, MF. et al. The association of carotid plaque burden and composition and the coronary artery calcium score in intermediate cardiovascular risk patients. Int J Cardiovasc Imaging 40, 1683–1692 (2024). https://doi.org/10.1007/s10554-024-03153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-024-03153-4