Abstract
Background
Premenstrual syndrome (PMS) consists of psychiatric or somatic symptoms negatively affecting the daily life. PMS treatment can involve the use of complementary-alternative approaches. Hydrogen-rich water (HRW) has antioxidant and anti-inflammatory properties that may treat PMS. This study aimed to investigate the effect of drinking HRW on the severity of premenstrual symptoms and the quality of life of women who suffer from PMS.
Methods
This study is a randomized controlled trial. Participants were randomized into two groups (intervention group=33, control group=32) using the block randomization method. Participants were requested to consume 1500-2000 mL of HRW daily in the intervention group and drink water in the placebo group. Participants began drinking either HRW or placebo water from day 16 of their menstrual cycle until day 2 of the following cycle for three menstrual cycles. The research data were collected using a Demographic Information Form, Premenstrual Syndrome Scale (PMSS), and Short form of the World Health Organization Quality of Life Questionnaire (WHOQOL- BREF).
Results
The intervention group had significantly lower mean scores than the control group in both the first and second follow-ups on the PMSS (P<0.05). In the first follow-up, the intervention group had significantly higher mean scores in the Physical Health and Psychological domains of the WHOQOL-BREF compared to the control group (P<0.05). Group × time interaction was significant for PMSS (F = 10.54, P<0.001). Group × time interaction was insignificant for WHOQOL- BREF (P>0.05).
Conclusions
The consumption of HRW reduces the severity of premenstrual symptoms and improves individuals' quality of life in physical and psychological domains.
Similar content being viewed by others
Introduction
Many women of reproductive age experience somatic and mental problems during the menstrual period [1]. Premenstrual syndrome (PMS) consists of psychiatric or somatic symptoms that develop during the luteal phase of the menstrual cycle, negatively affecting the individual's daily life [2]. In a systematic review examining the frequency of PMS in Turkey, the prevalence has been reported as 52.2% [3]. PMS, being such a widespread health issue, can negatively impact women's quality of life [1, 4,5,6,7], thus highlighting the importance of PMS treatment.
The pathogenesis of PMS is complex and not fully understood [8]. It is believed that the menstrual cycle and the underlying hormones influence the psychophysiological and biological processes related to PMS [9]. However, some studies have not shown differences in hormone levels between healthy and PMS women [9, 10]. One hypothesis suggested that the response alternated based on exposure or withdrawal from allopregnanolone, a progesterone metabolite and an agonist of gamma-aminobutyric acid [11]. Scientists are currently exploring another possible cause of PMS, which is an abnormal inflammatory response to different types of stimuli, such as biological or physical factors. This response may lead to oxidative stress, the imbalance between reactive oxygen species (ROS) production and their inactivation by antioxidants. Both inflammation and oxidative stress are being studied as possible factors in the development of PMS [8]. Therefore, it is believed that reducing inflammation and oxidative stress would be effective in the treatment of PMS. PMS treatment focuses on alleviating physical and psychiatric symptoms and can involve various dietary supplements and complementary alternative approaches [2]. Thus, one can assume the potential of hydrogen-rich water (HRW) to be benefit in the treatment of PMS.
Molecular hydrogen, the smallest molecule in the universe, is rapidly absorbed into the circulatory system following ingestion of HRW [12]. Hydrogen can penetrate biomembranes and permeate into the cytosol, mitochondria, nucleus, and blood-brain barrier. Consequently, tissues can rapidly absorb hydrogen [13, 14]. Molecular hydrogen (H2) is a light gas yet a selective antioxidant and has recently been recognized as a novel therapeutic agent [13]. H2 has been proposed as a potential treatment for certain neuromuscular disorders, cardiometabolic diseases, and types of cancer, primarily functioning as an anti-inflammatory agent and a selective antioxidant [15].
Molecular hydrogen can upregulate the enzymatic antioxidant systems, including glutathione peroxidase, superoxide dismutase, and catalase [14]. H2 can selectively scavenge the most harmful reactive oxygen/nitrogen species, e.g., hydroxyl radical and peroxynitrite, without affecting beneficial ones, e.g., hydrogen peroxide or nitric oxide [13, 15].
The consumption of HRW has been reported to be beneficial for daily life [16]. Due to the antioxidant and anti-inflammatory properties of HRW, the present study investigated if HRW can be used to treat PMS, a condition involving inflammation and oxidative stress in its pathophysiology. No study has been found in the literature investigating the effects of HRW consumption on PMS symptoms and quality of life. To the best of our knowledge, this study represents the first investigation conducted in this area.
Aim
This study aimed to determine the effect of HRW consumption on premenstrual symptoms and the quality of life of women (students) experiencing premenstrual syndrome.
Hypotheses
H01 There is no significant difference in the Premenstrual Syndrome Scale scores between participants consuming HRW and those in the control group.
H02 There is no significant difference in the Short form of the World Health Organization Quality of Life Questionnaire scores between participants consuming HRW and those in the control group.
Materials and methods
Design
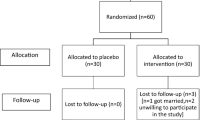
This study is a single-center, parallel-group, stratified, blocked, randomized, controlled experimental study. Every step of the study was conducted according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Fig. 1). This study was registered in the Clinical Trials registry system (https://clinicaltrials.gov/study/NCT05556252#study-record-dates) with the registration number NCT05556252 on 27/09/2022.
Population and sample
The study was conducted at the Faculty of Nursing, Ankara University (Turkey). The Faculty consists of departments of midwifery and nursing, with the majority of students being female. Initially, 775 volunteers were evaluated to assess those meeting the inclusion criteria for premenstrual syndrome (PMS) using the Premenstrual Syndrome Scale. Participants were classified as experiencing PMS if they scored 132 or above on the scale defined by Gençdoğan B. [17]. At this stage, it was found that 366 (47.2%) of the participants experienced PMS. Among these identified participants experiencing PMS, those willing to participate in the randomized controlled trial were evaluated based on the inclusion criteria for enrollment. The inclusion criteria for the study were having a menstrual cycle length within the normal range (21-35 days), regular menstruation in the last three cycles, scoring 132 or above on the Premenstrual Syndrome Scale [17], not receiving medical treatment for PMS, and not having any psychiatric diagnosis. The exclusion criteria included having received a psychiatric diagnosis, having any gynecological disease (such as abnormal uterine bleeding, myoma, ovarian cyst, etc.), using a hormonal contraceptive medication, non-compliance with the research plan by the participants, having pregnancy and participants initiating PMS treatment during the study period.
The study consisted of intervention and control groups. Individuals consuming HRW were assigned to the intervention group, while individuals consuming placebo water were assigned to the control group. G*Power 3.1.9 software was used to calculate the sample size for this study. No studies were found in the literature investigating the effects of HRW on premenstrual syndrome. Therefore, the study conducted by Taavoni et al. (2014) [18], which evaluated the effects of Royal Jelly on premenstrual syndrome, was used as a basis, and the minimum required sample size for a power of 0.9 and an alpha level of 0.05, with an effect size (d) of 0.73, was determined to be 33 for both the intervention and control groups, totaling 66 participants. A total of 98 individuals agreed to participate in the study. Among them, 10 had menstrual irregularities, 6 had a psychiatric diagnosis, 7 had a gynecological disease diagnosis, and 3 were using oral contraceptives. Therefore, a total of 72 volunteers were included in the study.
The volunteers were sorted in ascending order according to their student numbers. The block randomization method was utilized for assigning volunteers to groups in accordance with this sequence. The randomized block procedure was performed as follows: (a) block size of 6 was selected; (b) subjects were calculated as having 12 conditions (ABBBAA, ABABBA, BABBAA, BBABAA, BAABBA, ABBAAB, BBBAAA, ABABBA, BABAAB, BABABA, AABBAB and BBABAA); and (c) blocks were randomly selected to determine the assignment of all 72 participants, with an allocation ratio of 1:1. The study was completed with 33 participants in the intervention group and 32 participants in the control group (Fig. 1).
There was no statistical difference in the descriptive characteristics of the intervention and control groups (Table 1).
The study was conducted as a double-blind trial. For participant blinding, participants in the control group were provided with a bottle resembling a hydrogen water generator. HRW is colorless, odorless, and tasteless. Therefore, participants were not expected to discern the properties of the water they consumed [19]. Additionally, to prevent bias during the data collection process, the data collection forms used in the study were administered by a researcher unaware of the participants' group allocations.
The implementation of the study
The following interventions were implemented in the study groups.
Intervention group
Participants in this group were provided with HRW for consumption. Each participant was given a 500 mL portable hydrogen water generator (Novasmart, Turkey). Additionally, they were provided with drinking water (Sırma, Turkey) (0.5 L per PET bottle with 2.0 L per day) to prepare HRW. After filling the portable hydrogen water generator with drinking water, it was run for 5 minutes to achieve the saturation level of hydrogen [19, 20]. In a preliminary application conducted by researchers, it was observed that the H2 concentration of hydrogen-rich water (HRW) gradually decreased after preparation. Consistent with this observation, participants were instructed to consume HRW immediately after preparation [19]. Before starting the intervention, participants received training on how to use the hydrogen water generator. The consumption of HRW was individually tailored to each participant's menstrual cycle. Each participant commenced HRW consumption approximately on the 16th day of their menstrual cycle, coinciding with the onset of the secretory phase, and continued until the 2nd day of the menstrual phase. This pattern was maintained in the subsequent cycles. As part of the study, participants were instructed to consume 300-400 mL of HRW in the following time intervals: one hour before breakfast, one hour before lunch, two hours after lunch, one hour before dinner, and half an hour before bedtime. Thus, participants were requested to consume a minimum of 1500 mL and a maximum of 2000 mL HRW daily [21]. Since the application for changes in PMS symptoms should last for three menstrual cycles [1, 22], participants in this study were also provided with HRW for three cycles. A water intake tracking chart monitored participants' adherence to the program.
Control group
Participants in this group were provided with a flask similar to the hydrogen water generator but did not produce hydrogen or change the water properties. Participants in the control group (placebo) were instructed to consume the same amount of water (Sırma, Turkey) (0.5 L per PET bottle with 2.0 L per day) on the same days as the intervention group. A water intake tracking chart monitored participants' adherence to the program.
Data collection
The research data were collected using a Demographic Information Form, Premenstrual Syndrome Scale, and Short form of the World Health Organization Quality of Life Questionnaire.
Demographic information form
The form included questions to determine participants' socio-demographic characteristics and menstrual history. All participants completed this form after being assigned to their respective groups.
The Premenstrual Syndrome Scale (PMSS)
The Premenstrual Syndrome Scale (PMSS), developed, validity and reliability study was conducted by Gençdoğan (2006) based on DSM-III and DSM-IV-R, is a five-point Likert scale consisting of 44 items that measure the severity of premenstrual symptoms. The scale includes nine subscales: depressive mood, anxiety, fatigue, irritability, depressive thoughts, pain, appetite changes, sleep changes, and swelling. The Total Score of the PMSS can range from a minimum of 44 to a maximum of 220. An increase in the score indicates an increase in the intensity of PMS symptoms. Participants scoring 132 and above on the scale are considered to have PMS. The total Cronbach's alpha reliability coefficient of the scale is 0.75 [17]. In this study, Cronbach's alpha reliability coefficient for the initial measurement is 0.87.
The measurement times for assessing the severity of PMS were determined based on the study conducted by [22]. All participants' premenstrual symptoms were evaluated three times: before starting the intervention (on days 7-10 of their last menstrual cycle), after the application of three cycles of HRW/placebo water (on days 7-10 of menstruation), and one month after the completion of HRW/placebo water application (on days 7-10 of subsequent menstrual cycles).
Short form of the World Health Organization Quality of Life Questionnaire (WHOQOL- BREF)
In 1980, the World Health Organization (WHO) developed the WHOQOL-100 scale to define the quality of life and enable cross-cultural comparisons. The WHOQOL-BREF is a short form of 27 items derived from the WHOQOL-100 [23]. This form's Turkish validity and reliability study was conducted by [24]. The scale included 27 questions, each one scored on a scale of 1 to 5, with scores ranging from 0 to 100. The remaining questions were used to calculate scores for the physical health, psychological, social, and environmental domains, except for the first two general questions. Higher scores on the scale indicate a higher quality of life. The Cronbach's alpha values for the WHOQOL-BREF are as follows: physical health 0.83, psychological 0.66, social 0.53, and environment 0.73 [24]. In this study, Cronbach's alpha reliability coefficients for the initial measurement were calculated as follows: physical health 0.61, psychological 0.70, social 0.56, and environment 0.73.
The measurement times for WHOQOL-BREF were determined based on when the severity of PMS was assessed. All participants' premenstrual symptoms were evaluated three times: before starting the intervention (on days 7-10 of their last menstrual cycle), after the application of three cycles of HRW/placebo water (on days 7-10 of menstruation), and one month after the completion of HRW/placebo water application (on days 7-10 of subsequent menstrual cycles).
Data analysis
The statistical analysis was performed using the SPSS Windows 24.0 software package, and a significance level of P<0.05 was considered. The normality of the data was assessed by examining the skewness and kurtosis values, and values within the range of ±2 were considered indicative of a normal distribution [25]. The Chi-Square statistic was utilized to test relationships between categorical variables. Independent Samples t-test was employed to compare the independent groups in terms of menstrual characteristics, PMSS, Physical health, Social domain, Environment domain scores, and Psychological domain scores at the initial two measurements. Repeated-measures MANOVA was used to examine significant differences in the PMSS, Physical health, Social domain, and Environment domain scores across time among the groups. For the comparison of the independent groups in the Psychological domain at the third follow-up measurement, the Mann-Whitney U test was conducted, while the Friedman test was utilized to assess time-dependent changes.
Results
When comparing the mean PMSS scores between the intervention and control groups, it was found that the mean scores were not statistically different at the baseline (P>0.05), but in the first and second follow-ups, the intervention group had significantly lower mean scores compared to the control group (P<0.05) (Fig. 2). Group × time interaction was significant for PMSS (F = 10.54, P< 0.001) (Table 2).
When comparing the mean scores of the WHOQOL- BREF between the intervention and control groups, it was found that the mean scores in all subscales were not statistically different at the baseline (P>0.05). However, in the first follow-up, the intervention group had significantly higher mean scores in Physical Health (Fig. 3) and median scores in the Psychological Domain compared to the control group (P<0.05) (Table 3). In the first follow-up, the scores in the Social Domain (Fig. 4) and Environment Domain (Fig. 5) were similar in both groups (P>0.05), while in the second follow-up, all domains had similar scores in both groups (P>0.05). Group × time interaction was not significant for WHOQOL- BREF (P>0.05) (Table 3).
Discussion
This study represents the first investigation into the effects of consuming HRW on premenstrual symptoms and quality of life in students experiencing premenstrual syndrome (PMS). Our findings indicate that the consumption of HRW in this study reduced PMS symptoms and improved quality of life, particularly in the physical health and psychological domains. No previous studies have been found in the literature examining the effects of HRW consumption on PMS. However, Zhang et al. (2021) have determined that supplements containing exogenous H2 play essential roles in regulating the homeostasis of sexual organs in females. H2 supplementation has been shown to alleviate ovarian injuries induced by ischemia/reperfusion (I/R) and certain drugs [26]. Additionally, it can mitigate premature ovarian failure (POF) and promote follicle development. Furthermore, H2 has decreased inflammation in the uterus [26]. It has been revealed that HRW decreased the malondialdehyde, cortisol, and testosterone in female rats with polycystic ovary syndrome [27]. Another report revealed that HRW alleviated epithelial degeneration and necrosis in muscle fibers of tissues in animal models [28].
Increased oxidative stress [29, 30] and decreased antioxidant capacity may occur in PMS. Imbalance in the oxidant/antioxidant systems has been reported as a potential cause or consequence of various stress symptoms in PMS [30]. As a therapeutic medical gas, molecular hydrogen possesses antioxidant properties and selectively scavenges cytotoxic reactive oxygen species (ROS) in tissues, thereby reducing tissue inflammatory events [31]. Based on this information, it is hypothesized that HRW consumption may reduce PMS by restoring the balance of oxidant/antioxidant systems and reducing excessive inflammation levels.
Another action mechanism reported that H2 acts similarly to estrogen derivatives by upregulating the endogenous expression of antioxidant enzymes and peptides as an antioxidant [32]. During the late luteal phase of the menstrual cycle, estrogen hormone levels are low in preparation for menstruation. These low levels appear to be a factor in the onset of PMS symptoms [33]. Consumption of HRW may positively benefit PMS by temporarily increasing estrogen concentration without significantly altering the menstrual cycle.
The presence of PMS in women negatively affects physical health, psychological health, and social relationships, leading to a decrease in overall quality of life [34]. Premenstrual symptoms can cause impairments in physical functioning and psychological health. Studies comparing women with and without PMS have shown that women with PMS experience adverse effects on the physical and psychological domains of quality of life [4, 35, 36]. No studies that specifically investigate the impact of HRW consumption on the quality of life in women with PMS have been encountered. However, Kang et al. (2011) demonstrated in their study that consumption of HRW for six weeks reduced reactive oxygen metabolites in the blood, maintained blood oxidation potential, and significantly improved quality of life scores during radiotherapy in cancer patients compared to those who received a placebo [37]. Mizuno et al. (2017) also reported that four weeks of HRW application in adult volunteers improved mood, reduced anxiety, enhanced autonomic nervous system function, and strengthened quality of life [38]. In the literature, the consumption of HRW has been reported to reduce fatigue in healthy individuals [38] and improve cognitive function and anxiety levels [39]. These findings indicate that HRW may be effective in enhancing quality of life.
Conclusions
In conclusion, this study has demonstrated that consumption of HRW may reduce the severity of PMS symptoms and improve individuals' quality of life. Therefore, it is suggested that HRW consumption could be considered as an option for reducing the severity of PMS symptoms. HRW consumption is a simple practice that individuals can easily perform in their homes or workplaces. This method is believed to be easily applicable by everyone, practical, promoting individual responsibility for health, and cost-effective. Hence, it is recommended that nurses and other healthcare professionals consider the use of HRW in helping individuals cope with PMS and improving their quality of life.
Limitations: Participants could not be followed for over two menstrual cycles after completing the intervention. Therefore, the long-term effectiveness of the intervention could not be evaluated. The study participants were recruited from a single center and consisted of nursing students. Thus, the generalizability of the findings to other populations may be a limited issue. Therefore, it is recommended that more extended follow-up studies be conducted in broader populations.
Availability of data and materials
The data supporting this study's findings are available on request from the corresponding author.
Abbreviations
- PMS:
-
Premenstrual syndrome
- GABA:
-
Gamma-amino butyric Acid
- H2 :
-
Molecular hydrogen
- HRW:
-
Hydrogen-rich water
References
Behboudi-Gandevani S, Hariri FZ, Moghaddam-Banaem L. The effect of omega 3 fatty acid supplementation on premenstrual syndrome and health-related quality of life: a randomized clinical trial. J Psychosom Obstet Gynecol. 2018;39:266–72.
Hofmeister S, Bodden S. Premenstrual syndrome premenstrual dysphoric disorder. Am Fam Physician. 2016;94:236–40.
Çitil ET, Çitil Canbay F. Premenstrual syndrome severity in Turkey: a meta-analysis study. Psychol Health Med. 2023;28:1783–94.
Farrokh-Eslamlou H, Oshnouei S, Heshmatian B, Akbari E. Premenstrual syndrome and quality of life in Iranian medical students. Sex Reprod Healthc. 2015;6:23–7.
Sahin S, Ozdemir K, Unsal A. Evaluation of premenstrual syndrome and quality of life in university students. J Pak Med Assoc. 2014;64:915–22.
Al-Shahrani AM, Miskeen E, Shroff F, Elnour S, Algahtani R, Youssry I, et al. Premenstrual syndrome and its impact on the quality of life of female medical students at Bisha University, Saudi Arabia. J Multidiscip Healthc. 2021;14:2373–9.
Kustriyanti D, Rahayu H. Prevalence of premenstrual syndrome and quality of life among health science college student. Int J Public Heal Sci. 2020;9:15–9.
Granda D, Szmidt MK, Kaluza J. Is premenstrual syndrome associated with inflammation, oxidative stress and antioxidant status? A systematic review of case-control and cross-sectional studies. Antioxidants. 2021;10:604.
Nillni YI, Rasmusson AM, Paul EL, Pineles SL. The impact of the menstrual cycle and underlying hormones in anxiety and PTSD: what do we know and where do we go from here? Curr Psychiatry Rep. 2021;23:1–9.
Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017;174:980–9.
Appleton SM. Premenstrual syndrome: evidence-based evaluation and treatment. Clin Obstet Gynecol. 2018;61:52–61.
Alwazeer D. Consumption of Hydrogen-Treated Foods Provides Nutritional and Health Benefits. In: Slezak J, Branislav K, editors. Molecular Hydrogen in Health and Disease. Springer Cham; 2024. p. In press.
Alwazeer D, Liu FF-C , Wu XY, LeBaron WT. Combating oxidative stress and inflammation in COVID-19 by molecular hydrogen therapy: mechanisms and perspectives. Oxid Med Cell Longev. 2021. https://doi.org/10.1155/2021/5513868.
Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res. 2013;3:1–12.
Ostojic SM. Should hydrogen therapy be included in a musculoskeletal medicine routine? F1000Research. 2016;5:5–7.
Fukai Y. Molecular hydrogen for medicine: the art of ancient life revived. In: Springer Nature. 2020.
Gençdoğan B. Premenstrual sendrom için yeni bir ölçek. Türkiye’de Psikiyatr. 2006;8:81–7.
Taavoni S, Barkhordari F, Goushegir A, Haghani H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: a randomized, triple-blind, placebo-controlled study. Complement Ther Med. 2014;22:601–6.
Timón R, Olcina G, González-Custodio A, Camacho-Cardenosa M, Camacho-Cardenosa A, Guardado I. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol Sport. 2021;38:269–75.
Hatae T, Miwa N. Electrolytic hydrogen-generating bottle supplies drinking water with free/combined chlorine and ozone repressed within safety standard under hydrogen-rich conditions. Med Gas Res. 2021;11:61.
Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome—an open label pilot study. J Clin Biochem Nutr. 2010;46:140–9.
Siahbazi S, Behboudi-Gandevani S, Moghaddam-Banaem L, Montazeri A. Effect of zinc sulfate supplementation on premenstrual syndrome and health-related quality of life: clinical randomized controlled trial. J Obstet Gynaecol Res. 2017;43:887–94.
WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–8.
Eser E, Fidaner H, Fidaner C, Eser SY, Elbi H, Göker E. Psychometric properties of the WHOQOL-100 and WHOQOL-BREF. J Psychiatry Psychol Psychopharmacol. 1999;7:23–40.
George D, Mallery M. Using SPSS for Windows step by step: a simple guide and reference. 2003.
Zhang Y, Liu H, Xu J, Zheng S, Zhou L. Hydrogen gas: a novel type of antioxidant in modulating sexual organs homeostasis. Oxid Med Cell Longev. 2021;2021:8844346.
Makav M, Kuru M, Aras ŞY, Sarı EK, Bulut M, Alwazeer D. The effect of hydrogen-rich water on letrozole-induced polycystic ovary syndrome in rats. Reprod Biomed Online. 2023;47:103332.
Köktürk M, Yıldırım S, Eser G, Bulut M, Alwazeer D. Hydrogen-Rich Water Alleviates the Nickel-Induced Toxic Responses (Inflammatory Responses, Oxidative Stress, DNA Damage) and Ameliorates Cocoon Production in Earthworm. Biol Trace Elem Res. 2022;200:3442–52.
Balat O, Dikensoy E, Ugur MG, Atmaca R, Cekmen M, Yurekli M. Malon dialdehyde, nitrite and adrenomedullin levels in patients with premenstrual syndrome. Arch Gynecol Obstet. 2007;275:361–5.
Duvan CI, Cumaoglu A, Turhan NO, Karasu C, Kafali H. Oxidant/antioxidant status in premenstrual syndrome. Arch Gynecol Obstet. 2011;283:299–304.
Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11.
Russell G, Nenov A. Molecular hydrogen therapies and the benefits for menopausal and perimenopausal women: an aphoristic review. GSC Biol Pharm Sci. 2022;21:112–5.
Sanchez BN, Kraemer WJ, Maresh CM. Premenstrual syndrome and exercise: a narrative review. Women. 2023;3:348–64.
Çelik A, Uskun E. Premenstrüel sendrom prevalansı ve yaşam kalitesi ile ilişkisi: toplum tabanlı bir çalışma örneği. Pamukkale Med J. 2022;15:1–13.
Bakhshani NM, Aghashahi Z, Pour KL, Yaghmaei M. Relationship between symptoms of premenstrual syndrome (PMS) and quality of life (QOL) in the adolescents. Life Sci J. 2013;10(2s):265–8.
El-Masry NM, Abdelfatah NR. Quality of life and burden of women with premenstrual dysphoric disorder. Egypt J Psychiatry. 2012;33:45–50.
Kang K-M, Kang Y-N, Choi I-B, Gu Y, Kawamura T, Toyoda Y. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res. 2011;1:1–8.
Mizuno K, Sasaki A, Ebisu K, Tajima K, Kajimoto O, Nojima J, et al. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med Gas Res. 2017;7:247–55.
Lee D, Choi J-I. Hydrogen-rich water improves cognitive ability and induces antioxidative, antiapoptotic, and anti-inflammatory effects in an acute ischemia-reperfusion injury mouse model. Biomed Res Int. 2021;2021:9956938.
Acknowledgments
Not applicable.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Ankara University. This study was registered in the Clinical Trials registry system (https://clinicaltrials.gov/study/NCT05556252#study-record-dates) with the registration number NCT05556252 on 27/09/2022.
Funding
This research was supported by Ankara University Scientific Research Projects Coordination Unit with project ID 2438.
Author information
Authors and Affiliations
Contributions
Conceptualization, MA, İ.G., MB, and DA; methodology, MA and İ.G.; validation, MA and İ.G.; formal analysis MA..; investigation, MA and D.Ç.; writing—original draft preparation, MA and İ.G.; writing—review and editing, MA, İ.G., MB, DA, and TWL; supervision, DA; project administration, MA; funding acquisition, MA.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study obtained ethical approval and institutional permission from the Ankara University Ethics Committee. The study was carried out in accordance with the principles of the Helsinki Declaration. Throughout the entire process of the study, adherence to the principles of autonomy, prevention of harm, promotion of good, justice, objectivity, transparency, fairness, and integrity was maintained. Written informed consent was obtained from the participants prior to commencing the study. All researchers acknowledge that no situations arose that could lead to conflicts of interest with the participants; participants have the right to participate in the study, to provide or not provide information, and that this right has not been violated, and discrimination has not occurred among the volunteers. All researchers acknowledge that there were no financial interests or personal benefits that could affect or appear to affect the impartial and objective execution of their duties or that of the volunteers involved in the study. The volunteers' identities remained confidential and were not used in the research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aker, M.N., Gönenç, İ.M., Çalişici, D. et al. The effect of hydrogen-rich water consumption on premenstrual symptoms and quality of life: a randomized controlled trial. BMC Women's Health 24, 197 (2024). https://doi.org/10.1186/s12905-024-03029-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-03029-8