Abstract
Background
For acute ischaemic stroke patients, it is uncertain whether intravenous thrombolysis combined with statins might increase the therapeutic effect. Additionally, using high-intensity statins after thrombolysis may increase the risk of bleeding in patients. Asian stroke patients often take low-dose statins. It is speculated that reducing the dose of statins may improve the risk of bleeding.
Methods
Data from consecutive acute ischaemic stroke patients with intravenous thrombolysis were prospectively collected. Efficacy outcomes included NIHSS (National Institutes of Health Stroke Scale) score improvement at 7 days after admission and mRS (Modified Rankin Scale) improvement at 90 days. Safety outcomes included haemorrhage events (intracerebral haemorrhage and gastrointestinal haemorrhage) in the hospital and death events within 2 years.
Results
The study finally included 215 patients. The statin group had a higher percentage of NIHSS improvement at 7 days (p < 0.001) and a higher percentage of a favourable functional outcome (FFO, mRS < = 2) (p < 0.001) at 90 days. The statin group had a lower percentage of intracerebral haemorrhage (p < 0.001) and gastrointestinal haemorrhage (p = 0.003) in the hospital and a lower percentage of death events (p < 0.001) within 2 years. Logistic regression indicated that statin use was significantly related to NIHSS improvement (OR = 4.697, p < 0.001), a lower percentage of intracerebral haemorrhage (OR = 0.372, p = 0.049) and gastrointestinal haemorrhage (OR = 0.023, p = 0.016), and a lower percentage of death events (OR = 0.072, p < 0.001).
Conclusion
For acute ischaemic stroke patients after intravenous thrombolysis, the use of low-dose statins was related to NIHSS improvement at 7 days and inversely related to haemorrhage events in the hospital and death events within 2 years, especially for moderate stroke or noncardioembolic stroke patients.
Similar content being viewed by others
Background
For acute ischaemic stroke within the time windows, IV thrombolysis (intravenous thrombolysis) is a clinically effective and guideline-recommended therapeutic method [1]. After intravenous thrombolysis, statins are regularly used as medicine for ischaemic stroke patients during hospitalization [2].
However, several studies have shown different conclusions regarding whether intravenous thrombolysis combined with statins is related to functional outcomes in ischaemic stroke patients [3,4,5]. Several meta-analyses have suggested that using statins in combination with thrombolysis had no significant effects on the prognosis of ischaemic stroke patients or that the combination increased the rate of haemorrhage [6,7,8]. Therefore, it is uncertain whether intravenous thrombolysis combined with statins is effective for ischaemic stroke patients.
A study showed that high-dose statins could increase the risk of intracerebral haemorrhage in ischaemic stroke patients [9]. Other studies have suggested that high-intensity statins after thrombolysis may increase the risk of bleeding in ischaemic stroke patients [10, 11]. These findings further raise doubts about the safety of intravenous thrombolysis combined with statins. In these studies, the patients were mainly from Europe and America, and these stroke patients mainly took high-dose statins. However, Asian stroke patients often take low-dose statins. Therefore, the conclusions may not be suitable for Asian patients. Therefore, it is speculated that reducing the dose of statins may improve the risk of bleeding in stroke patients undergoing thrombolysis.
The present prospective observational cohort study aimed to further explore the relationship between using low-dose statins combined with intravenous thrombolysis and the effects and safety outcomes of Asian ischaemic stroke patients.
Methods
Participants
We consecutively recruited patients with acute ischaemic stroke who received IV thrombolysis in the Neurology Department of West China Hospital, Sichuan University. The stroke diagnosis of the patients met the WHO stroke diagnostic criteria. All patients had a stroke pack (head CT: CTA + CTP) examination. The patients were enrolled from November 1, 2018, to September 30, 2020 and followed up to December 31, 2020. The study is a prospective observational cohort design.
The patients receiving statins after onset were assigned to the low-dose statin group, and the other patients who did not receive statins after onset were assigned to the control group. Low-dose statins were defined as atorvastatin 20 mg, simvastatin 10 mg and rosuvastatin 10 mg daily after onset [12]. A total of 220 patients were needed to show a significant difference [5].
The inclusion criteria were as follows: (1) age over 18 years; (2) the diagnosis of stroke with evidence of neuroimaging (CT or MRI); (3) The patients did not receive statins before onset; (4) The patients in the statin group started statins after onset for 7 days or more and (5) IV thrombolysis therapy administration within 4.5 h after stroke onset.
The exclusion criteria were as follows: (1) mRS was ≥ 2 before onset; (2) the time between admission and onset was more than 4.5 h; (3) treatment with other doses of statins after the onset of the disease or the use of statins for less than 7 days; (4) stroke associated with trauma or surgery; and (5) intracerebral haemorrhage, subarachnoid haemorrhage, coagulopathy, cancer, cardiac failure, or severe hepatic or renal dysfunction.
The study was performed in accordance with the Declaration of Helsinki and the ethical standards of the institutional and/or national research committee. The study was approved by the Ethics Committee of West China Hospital, Sichuan University with approval number 2019 (319).
Definition of risk factors
Cardioembolic stroke was proven by definite history, electrocardiography, and cardiac colour ultrasound. The haemorrhage events included intracerebral haemorrhage and gastrointestinal haemorrhage. ICH (intracerebral haemorrhage) was proven by CT in the hospital regardless of whether the condition of the patient changed. Gastrointestinal haemorrhage included haematemesis and haematochezia. An mRS score of 0–2 was defined as a favourable functional outcome (FFO) at 90 days. A difference in NIHSS score greater than 4 was defined as an improvement. A NIHSS score of 0–4 was defined as a mild stroke, a NIHSS score of 5–15 was defined as a moderate stroke, and a NIHSS score of 16–40 was defined as a severe stroke.
Data collection and outcome
The baseline data were collected from electronic clinical records, and structured questionnaires were completed by patients or their relatives at the patients’ admission to the hospital. The data included age, sex, blood pressure, history of smoking and drinking, NIHSS score and mRS score at admission, history of diseases and history of drug use. Types of strokes and types of statins were also collected. We recorded laboratory data, including TC (total cholesterol), TG (triglyceride), HDL-C (high-density lipoprotein cholesterol), and LDL-C. at the time of hospital admission.
The efficacy outcome was the difference in the NIHSS score between admission and 7 days after admission and FFO (mRS < = 2) at 90 days after onset. The safety outcomes were intracerebral haemorrhage and gastrointestinal haemorrhage in the hospital and death events at 90 days after onset. We collected the NIHSS scores and in the hospital via face-to-face interviews. We collected the haemorrhage in the hospital via electronic clinical records. We collected the mRS scores and death events at 90 days stroke onset within 2 years via telephone (more than two different numbers of patients or relatives), WeChat (an instant messaging application) or e-mail. The two experienced neurologists who evaluated events and outcomes were blind to the patients’ condition and grouping.
Statistical analysis
To describe the baseline characteristics, the continuous variables were expressed as the mean and SD (standard deviation) or median and frequencies. Categorized data and ranked data are expressed as numbers and percentages. To compare the group differences, variance analysis was conducted for continuous data following the normal distribution. A nonparametric test (Mann–Whitney U test) was conducted for continuous data that did not follow a normal distribution. A chi-square test was conducted for categorical data and ranked data.
To analyse the outcome events, we compared the group differences using the chi-square test. We analysed the effects of risk factors on the outcome events using univariable and multivariable logistic regression methods. The eligible factors of multivariable logistic regression included (1) P of univariable logistic regression less than 0.1 and (2) a factor with clinical significance. We calculated the odds ratios (ORs), 95% CIs and p values using logistic regression methods. We compared the death events at different times with a Kaplan–Meier curve. We performed subgroup analysis via univariable logistic regression by differences in NIHSS scores at admission and differences in stroke type (cardioembolic stroke and noncardioembolic stroke). The threshold for statistical significance was set as P < 0.05. SPSS 23.0 for Windows was used to process the data.
Results
Patients
The study recruited 236 patients. We lost 21 patients’ data for no follow-up or withdrew the study, and finally, the data of 215 patients were acquired.
Among the 215 patients (mean age: 70.92 ± 12.458 years), 113 (52.6%) were female. The median and interquartile ranges of the NIHSS at admission were 8 (5–14). Comparing the baseline data of the different groups, the low-dose statin groups had a higher percentage of antiplatelet drug use in the hospital, a lower percentage of cardioembolic in the hospital, and lower NIHSS scores at admission than the control group. No significant difference in other baseline data was found between the two groups (Table 1).
Efficacy outcome
We found that the low-dose (p < 0.001) statin groups had a higher percentage of NIHSS improvement at 7 days than the control group. The low-dose (p < 0.001) statin groups had a higher percentage of FFOs at 90 days than the control group (Table 1).
In the univariate logistic regression analysis, we found that the use of statins (OR = 5.524, P < 0.001) and the use of antiplatelet drugs in the hospital (OR = 1.455, P = 0.032) were related to a higher percentage of NIHSS score improvement at 7 days after admission, and the history of hypertension (OR = 0.468, P = 0.018) and ICH in the hospital (OR = 0.266, P = 0.001) were inversely related to a higher percentage of NIHSS score improvement at 7 days after admission (Supplementary Table 1). Using statins (OR = 2.734, P = 0.008) and the use of antiplatelet drugs in hospitals (OR = 1.512, P = 0.008) were associated with FFO at 90 days. Higher NIHSS at admission (OR = 0.948, P = 0.011), higher NIHSS at 7 days after admission (OR = 0.938, P < 0.001), older age (OR = 0.975, P = 0.039), and higher systolic were inversely related to pressure (OR = 0.985, P = 0.007), and ICH in the hospital (OR = 0.452, P = 0.036) was inversely related to FFO at 90 days (Supplementary Table 2).
In the multivariable logistic regression analysis, after adjusting for risk factors, we found that the use of statins (OR = 4.697, P = 0.001) was still related to a higher percentage of NIHSS score improvement at 7 days after admission, and the history of hypertension (OR = 0.430, P = 0.015) and ICH in the hospital (OR = 0.367, P = 0.024) were still inversely related to a higher percentage of NIHSS score improvement at 7 days after admission (Table 2). Higher NIHSS at 7 days after admission (OR = 0.955, P = 0.022) and higher systolic pressure (OR = 0.986, P = 0.026) were still significantly inversely related to FFO at 90 days (Table 2).
Safety outcome
When we evaluated haemorrhage events in the hospital, we found that the low-dose statin groups had a lower percentage of ICH events (p < 0.001) and gastrointestinal haemorrhage(p = 0.003) than the control group (Table 1). Regarding death events at the 2-year follow-up, we found that the low-dose (p < 0.001) statin groups had a lower percentage than the control group (Table 1).
In the univariate logistic regression analysis, we found that the use of statins (OR = 0.157, P < 0.001), use of antiplatelet drugs in the hospital (OR = 0.287, P < 0.001) and higher value of platelets (OR = 0.988, P = 0.008) were inversely related to a higher percentage of ICH in the hospital (Supplementary Table 2). Cardioembolic stroke (OR = 2.676, P = 0.010) was related to a higher percentage of ICH in the hospital (Supplementary Table 2). Using statins (OR = 0.043, P = 0.006) and a higher value of TC (OR = 0.379, P = 0.048) were inversely related to a higher percentage of gastrointestinal haemorrhage in the hospital (Supplementary Table 2). Older age (OR = 1.192, P = 0.019), a higher value of platelets (OR = 1.014, P = 0.048) and a higher value of blood glucose (OR = 1.217, P = 0.020) were positively related to a higher percentage of gastrointestinal haemorrhage in the hospital (Supplementary Table 2). We also found that the use of statins (OR = 0.042, P < 0.001) and the use of antiplatelet drugs in hospitals (OR = 0.302, P < 0.001) were inversely related to a higher percentage of death events within 2 years. Older age (OR = 1.061, P = 0.002), higher NIHSS at admission (OR = 1.122, P < 0.001), higher NIHSS at 7 days after admission (OR = 1.130, P < 0.001) and cardioembolic status (OR = 2.779, P = 0.005) were positively related to a higher percentage of death events within 2 years (Supplementary Table 2).
In the multivariable logistic regression analysis, after adjusting for risk factors, we found that the use of statins (OR = 0.372, P = 0.049), use of antiplatelet drugs in the hospital (OR = 0.414, P = 0.006) and higher value of platelets (OR = 0.988, P = 0.010) were still inversely related to a higher percentage of intracerebral haemorrhage in the hospital (Table 3). Using statins (OR = 0.023, P = 0.016) was still inversely related to a higher percentage of gastrointestinal haemorrhage in the hospital. Older age (OR = 1.429, P = 0.022) and a higher value of blood glucose (OR = 1.407, P = 0.026) were still positively related to a higher percentage of gastrointestinal haemorrhage in the hospital (Table 3). Using statins (OR = 0.072, P < 0.001) was still inversely associated with a higher percentage of death events within 2 years. Older age (OR = 1.061, P = 0.009) and higher NIHSS at 7 days after admission (OR = 1.075, P = 0.006) were still positively related to a higher percentage of death events at 2 years (Table 4).
When we analysed death events in the different groups at different times with Kaplan–Meier curves, we found that the low-dose statin groups had a significantly higher survival rate than the control group at different times (Fig. 1).
Subgroup analysis
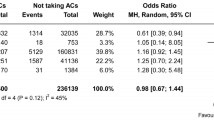
In the subgroup analysis, in mild stroke patients, statin use was only significantly inversely related to ICH (OR = 0.01) in the hospital and death event rates (OR = 0.01) at 2 years. Using statins were not related to other outcomes. In moderate stroke patients, using statins was significantly related to NIHSS improvement (OR = 7.222), ICH (OR = 0.201) and gastrointestinal haemorrhage (OR = 0.074) in the hospital and death event rates (OR = 0.047) at 2 years. Using statins was not related to FFO at 90 days. In severe stroke patients, statin use was significantly related to NIHSS improvement (OR = 4.833) and death event rates (OR = 0.083) at 2 years. Using statins was not related to ICH or gastrointestinal haemorrhage in the hospital or FFO at 90 days (Fig. 2). In the cardioembolic stroke subgroup, statin use was significantly related to NIHSS improvement (OR = 3.5) and death event rates (OR = 0.102) at 2 years. Using statins was not related to ICH in the hospital or FFO at 90 days. In the noncardioembolic stroke subgroup, statin use was not related to NIHSS improvement (OR = 9.143) or FFO at 90 days (OR = 3.514). Statin use was significantly inversely related to ICH (OR = 0.353) in the hospital and death event rates (OR = 0.102) at 2 years. Using statins was not related to gastrointestinal haemorrhage in the hospital (Fig. 3).
Discussion
We found that the low-dose statin groups had a higher percentage of NIHSS improvement at 7 days after admission and FFO at 90 days. The low-dose statin groups had a lower percentage of intracerebral haemorrhage and gastrointestinal haemorrhage in the hospital and a lower percentage of death events within 2 years than the control group. When we analysed risk factors through logistic regression, statins were significantly related to NIHSS improvement, fewer haemorrhage events in the hospital and death events within 2 years. When we evaluated death events through the K-M curve, we found that the low-dose statin groups had a higher survival rate than the control group in both 2 years. The effect of statins was more significant for moderate stroke patients or noncardioembolic stroke patients.
When the efficacy outcomes in the hospital were evaluated, our results were similar to those of Manuel Cappellari’s study [5]. The effect was significant in the different subgroups. Acute ischaemic patients after intravenous thrombolysis taking statins might have a short-term benefit for patients. When evaluating functional outcomes at 90 days, our results showed that using statins was not significantly related to FFO. The relationship in the difference subgroup had a similar result. The results were consistent with those of the low-dose statin subgroup of Manuel Cappellari’s study for 3 months of FFO [5]. However, the noncardioembolic stroke subgroup showed a significant difference. Therefore, low-dose statins might improve FFO at 90 days in noncardioembolic stroke patients after intravenous thrombolysis. We found that a higher value of SBP at admission was a risk factor for higher mRS at 90 days, and the results were not entirely consistent with Anderson’s study [13]. Anderson’s intervention was intensive blood pressure reduction, and our data were SBP. The different data types could partly explain the different conclusions. The results suggest that blood pressure management at admission plays a role in the prognosis of ischaemic stroke patients with intravenous thrombolysis.
When we evaluated safety outcomes, we found that low-dose statins were related to fewer intracerebral haemorrhage and gastrointestinal haemorrhage events through the chi-square test and logistic regression. In the mild and moderate stroke subgroups and noncardioembolic subgroups, statin use was significantly inversely related to ICH. It suggested the results had a statistic power. Our results showed that antiplatelet drug use and higher platelet counts were also related to fewer intracerebral haemorrhage events. The relationship between statin use and antiplatelet therapy was not consistent with that found in other studies [14, 15]. Patients not taking these drugs when they have an intracerebral haemorrhage might partly explain the inconsistent results. It is easy to understand the effect of platelets on intracerebral haemorrhage. The results of reduced gastrointestinal haemorrhage were consistent with a study that included myocardial infarction patients [16]. This finding suggested that statins might be a protective factor against gastrointestinal haemorrhage in acute ischaemic stroke patients with intravenous thrombolysis. However, for the different subgroups, the relationship was not statistically significant. Therefore, further study is needed to confirm this conclusion. In addition, our results suggested that increasing age and higher blood glucose levels were related to more gastrointestinal haemorrhage. This suggests that we should consider these factors when evaluating the risk of intravenous thrombolysis for acute ischaemic stroke patients.
When evaluating death outcomes, we found that using statins resulted in fewer deaths for acute ischaemic stroke patients with intravenous thrombolysis. The effect was significant for different subgroups of patients. The results were similar to Manuel Cappellari’s study about the effect of statins on the chance of death in acute ischaemic stroke patients with intravenous thrombolysis over 3 months [5]. Our results suggested that using statins to decrease death events had a longer-term effect. Within 2 years, the low-dose statin groups had significantly fewer deaths than the control group. This result suggested that low-dose statins might decrease long-term mortality for acute ischaemic stroke patients with intravenous thrombolysis. Nonetheless, more research is needed to confirm this conclusion.
Our study has several limitations. First, the study could not identify a causal relationship given the observational cohort design. However, the conditions of the prospective observational cohort were consistent with those of the real world, and the conclusions might be suitable for use in clinical practice. Second, we did not compare the specific doses of statins in our study because high-intensity statins are rarely used in Asian clinical practice, so it is difficult to obtain relevant data. Finally, the smaller number of patients in the control groups might have affected the statistical power of the results. Although we used logistic regression to increase the statistical power of the results, a study including more control group patients could further support the conclusions of the present study.
In conclusion, for acute ischaemic patients after intravenous thrombolysis, the use of low-dose statins was significantly related to NIHSS improvement, fewer haemorrhage events in the hospital and death events within 2 years. These relationships were significant, especially for moderate stroke patients and noncardioembolic stroke patients.
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Powers. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;2018(49):E138. https://doi.org/10.1161/str.0000000000000163.
Makihara N, Okada Y, Koga M, et al. Effect of serum lipid levels on stroke outcome after rt-PA therapy: samurai rt-PA registry. Cerebrovasc Dis. 2012;33:240–7. https://doi.org/10.1159/000334664.
Mowla A, Shah H, Lail NS, et al. Statins Use and Outcome of Acute Ischemic Stroke Patients after Systemic Thrombolysis. Cerebrovasc Dis 2020: 1–6. DOI: https://doi.org/10.1159/000510095.
Cappellari M, Bovi P, Moretto G, et al. The THRombolysis and statins (THRaST) study. Neurology. 2013;80:655–61. https://doi.org/10.1212/WNL.0b013e318281cc83.
Cappellari M, Deluca C, Tinazzi M, et al. Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J Neurol Sci. 2011;308:128–34. https://doi.org/10.1016/j.jns.2011.05.026.
Chroinin DN, Asplund K, Asberg S, et al. Statin therapy and outcome after ischemic stroke systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–56. https://doi.org/10.1161/strokeaha.112.668277.
Martinez-Ramirez S, Delgado-Mederos R, Marin R, et al. Statin pretreatment may increase the risk of symptomatic intracranial haemorrhage in thrombolysis for ischemic stroke: results from a case-control study and a meta-analysis. J Neurol. 2012;259:111–8. https://doi.org/10.1007/s00415-011-6137-3.
Meseguer E, Mazighi M, Lapergue B, et al. Outcomes after thrombolysis in AIS according to prior statin use a registry and review. Neurology. 2012;79:1817–23. https://doi.org/10.1212/WNL.0b013e318270400b.
Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59.
Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: A review of natural history and treatment. JAMA Neurol. 2014;71:1181–5.
Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–82. https://doi.org/10.1016/s0140-6736(07)60149-4.
Dong SJ, Guo J, Fang JH, et al. Low-dose statin pretreatment reduces stroke severity and improves functional outcomes. J Neurol. 2019;266:2970–8.
Anderson CS, Huang YN, Lindley RI, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019;393:877–88. https://doi.org/10.1016/s0140-6736(19)30038-8.
Lin T-C, Lin Y-K, Chen C-I, et al. Serum lipid level is not associated with symptomatic intracerebral hemorrhage after intravenous thrombolysis for acute ischemic stroke. PeerJ. 2018;6:e6021. https://doi.org/10.7717/peerj.6021.
Rocco A, Sykora M, Ringleb P, et al. Impact of statin use and lipid profile on symptomatic intracerebral haemorrhage, outcome and mortality after intravenous thrombolysis in acute stroke. Cerebrovasc Dis. 2012;33:362–8. https://doi.org/10.1159/000335840.
Atar S, Cannon CP, Murphy SA, et al. Statins are associated with lower risk of gastrointestinal bleeding in patients with unstable coronary syndromes: Analysis of the orbofiban in patients with unstable coronary syndromes-thrombolysis in myocardial infarction 16 (OPUS-TIMI 16) trial. Am Heart J. 2006;151(976):e1-6. https://doi.org/10.1016/j.ahj.2006.02.013.
Acknowledgements
We thank Professor Ning Chen for offering some guidance for the design of the study.
Funding
This study was supported by the project “National Key R&D Program of China” (Funding number: 2018YFC1311400 and 2018YFC1311401), the project “National Natural Science Foundation of China” (Funding number: 81772435) and the project “Post-Doctor Research Project” (Funding number: 2020HXBH032). All of these funds provide financial and resource support for the article.
Author information
Authors and Affiliations
Contributions
Professor LH, Dr YL and Dr CC conceived and designed the study. Dr CC, Dr SD, MD JB, and Dr LG screened and collected data for the article. Dr CC, Dr SD and MD JB conducted statistical analyses. Dr YL, Dr CC and Dr SD written and revised the manuscript. All authors reviewed the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and the ethical standards of the institutional and national research committees. The study was approved by the Ethics Committee of West China Hospital, Sichuan University, under approval number 2019 (319). Informed consent was obtained from all individual participants included in the study.
Consent for publication
The manuscript has been approved by all authors for publication.
Competing interests
All authors declare no conflicts of interest or competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, C., Li, Y., Bao, J. et al. Low dose statins improve prognosis of ischemic stroke patients with intravenous thrombolysis. BMC Neurol 21, 220 (2021). https://doi.org/10.1186/s12883-021-02259-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-021-02259-9