Abstract
To review the literature for randomized control trials (RCTs) and prospective cohort studies investigating the safety and efficacy of tirofiban and eptifibatide in patients with acute ischemic stroke (AIS). PubMed, Embase, and the Cochrane library were searched for available papers published up to September 2021. The efficacy was evaluated based on the 3-month favorable outcome [modified Rankin scale (mRS) = 0–1], functional outcome (mRS = 0–2), and the last available National Institutes of Health Stroke Scale (NIHSS) score measured in each study. Twelve studies (two RCTs and 10 prospective cohorts) and 2926 patients were included. Treatment with tirofiban or eptifibatide had no effects on the favorable outcome (RR = 1.09, 95% CI 0.89–1.35, P = 0.411), functional outcome (RR = 1.12, 95% CI 0.98–1.28, P = 0.010), and last available NIHSS (WMD = − 2.32, 95% CI − 5.14 to 0.50, P = 0.106), but might increase mortality (RR = 0.84, 95% CI 0.71–0.99, P = 0.121). The sensitivity analyses showed that the meta-analyses were robust. There was no significant publication bias. Tirofiban did not increase the risk of ICH (P = 0. 423) and sICH (P = 0. 990) but increased the risk of fatal ICH (RR = 3.59, 95% CI 1.62–7.96, P = 0.002). Thrombolysis/thrombectomy did not influence any of the outcomes. Adding tirofiban or eptifibatide to thrombolysis/thrombectomy was not significantly associated with a favorable outcome (mRS = 0–1) nor functional outcome (mRS = 0–2) in patients with AIS at 3 months, but might be associated with mortality, possibly due to fatal ICH. The NIHSS was also not significantly different between the intervention and control groups after treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
This meta-analysis assessed whether adding an anti-IIb/IIIA to the standard of care thrombolysis/thrombectomy (intervention group) had any benefit over thrombolysis/thrombectomy alone (control group).
-
Adding tirofiban or eptifibatide to thrombolysis/thrombectomy was not significantly associated with a favorable outcome (mRS = 0–1) nor functional outcome (mRS = 0–2) in patients with AIS at 3 months
-
Adding tirofiban or eptifibatide to thrombolysis/thrombectomy might be associated with mortality, possibly due to fatal ICH.
-
The NIHSS was also not significantly different between the intervention and control groups after treatments.
Introduction
Comprehensive treatments of acute ischemic stroke (AIS), including endovascular thrombectomy (ET), fibrinolytic drugs, and antiplatelet (AP) drugs, can improve clinical outcomes or recanalization rate [1]. Such treatments are rarely used alone but rather in combinations in selected and appropriate patients. ET can be used to revascularize occluded large arteries, thereby improving patients' functional outcomes with AIS [2]. Still, during ET, the platelets are already activated by the thrombus and can be further activated by the endothelial injuries caused by ET, possibly leading to platelet-mediated thromboembolic complications and early reocclusion [3,4,5]. Aggregation of platelets and activation of the coagulation cascade will lead to fibrin and thrombus formation in the AIS setting [6, 7]. The fibrin component of the thrombus will be sensitive to thrombolysis (e.g., using tissue plasminogen activators (t-pa)), but the aggregated platelets might limit the thrombolytic effect and complicate management [8]. Hence, AP drugs can be used to prevent platelet activation and aggregation. The most used AP drugs are aspirin and clopidogrel, but their action is slow [9]. Thus, in contrast with oral AP, early intravenous AP agents such as tirofiban or eptifibatide can be used to prevent new thrombus formation.
Tirofiban and eptifibatide are fast-acting non-peptide antagonists of the glycoprotein IIb/IIIa (GPIIb/IIIa) receptor located on thrombocytes. Tirofiban is administered via intravenous infusion and is indicated to reduce the rate of thrombotic cardiovascular events in patients with acute coronary syndrome [10, 11]. An important feature of tirofiban and eptifibatide is their short half-life, leading to a reversal of the effect to normal coagulation within 4 h after discontinuation [9, 12]. Several studies investigated the effect of the combination therapy of either tirofiban or eptifibatide with t-pa and recombinant human t-pa (rt-pa), including several meta-analyses [13,14,15]. Dubey et al. [13] reported that the functional outcomes at 90 days were not better with the combined therapy (t-pa and GP11b/IIIa inhibitors) compared with the control. Ciccone et al. [14] concluded that the use of GP11b/IIIa inhibitors (abciximab or tirofiban) in AIS did not reduce death or disability. Furthermore, they even revealed a significantly increased risk of symptomatic intracerebral hemorrhage (ICH) with abciximab but not with tirofiban [14]. Guo et al. [15] concluded that GP11b/IIIa inhibitors combined with endovascular treatment were not associated with the functional outcome or recanalization rate. Of note, two meta-analyses included several retrospective studies [13, 15]. Another meta-analysis was performed using only randomized control trials (RCTs), but it was published some years ago [14], and since then, new RCTs have been published. Three other meta-analyses were published after the present one was planned [16,17,18]. Zhou et al. [16] included all types of studies and examined the safety and efficacy of tirofiban without endovascular treatment. Gong et al. [17] included RCTs and two-arm cohort studies that compared treatment with and without tirofiban. Fu et al. [18] included only cohort studies and examined tirofiban in patients who underwent endovascular treatment.
Nevertheless, tirofiban and eptifibatide are still used in clinical practice to avoid potential thromboembolism during ET in patients with AIS. The 2018 American Heart Association (AHA)/American Stroke Association (ASA) guidelines stated that "further research testing the safety and efficacy of these medications in patients with AIS is required", and that the "The efficacy of IV tirofiban and eptifibatide is not well established. Further clinical trials are needed" [1]. Therefore, there is a need for additional updated data on the use of those drugs in AIS.
Therefore, this meta-analysis aimed to review the literature for RCTs and prospective cohort studies investigating the safety and efficacy of tirofiban and eptifibatide in patients with AIS. A subgroup analysis was carried out to examine the combined effect of thrombolysis and thrombectomy.
Methods
Literature search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. The search strategy was based on the PICO principle [20]. PubMed, Embase, and the Cochrane library were searched for available papers published up to September 2021 using the MeSH terms ‘Ischemia’ and ‘Stroke’ as well as relevant key words such as “eptifibatide OR tirofiban”.
Eligibility criteria
The eligibility criteria were (1) population: patients with a definitive diagnosis of AIS, for whom treatment with intravenous thrombolysis (IVT) within 4.5 h or ET < 6 h of symptom onset, (2) intervention: tirofiban or eptifibatide, (3) control: rt-pa thrombolysis and/or ET, (4) outcome: modified Rankin scale (mRS) and National Institutes of Health Stroke Scale (NIHSS), (5) study type: RCTs or prospective cohort studies, and (6) English language. The experimental group included the patients who underwent thrombolysis/thrombectomy with eptifibatide or tirofiban, while the control group included the patients who underwent thrombolysis/thrombectomy without eptifibatide or tirofiban. All included studies included patients treated with thrombolysis/thrombectomy or who underwent thrombectomy after the failure of thrombolysis. There is no study in which the control arm did not receive any treatment or placebo.
Data extraction
The study characteristics (authors, year of publication, country, study design, and sample size), patient characteristics (age of the patients and NIHSS score at admission), treatment parameters (number of cases with permanent stenting, thrombectomy, thrombolysis, time from onset to treatment, and GP11b/IIIa inhibitor intervention strategy, including doses and routes of administration), and mRS, last-recorded NIHSS, ICH, symptomatic ICH, and fatal ICH were extracted by two authors independently (Jingting Liu and Yihong Yang). Any discrepancy was solved by a discussion with a third author (Hongbo Liu). A favorable outcome was defined as mRS of 0 or 1. A functional outcome was defined as mRS of 0–2. Only the last-recorded NIHSS were extracted and analyzed.
Outcomes
The efficacy was evaluated based on the 3-month favorable outcome (mRS = 0–1), functional outcome (mRS = 0–2), and the last available NIHSS score measured in each study. The safety measures were ICH, symptomatic ICH (sICH), and fatal ICH. The definition of ICH varied among the studies. ICH was defined based on a 24-h safety computed tomography (CT) scan [21], European Cooperative Acute Stroke Study (ECASS)-1 [22], ECASS-2 [23], or ECASS-3 [24] definition or a ≥ 4-point increase in NIHSS [25].
Data synthesis
For continuous results, we recorded the number of cases and the means ± standard deviations. For the studies that did not present their results as means ± standard deviations, we estimated the results with the reported parameters (medians and interquartile ranges) [26].
Quality of the evidence
Ultimately, two RCTs and eight prospective cohort studies were included in the meta-analysis. The level of evidence of the RCTs was assessed according to the Cochrane Handbook [27]. The Newcastle–Ottawa scale (NOS) was used to assess the prospective cohort studies [28]. The quality assessments were completed independently by two authors (Jingting Liu and Yihong Yang). The discrepancies in the assessment were resolved through discussion until a consensus was reached.
Among the two RCTs, one had a high risk of bias for one item [21], and the other one had an unclear risk of bias for one item [29] (Supplementary Table S1a). Among the cohort studies, one scored 7 points [30] on the NOS, six scored 8 points [25, 31,32,33,34,35], and one scored 9 points [36] (Supplementary Table S1b).
Statistical analysis
All analyses were performed using STATA SE 14.0 (StataCorp, College Station, Texas, USA). Relative risk (RR) and weighted mean difference (WMD) and their corresponding 95% confidence intervals (CIs) were used to compare the outcomes. Statistical heterogeneity among the studies was evaluated using Cochran’s Q-test and the I2 index. An I2 > 50% and Q-test P < 0.10 indicated high heterogeneity, and the random-effects model was used; otherwise, the fixed-effects model was used. Meta-regression analyses were performed to examine the influence of age and NIHSS score at admission on the outcomes. P-values < 0.05 were considered statistically significant. Potential publication biases were evaluated using funnel plots and Egger’s and Begg’s tests.
Results
Characteristics of the included studies
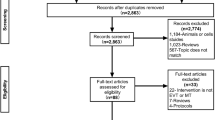
Figure 1 presents the study selection process. The initial search yielded 319 records, but 67 duplicates were removed. After screening the 252 records, 83 were excluded. Then, 169 papers were assessed for eligibility, and 157 were excluded: no full-text accessible (n = 29), study aim/design (n = 11), population (n = 68), intervention (n = 24), outcome (n = 16), meta-analysis (n = 7), and non-English (n = 2). Therefore, 12 studies were included.
Table 1 presents the 12 included studies [21, 25, 29,30,31,32,33,34,35,36,37,38]. There were two RCTs [21, 29] and ten prospective cohorts [25, 30,31,32,33,34,35,36,37,38]. A total of 2926 patients were included (n = 82–662/study). One study evaluated eptifibatide [21], and 11 evaluated tirofiban [25, 29,30,31,32,33,34,35,36,37,38]. Supplementary Table S1 presents the quality assessment of the included studies.
Favorable outcome
Nine studies reported favorable outcome data (mRS = 0–1) [21, 25, 29, 30, 32, 33, 35,36,37] (1917 patients). The study with eptifibatide was not included. Treatment with tirofiban had no effect on the favorable outcome of patients with AIS (RR = 1.09, 95% CI 0.89–1.35, P = 0.411; I2 = 58.0%, Pheterogeneity = 0.015) (Fig. 2A; Table 2).
Functional outcome
Nine studies presented functional outcome data (mRS = 0–2) [29,30,31,32, 34,35,36,37,38] (2532 patients). The study with eptifitabide was not included. Treatment with tirofiban had no effect on the functional outcome in patients with AIS (RR = 1.12, 95% CI 0.98–1.28, P = 0.010; I2 = 46.1%, Pheterogeneity = 0.063) (Fig. 2B; Table 2).
Last-recorded NIHSS
The admission NIHSS was not significantly different between the intervention and control groups. Three studies presented the last-recorded NIHSS [25, 29, 31] (443 patients). The study with eptifibatide was not included. Treatment with tirofiban had no effect on the last-recorded NIHSS in patients with AIS (WMD = − 2.32, 95% CI − 5.14 to 0.50, P = 0.106; I2 = 73.7%, Pheterogeneity = 0.022) (Fig. 3; Table 2).
Safety measures
Eleven studies presented death data [21, 25, 29, 30, 32,33,34,35,36,37,38] (2926 patients). Treatment with tirofiban or eptifibatide appeared to increase the risk of death in patients with AIS (RR = 0.84, 95% CI 0.71–0.99, P = 0.121; I2 = 16.8%, Pheterogeneity = 0.283) (Fig. 2C; Table 2). The results showed that tirofiban did not increase the risk of ICH (RR = 0.96, 95% CI 0.78–1.11, P = 0.423; I2 = 76.5%, Pheterogeneity < 0.001) (2115 patients) (Fig. 4A; Table 2) and symptomatic ICH (RR = 1.00, 95% CI 0.75–1.33, P = 0.990; I2 = 41.0%, Pheterogeneity = 0.084) (2776 patients) (Fig. 4B; Table 2), but increased the risk of fatal ICH (RR = 3.59, 95% CI 1.62–7.96, P = 0.002; I2 = 0%, Pheterogeneity = 0.575) (560 patients) (Fig. 4C; Table 2). Figure 4D suggests that tirofiban increased the risk of PH1/PH2 (RR = 0.15, 95% CI 0.04–0.67, P = 0.013; I2 = 0.0%, Pheterogeneity = 0.590) [29, 38], but without impact on PH2, IVH, and systemic bleeding (all P > 0.05).
Sensitivity analyses
The sensitivity analyses showed that all meta-analyses were robust, without studies being outliers to the calculated RRs and WMD (Supplementary Figure S1). Of note, when excluding the CLEAR trial [21] (i.e., the only included study that examined eptifibatide), similar conclusions were still reached for all analyses.
Subgroup analyses based on thrombolysis/thrombectomy
Supplementary Figure S2 shows that thrombolysis/thrombectomy did not influence any of the study outcomes.
Publication bias
The funnel plots (Supplementary Figure S3) and Begg's and Egger's tests (Supplementary Table S2) showed no significant publication bias for all four meta-analyses.
Meta-regression analysis
Supplementary Table S3 presents the meta-regression analyses for the influence of age and admission NIHSS scores on the outcomes. Age only influenced the relation of the treatment with mortality (coefficient: 0.087, 95% CI 0.021, 0.152, P = 0.009). The admission NIHSS score was not associated with the outcomes.
Discussion
Previous studies and meta-analyses reported conflicting results about tirofiban or eptifibatide combined therapy and the favorable outcomes of AIS [13,14,15,16,17,18]. Therefore, the present meta-analysis aimed to review the literature for RCTs and prospective cohort studies investigating the safety and efficacy of tirofiban and eptifibatide in patients with AIS. The difference between the two groups is that the patients included in the experimental arm were those who received eptifibatide or tirofiban. The experimental group included the patients who underwent thrombolysis/thrombectomy with eptifibatide or tirofiban, while the control group included the patients who underwent thrombolysis/thrombectomy without eptifibatide or tirofiban. Therefore, all patients underwent thrombolysis/thrombectomy. The present meta-analysis indicates that adding tirofiban or eptifibatide to thrombolysis/thrombectomy was not significantly associated with a favorable outcome (mRS = 0–1) nor functional outcome (mRS = 0–2) in patients with AIS at 3 months, but might be associated with mortality, possibly due to fatal ICH. The NIHSS was also not significantly different between the intervention and control groups after treatments.
GPIIb/IIIa receptor antagonists are used in patients with AIS undergoing ET because they are, theoretically, faster than aspirin and clopidogrel to dissociate the thrombus' platelet component [9, 12]. Tirofiban or eptifibatide are usually used in combination with t-pa or rt-pa. Initial studies and small trials suggested that they were effective and safe in selected populations [25, 39,40,41,42]. Still, the meta-analyses reported no apparent benefit from the use of tirofiban or eptifibatide [13,14,15], and a previous study even reported an increased risk of symptomatic ICH [36]. Wu et al. [33] showed that the risk of bleeding was dependent upon the dose of tirofiban. Nevertheless, those three previous meta-analyses included studies with a wide variety of study designs, which could decrease the results' reliability. Hence, the 2018 AHA/ASA guidelines specifically stated the need for additional high-quality evidence for the use of tirofiban and eptifibatide in patients with AIS [1]. The present meta-analysis included only RCTs and prospective cohort studies. Despite a higher level of evidence, this updated meta-analysis reached conclusions similar to those of the three previous meta-analyses [13,14,15]. Although the present study showed no increase in death with tirofiban and eptifibatide, the results indicate a higher risk of fatal ICH. Nevertheless, the present meta-analysis supports the statement of the 2018 AHA/ASA guidelines that "The efficacy of IV tirofiban and eptifibatide is not well established. Further clinical trials are needed" [1]. Still, three other meta-analyses were published after the present one was planned [16,17,18]. The meta-analysis by Fu et al. [18] did not select specific study types and only evaluated tirofiban combined with ET; the results showed that tirofiban combined with ET improved mortality, recanalization rates, and functional outcome. The meta-analysis by Gong et al. [17] included RCTs, and two-arm comparative studies showed that tirofiban did not improve the functional outcome; the risk of fatal ICH was increased, but the subgroup analysis showed that this was only observed with intra-arterial and not intravenous injections of tirofiban. Zhou et al. [16] also showed that the intravenous use of tirofiban as monotherapy was safe. Therefore, the available meta-analyses report conflicting results and suggest that the tirofiban administration strategy and methods might affect the efficacy and safety. Additional studies are necessary to determine the most optimal strategies for patients with AIS. Nonetheless, the ongoing MOST trial (ClinicalTrial.gov NCT03735979) of alteplase ± argatroban or eptifibatide could also provide additional insights into the use of GPIIb/IIIa receptor antagonists in patients with AIS.
The results of the present meta-analysis must be considered along with their limitations. Only two RCTs were included. Nevertheless, we were able to include a larger number of prospective cohort studies with larger numbers of patients. Second, among the 12 included studies, nine were conducted in China. This might introduce a potential bias since over 80% of the cases were from Chinese studies. This could limit the generalizability of the results because of differences in genetics and lifestyle habits. Third, only one study used eptifibatide, and it might underestimate the effect of eptifibatide in the analysis. Nevertheless, a study showed that both eptifibatide and tirofiban were non-inferior to abciximab in patients with myocardial infarction, but no direct comparison was made between eptifibatide and tirofiban [43]. Another study showed a non-significant difference of 26% in efficacy between eptifibatide and tirofiban [44]. Therefore, it is unlikely that the study about eptifibatide influenced the results, as suggested by the sensitivity analyses. Fourth, several studies did not report their results as means ± standard deviations. We had to estimate the means and standard deviations based on the medians and ranges [26], introducing some bias. Fifth, we could not add the results about extracranial hemorrhage because of a lack of consistency in reporting among the included studies. Finally, the quality of the included studies was not always high, which could introduce bias. The heterogeneity was high, and the differences in dosing strategies among the studies probably played a role. Indeed, as shown in Table 1, all studies included in the present meta-analysis used tirofiban or eptifibatide in different manners. Some studies used a bolus followed by infusion [21, 25, 32, 34], while others used only a continuous infusion [29,30,31, 33, 35] or only specified adapted to patients’ weight and creatinine clearance [36]. The infusion duration varies from 30 min to 24 h. The doses also varied widely among studies. In addition, some studies used tirofiban or eptifibatide in an upfront manner, while other studies used it as a bail-out strategy. Furthermore, as shown in Table 1, the definitions of symptomatic ICH varied among studies, probably participating in the heterogeneity of the safety measure analyses.
In conclusion, adding tirofiban or eptifibatide to thrombolysis/thrombectomy was not significantly associated with a favorable outcome (mRS = 0–1) nor functional outcome (mRS = 0–2) in patients with AIS at 3 months, but might be associated with mortality, possibly due to fatal ICH. The NIHSS was also not significantly different between the intervention and control groups after treatments. ICH and symptomatic ICH's occurrence were similar between the two groups, but the risk of fatal ICH was higher with tirofiban. Compared with previous meta-analyses [13, 15], we were able to limit the study types to RCTs and prospective cohorts, yet with a larger total number of patients. Although the present meta-analysis results are consistent with the previous meta-analyses [13,14,15], we only assessed the overall safety and efficacy based on the mRS, ICH, and last recorded NIHSS. Considering that tirofiban and eptifibatide are still used sometimes in the current clinical practice, future studies should investigate the specific adverse effects in patients with AIS undergoing treatment. Still, it should be used with caution and in highly selected patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke C (2018) 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):e46–e110. https://doi.org/10.1161/STR.0000000000000158
van den Berg LA, Dijkgraaf MG, Berkhemer OA, Fransen PS, Beumer D, Lingsma HF, Majoie CB, Dippel DW, van der Lugt A, van Oostenbrugge RJ, van Zwam WH, Roos YB, Investigators MC (2017) Two-year outcome after endovascular treatment for acute ischemic stroke. N Engl J Med 376(14):1341–1349. https://doi.org/10.1056/NEJMoa1612136
Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK (2014) Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis 37(5):350–355. https://doi.org/10.1159/000362435
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR, American Heart Association Stroke C (2015) 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 46(10):3020–3035. https://doi.org/10.1161/STR.0000000000000074
Rubiera M, Alvarez-Sabin J, Ribo M, Montaner J, Santamarina E, Arenillas JF, Huertas R, Delgado P, Purroy F, Molina CA (2005) Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 36(7):1452–1456. https://doi.org/10.1161/01.STR.0000170711.43405.81
Global Use of Strategies to Open Occluded Coronary Arteries I (1997) A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med 337(16):1118–1123. https://doi.org/10.1056/NEJM199710163371603
Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN (2000) Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part I-Pathophysiological and pharmacological features. Neurosurgery 46(6):1344–1359. https://doi.org/10.1097/00006123-200006000-00012
Yasuda T, Gold HK, Fallon JT, Leinbach RC, Garabedian HD, Guerrero JL, Collen D (1989) A canine model of coronary artery thrombosis with superimposed high grade stenosis for the investigation of rethrombosis after thrombolysis. J Am Coll Cardiol 13(6):1409–1414. https://doi.org/10.1016/0735-1097(89)90319-7
Niu J, Ding Y, Zhai T, Ju F, Lu T, Xue T, Yin D, Fang D, Chen H, Zhao G (2019) The efficacy and safety of tirofiban for patients with acute ischemic stroke: a protocol for systematic review and a meta-analysis. Medicine 98(9):e14673. https://doi.org/10.1097/MD.0000000000014673
Bansal AB, Sattar Y, Jamil RT (2021) Eptifibatide. In: StatPearls. Treasure Island (FL)
McClellan KJ, Goa KL (1998) Tirofiban: a review of its use in acute coronary syndromes. Drugs 56(6):1067–1080. https://doi.org/10.2165/00003495-199856060-00017
Harker LA (1998) Therapeutic inhibition of platelet function in stroke. Cerebrovasc Dis 8(Suppl 5):8–18. https://doi.org/10.1159/000047513
Dubey D, Banerjee C, Sawhney A, Sharma A, Alberts MJ (2014) Combination therapy of intravenous glycoprotein IIB/IIIA inhibitors and tissue plasminogen activator for acute ischemic stroke. Neurol India 62(6):631–634. https://doi.org/10.4103/0028-3886.149385
Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I (2014) Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev 3:CD005208. https://doi.org/10.1002/14651858.CD005208.pub3
Guo Y, Lin Y, Tang Y, Tang Q, Wang X, Pan X, Zou J, Yang J (2019) Safety and efficacy of early antiplatelet therapy in acute ischemic stroke patients receiving endovascular treatment: a systematic review and meta-analysis. J Clin Neurosci 66:45–50. https://doi.org/10.1016/j.jocn.2019.05.028
Zhou J, Gao Y, Ma QL (2020) Safety and efficacy of tirofiban in acute ischemic stroke patients not receiving endovascular treatment: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 24(3):1492–1503. https://doi.org/10.26355/eurrev_202002_20208
Gong J, Shang J, Yu H, Wan Q, Su D, Sun Z, Liu G (2020) Tirofiban for acute ischemic stroke: systematic review and meta-analysis. Eur J Clin Pharmacol 76(4):475–481. https://doi.org/10.1007/s00228-019-02817-8
Fu Z, Xu C, Liu X, Wang Z, Gao L (2020) Safety and efficacy of tirofiban in acute ischemic stroke patients receiving endovascular treatment: a meta-analysis. Cerebrovasc Dis 49(4):442–450. https://doi.org/10.1159/000509054
Selcuk AA (2019) A Guide for Systematic Reviews: PRISMA. Turk Arch Otorhinolaryngol 57(1):57–58. https://doi.org/10.5152/tao.2019.4058
Aslam S, Emmanuel P (2010) Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS 31(1):47–50. https://doi.org/10.4103/0253-7184.69003
Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, del Zoppo G, Kleindorfer D, Woo D, Khatri P, Castaldo J, Frey J, Gebel J Jr, Kasner S, Kidwell C, Kwiatkowski T, Libman R, Mackenzie R, Scott P, Starkman S, Thurman RJ, Investigators CT (2008) The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke 39(12):3268–3276. https://doi.org/10.1161/STROKEAHA.108.517656
Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH et al (1995) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 274(13):1017–1025
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352(9136):1245–1251. https://doi.org/10.1016/s0140-6736(98)08020-9
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(13):1317–1329. https://doi.org/10.1056/NEJMoa0804656
Li W, Lin L, Zhang M, Wu Y, Liu C, Li X, Huang S, Liang C, Wang Y, Chen J, Feng W (2016) Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke 47(10):2649–2651. https://doi.org/10.1161/STROKEAHA.116.014413
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane Collaboration, London
Lo CK, Mertz D, Loeb M (2014) Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14:45. https://doi.org/10.1186/1471-2288-14-45
Torgano G, Zecca B, Monzani V, Maestroni A, Rossi P, Cazzaniga M, Manganaro D, Boiti C, Zilioli E, Borutti G, Falaschi F, Mandelli C (2010) Effect of intravenous tirofiban and aspirin in reducing short-term and long-term neurologic deficit in patients with ischemic stroke: a double-blind randomized trial. Cerebrovasc Dis 29(3):275–281. https://doi.org/10.1159/000275503
Sun C, Li X, Zhao Z, Chen X, Huang C, Li X, Shan Y, Zou Y, Liu Y, Ibrahim M, Nyame L, Song B, Wang F, Zheng X, Hu J, Zhao Z, Zhou J, Zou J (2019) Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol 10:1100. https://doi.org/10.3389/fneur.2019.01100
Pan X, Zheng D, Zheng Y, Chan PWL, Lin Y, Zou J, Zhou J, Yang J (2019) Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur J Neurol 26(8):1105–1110. https://doi.org/10.1111/ene.13946
Wu C, Sun C, Wang L, Lian Y, Xie N, Huang S, Zhao W, Ren M, Wu D, Ding J, Song H, Wang Y, Ma Q, Ji X (2019) Low-dose tirofiban treatment improves neurological deterioration outcome after intravenous thrombolysis. Stroke 50(12):3481–3487. https://doi.org/10.1161/STROKEAHA.119.026240
Wu Y, Yin C, Yang J, Jiang L, Parsons MW, Lin L (2018) Endovascular thrombectomy. Stroke 49(11):2783–2785. https://doi.org/10.1161/STROKEAHA.118.022919
Yang M, Huo X, Gao F, Wang A, Ma N, Shi H, Chen W, Wang S, Wang Y, Miao Z (2020) Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol 27(6):1056–1061. https://doi.org/10.1111/ene.14170
Zhao W, Che R, Shang S, Wu C, Li C, Wu L, Chen J, Duan J, Song H, Zhang H, Ling F, Wang Y, Liebeskind D, Feng W, Ji X (2017) Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 48(12):3289–3294. https://doi.org/10.1161/STROKEAHA.117.019193
Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, Stampfl S (2013) Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke 44(5):1453–1455. https://doi.org/10.1161/STROKEAHA.111.000502
Huo X, Raynald WA, Mo D, Gao F, Ma N, Wang Y, Wang Y, Miao Z (2021) Safety and efficacy of tirofiban for acute ischemic stroke patients with large artery atherosclerosis stroke etiology undergoing endovascular therapy. Front Neurol. https://doi.org/10.3389/fneur.2021.630301
Ma G, Li S, Jia B, Mo D, Ma N, Gao F, Huo X, Luo G, Wang A, Pan Y et al (2021) Safety and efficacy of low-dose tirofiban combined with intravenous thrombolysis and mechanical thrombectomy in acute ischemic stroke: a matched-control analysis from a Nationwide Registry. Front Neurol. https://doi.org/10.3389/fneur.2021.666919
Siebler M, Hennerici MG, Schneider D, von Reutern GM, Seitz RJ, Rother J, Witte OW, Hamann G, Junghans U, Villringer A, Fiebach JB (2011) Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke 42(9):2388–2392. https://doi.org/10.1161/STROKEAHA.110.599662
Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D (2005) Intravenous glycoprotein IIb/IIIa inhibitor (tirofiban) followed by intra-arterial urokinase and mechanical thrombolysis in stroke. AJNR Am J Neuroradiol 26(10):2595–2601
Kwon JH, Shin SH, Weon YC, Hwang JC, Baik SK (2011) Intra-arterial adjuvant tirofiban after unsuccessful intra-arterial thrombolysis of acute ischemic stroke: preliminary experience in 16 patients. Neuroradiology 53(10):779–785. https://doi.org/10.1007/s00234-011-0939-y
Kim JW, Jeon P, Kim GM, Bang OY, Byun HS, Kim KH (2012) Local intraarterial tirofiban after formation of anterograde flow in patients with acute ischemic stroke: preliminary experience and short term follow-up results. Clin Neurol Neurosurg 114(10):1316–1319. https://doi.org/10.1016/j.clineuro.2012.04.022
Ottani F, La Vecchia L, De Vita M, Catapano O, Tarantino F, Galvani M (2010) Comparison by meta-analysis of eptifibatide and tirofiban to abciximab in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol 106(2):167-174 e161. https://doi.org/10.1016/j.amjcard.2010.03.012
Antoniucci D (2007) Differences among GP IIb/IIIa inhibitors: different clinical benefits in non-ST-segment elevation acute coronary syndrome percutaneous coronary intervention patients. Eur Heart J 9(Suppl_a):A32–A36
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LJT conceived and supervised the study; YYH searched for studies; LJT analyzed the data; XX, LJT wrote the manuscript; LHB made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., Yang, Y. & Liu, H. Efficacy outcomes and safety measures of intravenous tirofiban or eptifibatide for patients with acute ischemic stroke: a systematic review and meta-analysis of prospective studies. J Thromb Thrombolysis 53, 898–910 (2022). https://doi.org/10.1007/s11239-021-02584-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02584-3