Abstract
Purpose
To investigate oxidant/antioxidant status in premenstrual syndrome (PMS).
Methods
Study group (n = 20) consisted of PMS and control group (n = 21) consisted of normal menstruating women. The serum oxidant status was assessed by the lipid hydroperoxide (LHP), malondialdehyde (MDA) and protein carbonyl (PC); the antioxidant status was assessed by the total thiol (T-SH) and total antioxidant capacity (TAC).
Results
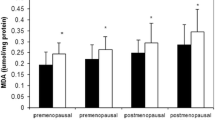
The study and control groups revealed no statistical difference, in terms of day 3 LHP, MDA, PC, T-SH and TAC levels. There were no significant differences between groups in terms of day 21 MDA, PC and T-SH levels. However, day 21 LHP levels were increased and TAC levels were decreased in the study group compared with the control group.
Conclusion
Increased oxidative stress and reduced antioxidant capacity may occur in PMS. It can be speculated that the imbalance of oxidant/antioxidant systems may be a cause or the consequence of the various stress symptoms in PMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Premenstrual syndrome (PMS) is a common disorder among women of reproductive age, which includes a broad group of physical, emotional and behavioral symptoms [1, 2]. The hallmark of PMS is the cyclic pattern of symptoms that begin in the late luteal phase of the menstrual cycle and subside shortly after the onset of menstruation [3]. Premenstrual symptoms have a significant negative impact on a woman’s quality of life and professional productivity [4, 5]. Actually, the exact cause of PMS is still not completely understood, but many theories are offered, including; diversified genetic vulnerability, sensitivity to hormonal fluctuations and altered brain processes [6, 7]. Although, none of these theories have gained universal acceptance, it is speculated that gonadal hormones appear to mediate changes in the activity of central neurotransmitters, such as serotonin and modulation of gamma-aminobutyric acid (GABA) receptors in the brain [8, 9]. Elucidation of the pathophysiologic mechanisms of PMS should make a more precise diagnosis possible and provide guidance for targeted therapeutic interventions.
In a healthy body, reactive oxygen species (ROS) and antioxidants remain in balance. Oxidative stress (OS) results from an oxidant/antioxidant imbalance, an excess of oxidants and/or a depletion of antioxidants. OS has been implicated in more than 100 diseases [10, 11]. PMS has a wide spectrum of stress symptoms, such as affective and somatic complaints thus differential diagnosis must be done before administration of any treatment. The most common psychiatric disorders confused with PMS are major depression and anxiety. Several studies demonstrated increased OS in psychiatric disorders [12, 13].
Despite the presence of many studies performed on oxidative status in psychiatric disorders, only few studies exist in the literature with limited measurable serum markers of the oxidant/antioxidant status in PMS. There is also growing literature on the effects of OS in gynecologic and obstetric disorders, such as etiopathogenesis of endometriosis, the pathophysiology of preeclampsia and hydatidiform mole [14–16].
Hence, we planned to evaluate oxidant/antioxidant status with more measurable serum markers in patient with PMS and to make suggest the possible relation of OS in the pathophysiologic mechanisms of PMS.
Materials and methods
Forty-one patients were enrolled in the study, between May 2007 and October 2007 in Fatih University Faculty of Medicine. Patients were allocated into two groups: group I was (study group) consisting of 20 patients with PMS, whereas group II was (control group) 21 normal women. The study was approved by the Institutional Review Board of Fatih University and written informed consents were obtained from each of the subjects. All women were aged between 22 and 39 years and had regular menses for at least six previous cycles. Diagnosis of PMS was confirmed by the Daily Symptom Rating Scale (DSR) that included 19 symptoms [17]. Both the PMS group and the control group completed the DSR for 2 months. We obtained rating for each symptom from each woman for 2 months and averaged the scores. Subjects were positively diagnosed with PMS if they scored above a cumulative threshold value of 70 on the last 6 days of the cycle in the rating system. DSR also measured the severity of common symptoms of PMS with each symptom rated on 1–5 scale. The DSR was included the following symptoms: irritability, nervous tension, anxiety, mood swings, feeling out of control, confusion, swelling, depression, fatigue, insomnia, poor coordination, crying, food craving, headache, other aches, breast tenderness, cramps, concentration difficulties and decreased interest in activities.
Before study each woman underwent a medical evaluation that included a physical and pelvic examination, laboratory tests. Women who were pregnant, lactation, under treatment for PMS or with a history of psychiatric disorders, such as any current or past psychotropic drugs use and/or who have ever undergone psychiatric therapy or those taking oral contraceptive pills, thyroid and other endocrine disorders were excluded.
Serum oxidant status was evaluated by measuring lipid hydroperoxides (LHP), malondialdehyde (MDA) and protein carbonyl (PC); serum antioxidant status was evaluated by measuring total thiol (T-SH) levels and total antioxidant capacity (TAC) in patients with PMS and in healthy controls. Ovulation was confirmed by means of day 21 progesterone level which was >3 ng mL−1. Blood samples were obtained from each woman at the 3rd and 21st day of menstrual cycle and oxidant and antioxidant parameters were assessed in these samples for each patient.
Measurement of plasma LPH levels
Plasma LHP levels were determined according to the method of ferrous oxidation with xylenol orange [18], 90 μL plasma was transferred into microcentrifuge tubes; 10 mM triphenylphosphine was added to vials to remove hydroperoxides; 10 μL methanol alone was added to the remaining vials. The LHP content in the plasma samples was determined as a function of the absorbance difference of samples with and without elimination of LHPs by TPP. The samples were vortexed and incubated at room temperature for 30 min. 900 μL FOX2 reagent was added into samples. After incubation at room temperature for 30 min, the samples were centrifuged at 12,000g at 25°C for 10 min; the supernatant was carefully placed into well plate. A Bio-Tek ELX800 absorbance microplate reader (Bio-Tek Instruments Inc., USA) was used to measure the absorbance at a wavelength between λ = 560 nm. Concentration of LHP was calculated using the extinction coefficient of blue-purple complex, 1.5 × 104 M−1 cm−1. Intra- and inter-assay coefficients for LHP assay were 9.8% n = 10 and 10.2% n = 12, respectively.
Measurement of plasma MDA levels
Thiobarbituric acid reactive substances (TBARS) such as MDA were determined according to the method described by Ohkawa et al [19]. 0.1 mL homogenate was mixed with 0.5 mL of 20% trichloroacetic acid, 1 mL of 0.67% thiobarbituric acid, and the mixture was incubated at 90°C for 30 min and then cooled. TBARS was extracted with 2 mL n-butanol and the mixture centrifuged at 3,000 rpm for 10 min. The resulting pink-stained TBARS was determined in a spectrophotometer at 532 nm. Calibration curve was performed using 1,1,3,3-tetramethoxypropane subjected to the same treatment as that of the samples. Intra- and inter-assay coefficients for TBARS assay were 4.5% n = 8 and 4.7% n = 10, respectively.
Measurement of plasma PC levels
A PC assay kit was utilized to measure the amount of PC in plasma samples; 800 μL volume of DNPH was added to the blank tube and 800 μL of 2.5 M HCL to the sample tube. After 1-h incubation, 1 mL of trichloroacetic acid was added for precipitation of proteins. Both tubes were centrifuged again to remove any debris and placed in the well plate. A plate reader was used to measure the absorbance at a wavelength between 340 and 370 nm. Concentration of PC was calculated using the extinction coefficient of DNPH, 2.2 × 104 mol−1 cm−1. According to PC assay kit protocol, intra- and inter-assay coefficient of variation were 4.7% n = 4 and 8.5% n = 4, respectively.
Measurement of T-SH plasma levels
Plasma total thiol levels were measured according to Sedlak and Lindsay method [20]. Plasma samples were mixed with Tris–EDTA (0.2 M), pH 8.2, 5,5′-dithiobis(2-nitrobenzoic acid) (0.01 M) and absolute methanol. A reagent blank (without sample) and a sample blank (without DTNB) were prepared in a similar manner. The reaction mixtures were centrifuged at 5,000g at room temperature for 10 min. The absorbances of the supernatants were read in a Shimadzu UV-1601 spectrophotometer at λ max = 412 nm. The molar extinction coefficient at λ max = 412 nm 13 100 mol−1 cm−1 was used for calculation of total thiol levels. Intra- and inter-assay coefficients for total thiol determination assay were 5.8% n = 10 and 6.2% n = 10 respectively.
Measurement of plasma TAC levels
Plasma antioxidant status was evaluated using FRAP assay [21]. In this assay, at low pH, a ferric-2,4,6-tripyridyl-s-triazine (FeIII–TPTZ) complex is reduced to the ferrous form, which is blue colored and monitored by measuring the change in absorbance at 593 nm. The change in absorbance is directly proportional to the reducing power of the electron-donating antioxidants present in the plasma. 300 mM of acetate buffer (pH 3.6), 10 mmol L−1 2,4,6-tri-pyridyl-s-triazine in 40 mM HCl and 20 mM FeCl3·6H2O in the ratio of 10:1:1 yield the working FRAP reagent. Fe(II) standards are used. 180 μL of working FRAP reagent is mixed with 30 μL plasma or standard in well plate. After exactly 5 min at room temperature, the absorbance at 593 nm was read against reagent blank in a plate reader. Intra- and inter-assay coefficients for FRAP assay were 7.2% n = 8 and 8.4% n = 20, respectively.
Statistical analysis
Data analysis was performed using SPSS (Statistical Package for Social Science, version 11.5) software package. Whether the continuous variables were normally distributed or not was determined using Shapiro–Wilk test. Descriptive statistics was shown as mean ± standard deviation for continuous variables. Inter-group comparisons regarding 3rd and 21st measurements were evaluated by independent samples t test or Mann–Whitney U test, where applicable. The differences between 3rd and 21st day measurements within groups were evaluated using paired sample t test or Wilcoxon sign rank test, where appropriate. A P < 0.05 was considered statistically significant.
Results
The mean age of women was 28.5 ± 4 years. Age, body mass index of study and control group was 29.23 ± 4.79 and 28.05 ± 4.66, 23.54 ± 3.85 and 22.82 ± 3.95, respectively. There were no significant differences between groups in terms of age and body mass index. There were no differences in the mean progesterone levels in the study and the control groups, respectively (15.5 ± 2.94, 11.0 ± 5.15, P > 0.05).
There was no statistical difference between the 3rd and the 21st day LHP, MDA, PC, T-SH and TAC levels in the control group (P > 0.05).
There were no statistical differences between the 3rd and the 21st day for MDA, PC and T-SH levels in the study group. However, while LHP level was increased, TAC level was decreased on the 21st day, compared with the 3rd day in study group, both changes being statistically significant, respectively (P = 0.02, P = 0.02).
Comparison between the study and control groups revealed no statistical difference in terms of day-3 LHP, MDA, PC and T-SH and TAC levels. In the study group, on 21st day, while LHP levels were increased, TAC levels were decreased and these differences were statistically significant (P = 0.01, P = 0.01) (Table 1).
Discussion
As a result of a wide range of affective and somatic complaints in PMS, patients may consult a variety of clinicians, including primary care physicians, gynecologists, psychiatrists or other specialists. A differential diagnosis is essential in evaluating a woman for a premenstrual disorder. It has been estimated that the symptoms reported by as many as three fourths of patients who present with PMS can be attributed to another cause, such as premenstrual exacerbation of a medical condition or psychiatric disorders [22]. The most common psychiatric disorders confused with PMS are major depression and anxiety. Several studies investigated the role of OS in psychiatric disorders. For example, Khanzode et al. [12] indicated that major depression is associated with increased levels of serum superoxide dismutase (SOD), serum MDA and decreased levels of plasma ascorbic acid. Arranz et al. found impaired immune function and increased cytokine release in anxious women. They considered that this might be related to increased cortisol secretion, which would lead to OS reflected in lowered plasma TAC [13].
Limited numbers of studies exist in literature about OS and PMS. In a study, Kalia et al. [23] investigated the levels of lipid peroxidation product-MDA and antioxidants, such as SOD, glutathione and ceruloplasmin in PMS patients. They did not detect any significant differences between the control and the PMS patients in terms of lipid peroxidation product and antioxidant levels. Likewise, in another study, Balat et al. [24] reported that there were no changes in the levels of MDA in PMS patients. They suggested that estrogens as well as progestin may exert a protective and adaptive effect as potent antioxidants. In contrast to the comments of these studies, it is the literature consensus that gonadal steroids are same in women with and without PMS [25, 26]. Different from these studies; our results are not correlated with them in terms of elevated LHP levels which is an early marker of the oxidation chain of lipids and decreased TAC levels which also consists of several antioxidant markers in patients with PMS. Both of the studies above, investigated only MDA levels as a marker of OS [23, 24]. However, measuring MDA levels is limited in detecting OS in some cases, and may not be sufficient enough to show OS in PMS [27]. In our study, besides MDA, we measured LHP levels, and detected an increase in LHP levels in PMS patients. We also investigated plasma levels of carbonyl-modified proteins which are the markers of oxidative damage. OS damages all intracellular components; not only lipids, but also proteins. However, we did not demonstrate any increase in PC levels both within and between groups, as an indication of protein oxidation. To our knowledge, there are no other studies in the literature investigating the protein oxidation in PMS. We also did not detect any differences between groups in terms of T-SH level, which is a member of the antioxidant markers. However, we found a significant reduction in TAC levels which consists of several antioxidant markers and reflect TAC in PMS group. As the TAC levels were reduced, and one of the indicators of oxidative status, i.e. LHPs levels, were significantly increased in PMS patients, the oxidant/antioxidant balance shifted significantly to the oxidant side.
Although pathophysiologic mechanisms in producing the complex symptoms of PMS still seem to be debating, several biological mechanisms are suggested. Recently, failure to identify gross aberrations in plasma concentrations of the reproductive hormones has led investigators to search for a common link between the dynamic neuroendocrine secretory events that characterize the menstrual cycle and central mechanisms regulating behavior and mood states. Especially, GABAergic system has been implicated in the pathophysiology of menstrual cycle-linked disorders [9, 28] as well as anxiety disorders [27]. It is well known that neuronal membrane contains a high proportion of polyunsaturated fatty acid and more vulnerable to the toxic effects of free radicals, as they have a high rate of catecholamine oxidative metabolic activity. Neuronal membrane dysfunction can be secondary to free radical-mediated pathology, and may effect GABAerjic system in PMS or existing impaired GABAerjic activity in the brain can induce metabolic processes that secondary lead to increase free radical production in the PMS patients. Experimental trial with mice strengthen of this suggesting that excessive opioid exposure to the brain during the luteal phase cause OS, such as increased cellular levels of ROS over the antioxidant capacity. This can lead in triggering the oxidative cell damage (i.e. DNA breaks, protein inactivation, altered gene expression, loss of membrane lipid-bound essential polyunsaturated fatty acids and often apoptosis) and vulnerability factors generating to abnormal neuronal excitability and causes affective symptoms of PMS [29].
When we interpret all our results, we can suggest that increased OS and reduced antioxidant capacity may occur in patients with PMS, but whether the imbalance of oxidant/antioxidant status is one of the reasons of distribution of GABAergic activity in PMS patients or a consequence of the excessive opioid activity in PMS is still not known.
The limitation of this study is that the patients were not screened for other possible diagnoses, such as depression and premenstrual exacerbation when they reported PMS-like symptoms. Practically, many clinicians do not routinely screen for psychiatric disorders but, diagnosis of PMS requires the exclusion of any psychiatric disorders that might explain the symptoms. On the other hand, in the study group mean actual differences (95% CIs) between days 3 and 21 regarding LHP and TAC were calculated as −0.04 (−0.07 to −0.01) and 165.6 (32.1–299.2), respectively. Although the difference in TAC was both statistically and clinically significant, the difference in LHP might be considered only statistically significant. We think that studies with larger numbers of patients are needed. Thus, the statistically significant result in LHP is clinically significant as well.
In conclusion, we demonstrated an imbalance in oxidant/antioxidant status in patients with PMS. These results give us to think that the role of impaired oxidative balance in the biochemical basis evoked in the pathophysiologic mechanisms of PMS, as well as the role of antioxidants in the therapeutic strategy and their implication in preventive approaches in patients with PMS. Before any other suggestions, further clinical researches with larger numbers of patients are required to clarify the relation of oxidant/antioxidant balance and pathophysiologic mechanisms of PMS.
References
Freeman EW (2003) Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology 28:25–37
Halbreich U (2004) The diagnosis of premenstrual syndromes and premenstrual dysphoric disorder-clinical procedures and research perspectives. Gynecol Endocrinol 19:320–334
Steiner M, Pearlstein T, Cohen LS, Endicott J, Kornstein SG, Roberts C et al (2006) Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: the role of SSRIs. J Womens Health 15:57–69
Halbreich U, Borenstein J, Pearlstein T, Kahn LS (2003) The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology 28:1–23
Alpay FB, Turhan NO (2001) Intermittent versus continuous sertraline therapy in the treatment of premenstrual dysphoric disorders. Int J Fertil Womens Med 46:228–231
Halbreich U (2003) The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology 28:55–99
Kendler KS, Karkowski LM, Corey LA, Neale MC (1998) Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry 155:1234–1240
Reame NE, Marshall JC, Kelch RP (1992) Pulsatile LH secretion in women with premenstrual syndrome (PMS): evidence for normal neuroregulation of the menstrual cycle. Psychoneuroendocrinology 17:205–213
Bäckström T, Andersson A, Andreé L, Birzniece V, Bixo M, Björn I et al (2003) Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 1007:42–53
Evans MD, Dizdaroglu M, Cooke MS (2004) Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 567:1–61
Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25:29–38
Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R (2003) Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep 8:365–370
Arranz L, Guayerbas N, De la Fuente M (2007) Impairment of several immune functions in anxious women. J Psychosom Res 62:1–8
Harma M, Harma M, Erel O (2005) Measurement of the total antioxidant response in preeclampsia with a novel automated method. Eur J Obstet Gynecol Reprod Biol 118:47–51
Harma M, Harma M, Kocyigit A (2004) Comparison of protein carbonyl and total plasma thiol concentrations in patients with complete hydatidiform mole with those in healthy pregnant women. Acta Obstet Gynecol Scand 83:857–860
Lagod L, Paszkowski T, Sikorski R, Rola R (2001) The antioxidant prooxidant balance in pregnancy complicated by spontaneous abortion. Ginekol Pol 72:1073–1078
Freemen E, Rickels K, Sondheimer SJ, Polansky M (1990) Ineffectiveness of progesteron suppository treatment for premenstrual syndrome. JAMA 264:349–353
Zadeh-Nourooz J, Tajaddini-Sarmadi J, Wolff S (1994) Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 220:403–409
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol 299:15–27
Korzekwa MI, Steiner M (1997) Premenstrual syndromes. Clin Obstet Gynecol 40:564–576
Kalia G, Sudheendran S, Rao A (2001) Antioxidant status and lipid peroxidation in premenstrual syndrome: a preliminary study. Clin Chim Acta 309:97–99
Balat O, Dikensoy E, Ugur MG, Atmaca R, Cekmen M, Yurekli M (2007) Malon dialdehyde, nitrite and adrenomedullin levels in patients with premenstrual syndrome. Arch Gynecol Obstet 275:361–365
Bäckström T, Sanders D, Leask R, Davidson D, Warner P, Bancroft J (1983) Mood, sexuality, hormones, and the menstrual cycle II: hormone levels and their relationship to the premenstrual syndrome. Psychosom Med 45:503–507
Halbreich U, Endicott J, Goldstein S, Nee J (1986) Premenstrual changes and changes in gonadal hormones. Acta Psychiatr Scand 74:576–586
Bremner JD, Innis RB, White T, Fujita M, Silbersweig D, Goddard AW et al (2000) SPECT [I-123] iomazenil measurement of the benzodiazepine receptor in panic disorder. Biol Psychiatry 47:96–106
Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM (2003) Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry 54:757–762
Rammal H, Bouayed J, Younos C, Soulimani R (2008) Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun 22:1156–1159
Conflict of interest statement
I certify that no actual or potential conflict of interest in relation to this article exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duvan, C.I., Cumaoglu, A., Turhan, N.O. et al. Oxidant/antioxidant status in premenstrual syndrome. Arch Gynecol Obstet 283, 299–304 (2011). https://doi.org/10.1007/s00404-009-1347-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-009-1347-y