Abstract
Groundwater and soil contaminated with arsenic (As) are significant environmental health concerns worldwide and have become a serious health issue for millions of Bangladeshis. This contaminated groundwater is commonly used for Boro rice production during the dry season in Bangladesh, leading to long-term As deposition in soils. The consequences of long-term irrigation with As-contaminated groundwater for bioaccumulation in food crops and hence dietary exposure to As and other metals are a threat to the resident. Highly arsenic (292 µg L−1) contaminated groundwater used to irrigate in the study area increases the As content (mean: 24.5 mg kg−1) in irrigated soil than non-irrigated soil (mean: 8.43 mg kg−1). The high enrichment factor (mean: 16.8) and geo-accumulation index (mean: 2.33) revealed that the soil of that particular area is strongly contaminated by As. In paddy, roots showed the maximum As concentration (mean: 139.8 mg kg−1) followed by straws (mean: 5.25 mg kg−1) and grains (mean: 1.23 mg kg−1) where 85–95% As exist in the roots of paddy, and only 0.8–1% As translocate to the harvesting part of the rice in the study area. In food samples, two rice grains (R-1, R-3) and a drumstick (FS-5) exceed the Indian standard of As concentration (As: 1.1 mg kg−1) in food. According to World Health Organization, the metals concentrations in all food samples exceeded the permissible limit except for Cu and Pb in red amaranth; Cr, Cd, and Pb in guava; and Cu and Cd in drumsticks. Higher values of bioaccumulation factor (BAF: 2.94) and the net translocation coefficient (NTC: 6.17) indicate As-accumulation in food from adjacent contaminated soil. Daily metal intake from food consumption suggests that the heavy metal(loid)s like As, Ni, and Pb represent high concentrations and exceed the daily ingestion limits (As: 3 µg kg−1 day−1, Ni: 1.3 µg kg−1 day−1, and Pb: 3.57 µg kg−1 day−1) developed by the Food and Agriculture Organization and European Food Safety Authority. Carcinogenic (> 10–6) and non-carcinogenic (HQ > 1) health risks of As, Pb, Cd, and Cu suggest that the community was at potential health risk according to the United States Environmental Protection Agency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As), a group-1 carcinogen is considered as one of the most significant global environmental toxicants which naturally occurs in rocks and soils [57, 78]. Although it is frequently present as a component of minerals, it is incredibly hard to find as a pure element. The primary As-bearing minerals are realgar (As4S4), arsenopyrite (FeAsS), niccolite (NiAs), and orpiment (As2S3), but it can also be found bonded to iron, manganese (oxy-)hydroxides, and sulfides in sedimentary rocks and soil [72, 74]. The levels of arsenic in foods generally reflect normal accumulation from the environment [80]. The problem of As contamination in agricultural soils occurs frequently and is likely to get worse in the near future. High concentrations of As in groundwater and surface soil have been reported in many countries of the world including Bangladesh, India, China, and Vietnam [42, 77]. There have been reports of high As concentrations in groundwater up to 2621 g L−1 [19, 20] and paddy soils with a range between 0.1 and 950 mg kg−1 [40, 86]. Domestic sewage, industrial wastewater, and irrigation utilizing As-enriched groundwater may be the main sources of As contribution to the soil-crops environment that influences food production all over the world [42]. High levels of As in soil pore water and crops have been associated with irrigation using As-enriched groundwater and the subsequent reductive dissolution of iron (Fe) (oxy)hydroxide minerals [108]. It has been observed that irrigation groundwater contains As, which accumulates in soil and not only affects agricultural growth but also puts food safety at risk across the food chain [85]. The increasing quantity of As in groundwater may endanger more than 200 million people worldwide, primarily in Asia [63, 84]. In South and Southeast Asia, especially in Bangladesh, India, Cambodia, Vietnam, and Taiwan, a major pathway of human exposure to As is via drinking water and intake from rice [40, 91]. Therefore, As contamination in drinking water and food is considered as one of the most serious issues associated with public health in Bangladesh [68]. Currently, in Bangladesh, 62 districts out of 64 have been affected by As and face a serious threat to public health, and 97% of Bangladesh's rural population relies on tube wells as their primary source of drinking water, and the country's entire supply of these wells is contaminated with As [1]. Together, 85 million people are at risk, and several thousand of them have shown symptoms of arsenicosis. Paddy rice, the main staple food for the inhabitants, is also irrigated with that As-contaminated groundwater, which causes a gradual increase of As in the soil. Therefore, few studies have reported that the As concentration in Bangladesh’s groundwater, soil, and plants is higher than the permissible limit and that 85–95% of the total As in rice and a vegetable was inorganic [29].
Bangladesh is mostly situated in the Bengal Basin, formed by the three mighty Ganga–Brahmaputra–Meghna (GBM) river systems. The Ganga–Meghna–Brahmaputra (GMB) river basin is at risk due to the presence of high concentrations of arsenic in groundwater [26]. This sedimentary basin was developed by the deposition of significant amounts of sediments containing As, primarily from the Himalayas, during the Pleistocene and Holocene periods. In the fan deposit areas and the Holocene alluvium, arsenic is eroded from these sediments and entered groundwater aquifers. Although it is unclear how exactly As leaches into groundwater, most researchers agree that the As contamination source is geological, but there is still controversy over identifying the source. The source of As in the groundwater of Bangladesh remains obscure [17]. To date, three significant theories have reported the mechanisms of As contamination in underground water in the GMB basin: oxidation of As-bearing pyrite, reductive dissolution of FeOOH and MnOOH, and competitive exchange of phosphate anions in the aquifer [1, 2, 50, 90, 111]. The second mechanism has been more acceptable in the last couple of years [73].
Massive extraction of groundwater contaminated with As for irrigation has led to high concentrations of As in top soils [52]. Arsenic contamination of the soil raises the amount of As that accumulates in crops [58]. According to European Community (EC), the maximum permissible limit of arsenic for agricultural soils is 20 mg kg−1 and a high concentration of arsenic is toxic for plants, gradually reducing crop yields [23, 35, 66]. Natural As concentrations in soils are approximately 5 mg kg−1 globally but can be elevated depending on the nature of the soil and anthropogenic loading of As from different chemicals and pesticides [48]. Contaminated soil is the primary pathway to exposure to poisonous metals in the human body. Human dietary products like crops, vegetables, and fruits grow in contaminated soils capable of translocating metals from soil to crops, resulting in a probable human health hazard.
Geological and geochemical studies revealed that the release of As in various parts of the earth is caused by both anthropogenic activities and natural rock or mineral weathering processes [10]. The situation is exacerbated by the fact that As, once present in groundwater and soil, can be bioaccumulated by terrestrial and aquatic biota, which are either consumed by humans or absorbed into cattle feed [51]. The uptake of As in plants from irrigated water and soils is large enough to cause potential health hazards due to extensive contamination by As. Chronic ingestion of heavy metals can lead to a deleterious health hazard for a human being, even in a trace amount [11]. Arsenic is phytotoxic in high quantities and is not necessary for plant growth. Typical As concentrations are 1–1.7 mg kg−1 for plant shoots growing in unpolluted soils [34]. Soil–plant exposure to As can occur in humans through As transfer [25]. Some common vegetables have regulatory limits on their As content, such as Poland’s standard for fresh vegetables is 0.2 mg kg-1, China’s standard for rice, beans, and vegetables is 0.5 mg kg−1 [59] and Japan’s is 1.0 mg kg−1 for spinach, tomatoes, and cucumbers. The European Commission and the US Food and Drug Administration proposed a maximum As-acceptable limit of 0.1 mg kg−1 for rice [36]. Aside from drinking arsenic-contaminated water, residents are also exposed to arsenic through dietary intakes of foods and vegetables, particularly rice cultivated on tainted fields in the region [33, 81]. Arsenic accumulated in rice from contaminated groundwater and soils is still considered the major dietary contributor to As intake for people [79].
Arsenic exposure has many adverse human health effects. Nearly all human body systems are impacted by exposure to arsenic, but the cardiovascular, neurological, hepatic, renal, hematological, endocrine, respiratory, and reproductive systems are the most severely affected by arsenic [65, 68, 84]. In addition, exposure to As during pregnancy increases the risk of infant death, spontaneous abortion, stillbirth, preterm birth, low birth weight, delayed child growth, a compromised immune system, a lower IQ, neurotoxicity, and neurodevelopmental impairment [102]. Chronic poisoning symptoms include skin diseases such as keratosis and possibly amputation due to gangrene. In Bangladesh, one of the most typical effects of long-term exposure to arsenic is skin lesions, i.e., arsenicosis, or hardening of the skin with lots of black and white spots [43]. High concentrations of Pb and Cd in food cause nerve, skeletal, circulatory, enzymatic, endocrine, and immunological system damage, hypertension, as well as pulmonary and renal impairment in the human body [45].
Most of the published paper is on As concentration in groundwater, soil, and crops, but there is little information on the bioaccumulation factor and net translocation coefficient of As from soil to crops and the associated carcinogenic health risk in the study area. Although some studies have suggested arsenic toxicity in rice cultivation, there have been very few investigations on its transfer from soil to rice and vegetables. Arsenic accumulation in food crops, notably rice and vegetables irrigated with As-contaminated groundwater, has been extensively observed in recent years [75, 76]. Little work has been conducted to investigate the translocation mechanism of As from root to grain in that As hotspot area where the As-affected people are found more. Moreover, only some authors have published a paper on As in soil and paddy. Still, no one has published an article on As and other metals in consumable foods and their impact on human health in this arsenic hotspot zone. To assess the health and environmental concerns, it is essential to investigate the transfer of arsenic from soil to edible parts of rice and vegetables in the study area. The current research work aimed to (1) assess the contamination level of As and other metals in soils and crops in the study area, (2) identify the possible sources and correlation among the metals with multivariate approaches, (3) measure the transfer of As from soils to the edible parts of various vegetables and rice grains, and (4) assess the human health risk associated with As-contaminated rice and vegetable consumptions.
Materials and methods
Site description
This research area is located in the Gangni Sub-District of Meherpur, Bangladesh, and is known as Bholadanga. This location is 15 km distance from Meherpur city and is situated on the boundary of the Meherpur-Kushtia-Chuadanga district. Agricultural activities are the primary source of livelihood for local inhabitants. It is located amid north and southwestern Bangladesh between the graphical coordinates of 23º51′00″ to 23º51′15’’north latitudes and 88º51′35″ to 88º51′50″ east longitudes (Fig. 1). It is bordered on the north by Doulatpur Upazila, on the east by Mirpur Upazila, on the south by Alomdanga Upazila, and the west by Meherpur Sadar Upazila. The Mathabhanga is a transboundary river between India and Bangladesh that begins on the right bank of the Padma near Munshiganj, Kushtia District. Like the rest of Bangladesh, the study area has a tropical Indian Monsoon climate with a tropical wet and dry, or savanna, climate. The district’s yearly temperature is 28.33 ℃ (maximum 39 ℃ in April and minimum 10.5 ℃ in January), and it is 0.59% higher than Bangladesh’s averages. The study area is situated at an elevation of 23 m (76 feet) above sea level, whereas the topographic gradient runs north to south [101]. The majority of groundwater flows in the same direction as the topographic slope.
Sampling
Groundwater sample was collected from a nearby shallow tube well used to irrigate the agricultural land and village area. Before sampling, an electric pump was run for about 2–3 min to obtain fresh groundwater. A previously cleaned 500-mL plastic bottle was used to collect groundwater and then acidified with concentrated HNO3 (1 mL). Soil samples (n = 8) and human consumable food samples (n = 9), along with paddy, fruits, and vegetables, were collected randomly from the nearby agricultural land and village area. Soil samples at 0–15 cm depth were collected (250 g each for different analyses) from adjacent fields of food samples. To create a homogeneous composite sample, triplicate soil samples were collected at random from each site and stored in a polythene bag. Four rice plants (RP) and five vegetables and fruits (FS) with edible parts were collected randomly from each field. All the food samples were kept in clean air-protected polyethylene bags with an icebox for carrying to the laboratory and stored at 4 ℃ until analysis. At the time of sampling, biodata (age, body weight, and sex) and food consumption rates and behaviors of the local people were collected to obtain individual As exposure assessments. A detail of the collected food samples is shown in Table 1.
Sample preparation
After collecting, the soil samples in the laboratory were dried up in the air, ground and passed through a 2-mm sieve, and finally stored in plastic pots. All soil samples were then oven-dried at 105 ℃, and the collected triplicate samples were carefully mixed entirely to get a composite sample. Then, prepared soil samples were kept in polyethylene bags for analysis. To extract the total metal concentration, about 10 g of dry soil sample was weighed into a 100-mL glass beaker. Then, 20 mL of concentrated HNO3 was added to the samples, and the suspension was heated for approximately 2 h at 120 ℃ in a hot plate. Then, 5–10 mL of concentrated HClO4 was added and again digested at 100 ℃ until it was a clear solution. The solution was then filtered through a Whatman™ 41 (150 mm) filter paper in the calibrated volumetric flask (100 mL) through multiple washing of the glass beaker and finally, it was stored in the plastic bottle for chemical analyses.
All food samples were rinsed with distilled water and washed with 0.05 M HCl solution to remove outside metals adhered in the free dust and soil particles on the root, straw, and grain (paddy). All crops, vegetable, and fruit samples were then dried up in the oven for 48 h at 110 ℃ to remove moisture. The food samples were then crushed to make powder. For metal content determination, about 10 g of dried powder samples were weighed in a 100 mL beaker and gradually heated up to 500 ℃ in a muffle furnace for ashing. After complete ashing, the samples were digested with 10 mL HNO3 and 5 mL HClO4 at around 100 ℃ in a hot plate and then filtered after cooling in a 50-mL volumetric flask and stored at 4 ℃ for analysis.
Sample analysis
Metal concentrations in soil and food samples were analyzed at the Institute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh, which is an ISO/IEC 17025:2017 accredited laboratory. The concentrations of As, Cu, Zn, Fe, and Mn in the soil and food samples were measured using an atomic absorption spectrometer (models: Spectra AA220 and AA240FS, Varian, Australia) equipped with electro-thermal temperature controller and flames, whereas Pb, Cd, Co, Ni, and Cr were determined using a Zeeman atomic absorption spectrometer (model: GTA 120-AA240Z, Varian, Australia) equipped with a graphite furnace. The certified reference materials (CRM) of metals of 1000 ± 4 mg L−1 stock solution (Fluka Analytical, Sigma-Aldrich, Germany) were used to construct the calibration curves, and the samples were measured against the prepared curves. All samples were measured in duplicate and the mean concentrations with a relative standard deviation (RSD) of less than 5% were considered for reporting.
Analytical quality control
Throughout the study period, from sample collection to laboratory analysis, the quality of the analysis was carefully monitored. For sample preparation, analytical-grade HNO3 and HClO4 from Merck Germany, and deionized water (electrical conductivity < 0.2 µS cm−1, resistance 18.2 MΩ cm at 25 ℃) were used. All glassware used for the analysis, such as the pipette and volumetric flask, were calibrated and cleaned prior to use by soaking in 10% (v/v) HNO3 overnight and then rinsing with deionized water. For sample weighing, a digital electrical balance (GR-200, A&D Company Limited, Tokyo, Japan) was used. The atomic absorption spectrometer was calibrated using the certified reference material (CRM) of the elements. The analytical accuracy and precision were ensured through the replicate measurement of CRM and the samples where the RSD was less than 5%. The measurement of sample blank and quality control standards were performed sequentially. The spike recovery in the analysis of metals was in the range of 96–104%. The detection limit for the analyzed metals were 0.2, 67.7, 3.1, 2.1, 2.2, 6.2, 7.5, 0.4, 0.3, and 1.2 µg/L for As, Fe, Mn, Co, Ni, Cu, Zn, Cr, Cd, Pb, respectively. Further details about quality assurance and quality control are described in our previous works [3, 28, 87].
Data analysis
Statistical analysis and software used
Analytical data were statistically analyzed with IBM SPSS Statistics software (Version 20, IBM Corporation, USA). To identify the source(s) of metals, multivariate statistical analyses such as principal component analysis (PCA), hierarchical cluster analysis (HCA), and correlation analysis were used. The results of HCA were expressed with a dendrogram. For spatial distribution, mapping, and other analytical calculations, Arc-GIS (version: 10.3.1), Adobe Illustrator (version: 2017), and Microsoft Excel 2013 were used usually.
Assessment of soil contamination
The enrichment factor (EF) and geo-accumulation (Igeo) index are the most often used methods for determining soil contamination degree. Enrichment factor and Igeo were used to assess the status of elemental contamination in the studied soil.
Enrichment factor (EF)
The most often used approach for estimating the impact of anthropogenic input on soil contamination is an enrichment factor (EF) for metal contamination levels above uncontaminated background levels [9, 103]. This study has potentially explained the metal associations and enrichment pattern throughout the period [14]. According to Taylor [93], the enrichment factor for each specific metal in the sample is typically calculated using the following relationship:
Iron (Fe) was considered as the reference metal for geochemical normalization. The degree of soil contamination using enrichment factors can be classified into seven classes such as 0–1 (no enrichment), 1–3 (small enrichment), 3–5 (medium enrichment), 5–10 (medium to acute enrichment), 10–25 (acute enrichment), 25–50 (very acute enrichment), and greater than 50 (extremely acute enrichment).
Geo-accumulation index (Igeo)
Muller [55] introduced the equation to represent the geo-accumulation index (Igeo), which is commonly used to measure additional metal concentrations in soil:
where Cn refers to the metal concentration from analysis and Bn is the geochemical background value of that metal. Muller [56] classified the geo-accumulation index as follows: 0 (uncontaminated), 0–1 (uncontaminated to moderately contaminated), 1–2 (moderately contaminated), 2–3 (moderately to strongly contaminated), 3–4 (strongly contaminated), 4–5 (strongly to extremely contaminated), and ≥ 5 (extremely contaminated). For the calculation of EF and Igeo by Krauskopf and Bird [41], the crustal assemblage was used as the background values.
Degree of metal contamination in foods
Bioaccumulation factor (BAF) of metals
Metals can enter the human body through three routes: natural or anthropogenic metal loads on soil, soil to food crops, and food crops to humans. The BAF is the potential ability of a plant species to transfer or accumulate a specific metal from adherent soil to edible sections of plants and can be described as the concentration ratio of metal in the plant material to that in the relevant soil [53, 78, 103]:
where Cplant and Csoil represent metal concentrations in green plants and soil, respectively, on a dry weight basis. BAF can control the metal’s exposure to humans via the food chain [15].
Net translocation coefficient (NTC)
Previously, the term “translocation coefficient” (TC) was used to describe a plant’s ability to absorb and translocate elements from water and soil via their root to the consumable part [49]. It was estimated on a dry weight basis for each soil–plant sample using the equations listed below [92, 104]:
where Csoil, Croot, Cstraw, and Cgrain are the total concentrations of metal in soil, root, straw, and grain, respectively.
Daily intake of metals (DIM)
The daily ingestion of metals (DIM) typically depends on the amount of a particular metal in the food sample and the ingestion rate of that respective food. The DIM for adults was calculated by the following relationship [54]:
where Cmetal is the metal concentration in food samples, Cwood is the average ingestion rate per day of the respective food, and Bw is the average body weight. The average daily ingestion of rice (grain), vegetables, and fruit for adults was considered to be 372 g, 274 g [112], and 200 g per person per day, respectively, while the average body weight for adults in the study was considered as 65 kg.
Health risk assessment
Non-carcinogenic risk
Non-carcinogenic risk assessments are often used to determine the possible health consequences of pollutants using the hazard quotient (HQ) [94]. The HQ values for each heavy metal can thus be measured through food consumption by nearby residents, and calculations were done using the conventional assumptions for an integrated USEPA risk study, as shown below [89, 96, 97]:
where EF is the exposure frequency (365 d year−1); ED is the exposure duration refers to life expectancy (72 years for Bangladesh); FIR is the food ingestion rate (g d−1). The daily ingestion of rice, vegetables, and fruits is considered to be 372, 274, and 200 g, respectively [112]. MC is the metal concentration of every individual sample (mg kg−1,RfD oral reference dose (mg kg−1 d−1). RfD value is 0.003, 1.5, 0.001, 0.004, 0.02, 0.04, and 0.3 for As, Cr, Cd, Pb, Ni, Cu, and Zn, respectively [99, 100]. BW is the average body weight (adult) in the study area. AT refers to the average lifetime for non-carcinogenic health hazards which is EF*ED = 365*72 = 26,280 days. HQ value of less than one suggests that daily exposure to a given metal through food consumption is unlikely to induce non-carcinogenic health impacts, whereas an HQ value larger than one indicates that chronic health concerns may develop in the exposed population [6].
Carcinogenic risk
Carcinogenic risk (CR) refers to an individual’s lifetime risk of developing cancer as a result of exposure to a potential carcinogen. Cancer risk from As, Pb, and Cd exposure over a lifetime was calculated using the cancer slope factor (CSF) given by the USEPA [97]. A carcinogenic hazard value greater than 10–6 indicates that the people in the respective area were at high carcinogenic risk according to USEPA [98]:
or,
CSF is the carcinogenic slope factor, which measures the upper-bound possibility of a single person developing cancer due to lifetime exposure to a specific level of probable carcinogen through an ingestion route. The CSF value of As, Pb, and Cd is 1.5, 0.0085, and 15 mg kg−1 day−1, respectively [98].
Results and discussion
Contamination assessment of heavy metals in soil
The analytical results of metal(loid)s concentration in soil samples of the research area are shown in Table 2. The range of arsenic (As) concentration in irrigated land is 16.6–40.2 mg kg−1 (mean ± SD: 24.5 ± 10.9) and in non-irrigated/partially irrigated land is 4.54 to 10.3 mg kg−1 (mean ± SD: 8.43 ± 2.64). Except for all other metals, the concentration of As (mean ± SD: 16.5 ± 11.3) was found higher value than the average concentration of As (4 to 8 mg kg−1) in soils of Bangladesh [4, 107]. The rest of the metal concentration shows average crustal values, which are naturally present as an earth material. Heavy metal(loid)s contamination of agricultural soil is one of the most serious obstacles to sustainable development, particularly in developing nations like Bangladesh. The degree of soil contamination in the study area is represented in Table 3. The enrichment factor (EF) is a widely used statistic for measuring how much the amount of an element in a sampling medium has risen due to human activity relative to normal natural abundance. Results of the enrichment factor (EF) of Co, Cd, and Cu show no enrichment (EF < 1). Fe, Mn, Zn, and Pb show slight enrichment (1 ≤ EF < 3), and the results of arsenic show acute enrichment (10 ≤ EF < 25). EF value ranges from 0.05 to 1.5, revealing a natural process, i.e., the metal supply is entirely from the crustal materials whereas, values greater than 1.5 indicate metals are mostly from the anthropogenic origin [110]. Significant enrichment of arsenic suggests that the soils were contaminated, primarily due to anthropogenic activity like pumping groundwater from the arsenic-bearing aquifer for irrigation purposes. The metal enrichment factor of less than 1.5 suggested that these metals did not significantly contaminate the soils in the studied area and that their small accumulation is entirely natural in origin. By comparing the current concentration to the background level, the geo-accumulation index (Igeo) was utilized to assess the level of heavy metal and metalloid components in the sediment. The results of the geo-accumulation index (Igeo) for Fe, Mn, Co, Cu, Zn, Cd, and Pb show particularly uncontaminated (Igeo ≤ 0), and As show moderately to strongly contaminated (2 < Igeo < 3), which occurs due to use of high As-contaminated groundwater. The As load on soil in the study area is a result of anthropogenic activities such as the use of wood preservatives in electric poles, the overuse of As-based fertilizers and pesticides in agriculture, and irrigation with As-contaminated groundwater [5, 12, 37, 67].
Spatial distribution maps of trace metals were developed to identify the spatial signature of trace metals in soil. For all trace metals, concentrations are higher in the northern portion among the sampling points SS-1, SS-2, SS-3, and SS-4. All of the samples above were collected from irrigated land where staple food rice was grown all year. Groundwater contains trace metals that accumulate on the soil over time. Trace metals concentration is lower in the southern portion of the study area among the sampling points SS-5, SS-6, SS-7, and SS-8, primarily in the residential area where local people used to cultivate seasonal fruits and vegetables that are partially irrigated, explaining why metals concentration is lower in the southern portion than the northern portion (Fig. 2).
Contamination assessment of heavy metals in food
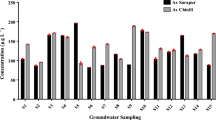
The analytical results of the metal concentration of collected food samples are presented in Table 4. A comparison of those metal accumulations with food standards established by WHO [24] found that only rice grain (R-1 to R-4) arsenic concentration is considered instead of rice plants. Two rice grains (R-1, R-3) and Sajna (drumstick) exceed the Indian standard (As: 1.1 mg kg−1) [7] limit for As in human consumable foods (Fig. 3a). For a nickel, all food samples exceed the Indian standard (Ni-1.5 mg kg−1) value except Lalshak (red amaranth) and Dalim (pomegranate) (Fig. 3b). Except for Lalshak (red amaranth) and Sajna (drumstick), all the samples exceed the WHO limit (10 mg kg−1) for Cu in food (Fig. 3c) [105]. All the samples exceed the WHO standard (1 mg kg−1) for Cr and Pb except Peyara (Guava) (Fig. 3d and f). For all paddy (grain) samples (R-1to R-4) and Dalim (pomegranate), Cd concentration exceeds the WHO limit (0.2 mg kg−1) for food samples (Fig. 3e). Comparison of As concentration in soil, rice, and vegetable/fruits with relevant literature data is presented in Table 5 which is the most essential to understand the status of regional arsenic contamination level.
Multivariate statistical analysis
Multivariate statistical approaches, such as principal components analysis (PCA), hierarchical cluster analysis (HCA), and correlation analysis are excellent tools for identifying and categorizing the many causes of soil metal pollution [87]. These analyses are commonly used to anticipate heavy metal variability and its governing factors, as well as to emphasize the impact of human activities on soil enriched with heavy metal [44]. Tables 6 and 7 show the findings of the PCA of metal contents in soil and food (paddy) samples, respectively. In this study, two principal components were identified from the analytical data of soil samples, which narrated the cumulative variance of 68.166 and 90.532%, respectively (Fig. 4a and b). In food samples (paddy), two principal components were also extracted, which explain the cumulative variance of 68.071 and 97.063%, respectively (Fig. 5a and b). Two principal components (PCA-1 and PCA-2) were noted for soil samples and food samples individually. In PCA-1 of soil samples, As, Co, Ni, Cu, Fe, Mn, Cr, and Pb showed positive loading, whereas only Zn showed a negative value. The positive loadings of trace metals on soil suggest that human activity is to blame for the accumulation in the study area, which is most likely derived from irrigation water. Factor loadings larger than 0.71 are generally regarded as outstanding, while those less than 0.32 are regarded as extremely poor [22].
In PCA-1, an eigenvalue greater than 2 with a total variance of 68.166% and significant loadings of As (0.940), Fe (0.956), and Mn (0.974) revealed that the source of those trace elements in the soil sample is the same [64]. In PCA-2, Zn, Cd, and Mg showed positive loading, which also suggests anthropogenic loading on soil due to the use of different types of fertilizer [39]. The highest values of Zn (0.963) and Mg (0.897) in PCA-2 introduce phosphate and magnesium sulfate fertilizers used for agricultural practice in that area. Organic and phosphate fertilizers contain higher amounts of Pb and Cd, respectively [46]. Pb and Cd showed almost similar loading in both PCA-1 and PCA-2, which mainly came from both geogenic and anthropogenic origins. Total factor accumulation values greater than 0.75, 0.75–0.50, and 0.50–0.30 were graded as high, medium, and low, respectively [3]. In PCA-1 of food samples, Co, Ni, Fe, Mn, Cr, Pb, and Mg showed positive loading, which is almost similar to the PCA-1 of soil samples, while As and Mn are dominant in PCA-2. In both factors of PCA-1 and PCA-2, As (PCA-1: 0.566; PCA-2: 0.817) and Mn (PCA-1: 0.586; PCA-2: 0.758) showed close positive loading, which suggests both natural and anthropogenic origins. The similar loadings of metals in soil and food samples in PCA-1 indicate homogeneity of origin. From PCA analysis, metal contents in entire soil and food samples are both geogenic and anthropogenic in origin because of the wide range of analytical testing of fertilizer products such as phosphate, micronutrient fertilizers, liming materials, insecticides, and pesticides that contain an elevated level of As, Ni, Cd, and Pb compared to other fertilizer types. The distribution of the same sorts of metals in soil samples and paddy samples was not analogous, which might be the result of distinct emission and accumulation characteristics of the considered metals by plants from the source to the environment [15].
Moreover, hierarchical cluster analysis (HCA) was executed with Ward’s methods to distribute the metals into different groups, representing the result with a dendrogram for soil samples (Fig. 4c) and food samples (Fig. 5c). For both soil and food samples, two clusters were identified with the Phenon line set to a rescaled distance of about 10 to show statistical similarity. In soil samples, Cluster-1 and Cluster-2 included Co, Ni, Cu, Fe, Mn, As, Pb, and Cr, and Zn, Mg, and Cd, which showed positive loadings on PCA-1 and PCA-2, respectively. It also exhibits the similarity between PCA and HCA. In food (paddy) samples, Cluster-1 showed positive loading of Fe, Ni, Cr, Pb, Co, Mg, As, and Mn, whereas Zn, Cd, and Cu are the dominant metals in Cluster-2. Only Cu and Mg showed dissimilarity between PCA and CA analyses in the paddy sample, which may happen due to the accumulation and resistance to the accumulation of metals in plant cells from different sources [15].
A mutual correlation among the studied metal concentrations in the soil and food samples was identified to determine their interrelationships, as presented in Table 8. In the case of soil samples, As showed strong positive correlations with Co (r = 0.831), Ni (r = 0.854), Cu (r = 0.792), Fe (r = 0.871), Mn (r = 0.905), Cr (r = 0.903), and Pb (r = 0.829) and negative correlations with Zn (r = −0.135). Fe showed a strong correlation with Mn (r = 0.973), Cr (r = 0.981), and Pb (r = 0.913). Homogeneous behavior among the metal groups revealed a similar source in origin [9]. In food samples, As showed a strong positive correlation with Co (r = 0.942), Ni (r = 0.972), Fe (r = 0.978), Mn (r = 0.983), Zn (r = 0.796), Cr (r = 0.963), and Pb (r = 0.960) and an insignificant correlation with Cu (r = 0.460). Fe showed a strong correlation with Mn (r = 0.963), Cr (r = 0.969), and Pb (r = 0.970), which indicate similarity in soil and food samples as a common source.
Accumulation of arsenic in soils and plants
Agricultural activities frequently result in heavy metal deposition in soils, and this includes non-point sources of contaminated inputs such as fertilizers, insecticides, sewage sludge, organic manures, and composts [88]. Groundwater contaminated with As used for drinking purposes and the agricultural water supply is considered the major ingestion route of As to the human body and plants. Several hypotheses have been made to understand the origin (primary) and mobilization (secondary) causes of elevated arsenic in groundwater. Arsenic in the Bengal Delta, especially in GMB (Ganges–Brahmaputra–Meghna) flood plains, came from the Himalayas and the highlands in the neighboring areas. Sulfides washed out and accumulated in these GMB floodplain areas from the Himalayan arsenic-bearing pyrite. Contaminants primarily affect the Holocene aquifer at depths ranging from 20 to 120 m, which cover southwest and south-central Bangladesh [71]. Arsenic can be found in Holocene sediments, which eventually mixed with groundwater as a result of microbial activity and the reductive dissolution of iron oxyhydroxides (FeOOH). A very low concentration of As in plants may have beneficial impacts on plant species [27]. Although the jury is still out on whether As is a natural element of some plants, it is widely accepted that As is not needed for plants [37]. The flooded method of irrigation is used in this region. As a result, a considerable amount of arsenic is available to both form adjacent soil and water to the rice plant. High concentration of As was found in root area, due to the synergistic effect of As from contaminated soil and groundwater [13]. The overall As content in water, soil, paddy roots, straw, and grain is presented in Table 9. A proposed model of arsenic circulation in an agronomical system modified from Sandberg and Allen [82], is shown in Fig. 6.

Source: modified from [82]
A proposed model for the arsenic cycle in an agronomical ecosystem in the study area.
Metals transfer from soils to crops
The results of the bioaccumulation factor of metals showed that the BAF values for rice plants are higher than those for other vegetable samples shown in Table 10. For rice plants (RP), As, Cd, and Mn showed greater concentrations; Zn, Cu, Fe, Cr, and Pb showed moderate concentrations; and Co and Ni showed low concentrations, which are moderately similar to their concentration in soil samples. The higher values of As for rice plants due to both soil As accumulated in previous years and As freshly introduced with irrigation water influence As uptake during rice growth [18]. Cadmium is the most accumulated metal and showed the highest BAF values among all the metals analyzed [83]. If the Cd enters the plant vessel, it interludes with the enzymes and takes the place of Zn, which results in an easy transfer of this metal from soil to the consumable part of the plant species compared to Zn [53]. Cd showed high accumulation in all the samples except Peyara without any visual effects. Cadmium transfer is highly susceptible due to its significant toxicity. Although soil samples were found to be low to moderately contaminated with Cd, with higher BAF values for rice plants and vegetable samples. For vegetable samples, Cu and Zn showed higher concentrations than all other metals. Comparatively low to moderate BAF values for Cr, Co, Fe, and Ni are probably due to their low mobility and phytoavailability, expressing their strong sorption in soils, which make lowers the free ion concentrations in the soil solution and makes them less available for plant accumulation [83]. The overall results strongly expressed that plant samples, especially rice plants, are mainly contaminated with As, Cd, and Mn, and other vegetable samples are also contaminated with cadmium, which showed a higher BAF value for all the samples except Peyara (guava).
The results of the net translocation coefficient (NTC) of arsenic for rice plants are almost similar to the transfer factor of arsenic in rice plants shown in Table 11. NTC soil/root has been thought to signify a plant's ability to accumulate a specific element. The translocation coefficient between soil and root (soil–root) showed a higher value than root–straw and straw–grain. On this basis, the NTC soil/root in the current study's data might be interpreted as indicating that rice has a greater capacity for As uptake. Such a high value (mean: 6.17) of TC root/soil for rice plants indicates arsenic sinks on soil and that soil arsenic can accumulate through the plant root to the harvesting part in the study area. The overall analytical and statistical results strongly indicate that food samples from Bholadanga village in Meherpur district, southwestern Bangladesh, are badly contaminated with As.
Health risks of local people
In the research area, humans were exposed to As toxicity through consumable food and drinking water. The schematic diagram of As exposure to the human body is shown in Fig. 7. The daily intake of metals (Table 12) for rice and vegetables shows terrible value. Highly toxic metal(loid)s like As, Ni, and Pb represent the maximum value. They all exceed the daily ingestion limits of As (3 µg/kg bw/day) according to the benchmark dosage for a 0.5% increased incidence of lung cancer (BMDL0.5) of 3.0 µg/kg bw/day, which varied from 2 to 7 µg/kg bw/day [106], Ni (1.3 µg/kg/day), and Pb (3.57 µg/kg/day) for adults recommended by FAO/WHO [24] and EFSA [21]. The US Environmental Protection Agency (USEPA) has used HQ as another index for computing human dietary risk [30]. The risk for any non-carcinogenic health hazard through intake of arsenic-contaminated drinking water and foodstuffs is denoted when the hazard quotient (HQ) value is greater than 1 [8, 16, 33, 109]. The results of the HQ (Table 13) for contaminated metals in foodstuff revealed that the values of As in rice (RP-1 to RP-4) and drumstick (FS-5), Pb in rice (RP-2), pomegranate (FS-3), arum (FS-4), drumstick (FS-5), and Cu in arum (FS-4) ingestion rates were higher than 1 (HQ > 1; bold typed face), and it was noted that the local people in the research area seem to be exposed to the significantly non-carcinogenic health risk of As and Pb. Carcinogenic risk (CR) is used to calculate the likelihood of an individual developing cancer as a result of consuming contaminated food. The CR was calculated for As, Pb, and Cd according to the equation developed by USEPA [98] presented in Table 14. The carcinogenic risk value is greater than 10–6, which suggests that the people in the vicinity are at high carcinogenic risk [62]. The carcinogenic risk from food consumption of the studied population is highly unacceptable and values were much beyond the safety standard value of 1 × 10–6 to 1 × 10–4 [100]. The results of CR for food indicate that for food samples, the values of As, Pb, and Cd are greater than 10–6 and the mean CR values for As, Pb, and Cd are 6.53 × 10–3, 4.04 × 10–5, and 7.72 × 10–3, respectively, which suggest that the people of the study area are at high carcinogenic risk according to USEPA-2005. Priority must be given to focusing on the carcinogenic risks to the populations residing in the study area, as they are at high risk of developing skin, bladder, and lung cancers as a result of arsenic exposure pathways [38]. Aside from drinking water, the potential health risk of arsenic from rice was a substantial threat in arsenic-affected areas [81].
Conclusions
Analytical results from this research indicate that the availability of As in irrigation water led to significant enrichment of As in the irrigated soil, mainly on the upper surface (0–10 cm), and through translocation processes or bioavailability of As gets into the human consumable food chain. This study also suggests gradual As poisoning in irrigated soil has increased. Local people in the study area are possibly affected by high As contamination through drinking water and ingestion of food for a couple of years and increased probability of health risk. High soil As is found in irrigated land of the northern portion whereas the southern portion is less contaminated. Strong correlations between dissolved Fe, As, and Mn in soil suggest that high elemental affinity is present between Fe, Mn, and As and from which As can strongly accumulate over the soil. According to principal component and cluster analysis, metal concentrations in soil are natural (As, Co, Ni, Cu, Fe, Mn) and anthropogenic (Zn, Cd, and Mg). Soil contamination indices like enrichment factor and geo-accumulation index indicate As is the dominant metalloid that contaminates soil in the study area. Among the food samples, paddy contains the highest As concentration compared to other vegetables or fruit samples. A higher value of BAF (Bioaccumulation Factor) and NTC (Net translocation coefficient) indicates plants absorb metals from adjacent soil. The toxicity of metals in humans is determined by their daily ingestion rate. Daily intake of metals shows harmful metal(loid)s like As, Ni, Cr, and Pb are the highest accumulated elements in the human body through the consumable food in the affected areas. Arsenic and Pb show the maximum potential hazard quotient from the food sample. The mean values of As and Pb for HQ are greater than 1 which indicates local people are at non-carcinogenic health risk from the intake of contaminated food. Individual carcinogenic health risks from As, Pb, and Cd indicate that the people in the Bholadanga village were at high carcinogenic risk and which exceed the cancer risk value (10–6) recommended by the United States Environmental Protection Agency (USEPA). However, it is suggested to install tube wells at a higher depth (> 150 m) to get As-free groundwater, and also surface water could be used for irrigation purposes, which is free from As.
Availability of data and materials
Data are available in the text of the manuscript.
References
Ahmad SA, Khan MH, Haque M (2018) Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Healthc Policy 11:251. https://doi.org/10.2147/RMHP.S153188
Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Alam SMM, Bhuyian MAH, Imam MB, Khan AA, Sracek O (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. ApplGeochem 19:181–200
Ahsan MA, Satter F, Siddique MAB, Akbor MA, Ahmed S, Shajahan M, Khan R (2019) Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ Monit Assess 191:575
Alam MB, Sattar MA (2000) Assessment of arsenic contamination in soils and waters in some areas of Bangladesh. Water Sci Technol 42(7–8):185–192
Alam MGM, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308:83–96
Avigliano E, Lozano C, Pla RR, Volpedo AV (2016) Heavy element determination in fish from Paraná River Delta (Argentina) by neutron activation analysis: tissue distribution and accumulation and health risk assessment by direct consumption. J Food Compos Anal 54:27–36
Awasthi SK (2000) Prevention of food adulteration act no 37 of 1954, Central and state rules as amended for 1999. Ashoka Law House, New Delhi
Biswas A, Swain S, Chowdhury NR, Joardar M, Das A, Mukherjee M, Roychowdhury T (2019) Arsenic contamination in Kolkata metropolitan city: perspective of transportation of agricultural products from arsenic-endemic areas. Environ Sci Pollut Res 26:22929–22944
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392
Bundschuh J, Nath B, Bhattacharya P, Liu CW, Armienta MA, Lopez MVM, Lopez DL, Jean JS, Cornejo L, Macedo LFL, Filho AT (2011) Arsenic in the human food chain: the Latin American perspective. Sci Total Environ 429:92–106
Cadar O, Miclean M, Cadar S, Tanaselia C, Senila L, Senila M (2015) Assessment of heavy metals in cow’s milk in Rodnei Mountains area. Romania EEMJ 14(11):2523–2528
Chandrakar V, Naithani SC, Keshavkant S (2016) Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: a review. Biologia 71:367–377. https://doi.org/10.1515/biolog-2016-0052
Chowdhury NR, Das R, Joardar M, Ghosh S, Bhowmick S, Roychowdhury T (2018) Arsenic accumulation in paddy plants at different phases of pre-monsoon cultivation. Chemosphere 210:987–997. https://doi.org/10.1016/j.chemosphere.2018.07.041
Chowdhury NR, Das A, Mukherjee M, Swain S, Joardar M, De A, Mridha D, Roychowdhury T (2020) Monsoonal paddy cultivation with phase-wise arsenic distribution in exposed and control sites of West Bengal, alongside its assimilation in rice grain. J Hazard Mater 400:123206. https://doi.org/10.1016/j.jhazmat.2020.123206
Chowdhury N, Rasid MM (2016) Heavy metal contamination of soil and vegetation in ambient locality of ship breaking yards in Chittagong, Bangladesh. J Environ SciHeavyol Food Technol 10(10):20–27
Das A, Das SS, Chowdhury NR, Joardar M, Ghosh B, Roychowdhury T (2020) Quality and health risk evaluation for groundwater in Nadia district, West Bengal: an approach on its suitability for drinking and domestic purpose. Groundw Sustain Dev 10:100351. https://doi.org/10.1016/j.gsd.2020.100351
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30:383–387. https://doi.org/10.1016/j.envint.2003.09.005
Dittmar J, Voegelin A, Maurer F, Roberts LC, Hug SJ, Saha GC, Ali MA, Badruzzaman ABM, Kretzschmar R (2010) Arsenic in soil and irrigation water affects arsenic uptake by rice: complementary insights from field and pot studies. Environ Sci Technol 44:8842–8848
Du Y, Deng Y, Ma T, Lu Z, Shen S, Gan Y, Wang Y (2018) Hydrogeochemical evidences for targeting sources of safe groundwater supply in arsenic-affected multi-level aquifer systems. Sci Total Environ 645:1159–1171. https://doi.org/10.1016/j.scitotenv
Duan Y, Gan Y, Wang Y, Deng Y, Guo X, Dong C (2015) Temporal variation of groundwater level and arsenic concentration at Jianghan Plain, central China. J Geochem Explor 149:106–119. https://doi.org/10.1016/j.gexplo.2014.12.001
EFSA (European Food Safety Authority) (2011) Use of the EFSA comprehensive European food consumption database in exposure assessment. EFSA J 9(3):2097
El Nemr A, Khaled A, El Sikaily A (2006) Distribution and statistical analysis of leachable and total heavy metals in the sediments of the Suez Gulf. Environ Monit Assess 118(1–3):89–112
FAO (2006) Arsenic contamination of irrigation water, soil and crops in Bangladesh: risk implications for sustainable agriculture and food safety in Asia. In: Heikens A (ed) Regional office for Asia and The Pacific, Rap publication 2006/20. Food and Agriculture Organization of the United Nation, Bangkok, Thailand
FAO/WHO (Food and Agriculture Organization/World Health Organization). Evaluation of certain food additives and the contaminants mercury, lead and cadmium. (1989). WHO TechnicalReport: 505.
Geng CN, Zhu YG, Tong YP, Smith SE, Smith FA (2006) Arsenate (As) uptake by and distribution in two cultivars of winter wheat (Triticum aestivum l.). Chemosphere 62:608–615
Goswami R, Kumar M, Biyani N, Shea PJ (2020) Arsenic exposure and perception of health risk due to groundwater contamination in Majuli (river island), Assam, India. Environ Geochem Health 42(2):443–460
Gulz PA, Gupta SK, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347. https://doi.org/10.1007/s11104-004-5960-z
Hasan AB, Reza AHMS, Kabir S, Siddique MAB, Ahsan MA, Akbor MA (2020) Accumulation and distribution of heavy metals in soil and food crops around the ship breaking area in southern Bangladesh and associated health risk assessment. SN Appl Sci 2(2):155. https://doi.org/10.1007/s42452-019-1933-y
Hossain MF (2006) Arsenic contamination in Bangladesh—an overview. Agr Ecosyst Environ 113(1–4):1–16. https://doi.org/10.1016/j.agee.2005.08.034
IRIS (2020) Integrated risk information system-database. US Environmental Protection Agency, Washington, DC
Islam MS, Ahmed MK, Mamun MHA, Islam KN, Ibrahim M, Masunaga S (2014) Arsenic and lead in foods: a potential threat to human health in Bangladesh. Food Addit Contam Part A. https://doi.org/10.1080/19440049.2014.974686
Jiang Y, Zeng X, Fan X, Chao S, Zhu M, Cao H (2015) Levels of arsenic pollution in daily foodstuffs and soils and its associated human health risk in a town in Jiangsu Province, China. Ecotoxicol Environ Saf 122:198–204. https://doi.org/10.1016/j.ecoenv.2015.07.018
Joardar M, Das A, Chowdhury NR, Mridha D, De A, Majumdar KK, Roychowdhury T (2021) Health effect and risk assessment of the populations exposed to different arsenic levels in drinking water and foodstuffs from four villages in arsenic endemic Gaighata block, West Bengal, India. Environ Geochem Health 43:3027–3053. https://doi.org/10.1007/s10653-021-00823-3
Kabata Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC, Boca Raton, p 53
Kabata-Pendias A, Pendis H (2001) Trace elements in soils and plants, 3rd edn. CRC Press, London, pp 38–45
Karagas MR, Punshon T, Davis M, Bulka CM, Slaughter F, Karalis D, Argos M, Ahsan H (2019) Rice intake and emerging concerns on arsenic in rice: a review of the human evidence and methodologic challenges. Curr Environ Health Rep 6:361–372
Khalid S, Shahid M, Niazi NK, Rafiq M, Bakhat HF, Imran M, Abbas T, Bibi I, Dumat C (2017) Arsenic behaviour in soil-plant system: biogeochemical reactions and chemical speciation influences. Enhancing cleanup of environmental pollutants. Springer, Berlin, Germany, pp 97–140
Khan KM, Chakraborty R, Bundschuh J, Bhattacharya P, Parvez F (2020) Health effects of arsenic exposure in Latin America: an overview of the past eight years of research. Sci Total Environ 710:136071
Khan R, Parvez MS, Jolly YN, Haydar MA, Alam MF, Khatun MA, Sarker MMR, Habib MA, Tamim U, Das S, Sultana S, Islam AM, Naher K, Paul D, Akter S, Khan MHR, Nahid F, Huque R, Rajib M, Hossain SM (2019) Elemental abundances, natural radioactivity and physicochemical records of a southern part of Bangladesh: implication for assessing the environmental geochemistry. Environ Nanotechnol Monit Manag 12:100225
Khanam R, Kumar A, Nayak AK, Shahid M, Pathak H (2019) Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: bioavailability and potential risk to human health. Sci Total Environ 699:134330
Krauskopf KB, Bird DK (1995) Introduction to geochemistry, 3rd edn. McGraw-Hill Inc, New York
Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J (2018) Arsenic speciation dynamics in paddy rice soil-water environment: sources, physico-chemical, and biological factors—a review. Water Res 140:403–414
Kurasaki M, Ichihara S (2021) Environmental arsenic exposure and its contribution to human diseases, heavyity mechanism and management. Environ Pollut 289:117940. https://doi.org/10.1016/j.envpol.2021.117940
Li X, Feng L (2012) Multivariate and geostatistical analyzes of metals in urban soil of Weinan industrial areas, Northwest of China. Atmos Environ 47:58–65
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Liao S, Jin G, Khan MA, Zhu Y, Duan L, Luo W, Jia J, Zhong B, Ma J, Ye Z, Liu D (2020) The quantitative source apportionment of heavy metals in peri-urban agricultural soils with UNMIX and input fluxes analysis. Environ Technol Innov. https://doi.org/10.1016/j.eti.2020.101232
Lin SC, Chang TK, Huang WD, Lur HS, Shyn GS (2015) Accumulation of arsenic in rice plant: a study of an arsenic-contaminated site in Taiwan. Paddy Water Environ 13:11–18. https://doi.org/10.1007/s10333-013-0401-3
Mandal BK, Suzuki KT (2002) Arsenic around the world: a review. Talanta 58:201–235
Mattina MI, Lannucci-Berger W, Musante C, White JC (2003) Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ Pollut 124:375–378
McArthur JM, Banjeree DM, Hudson-Edwards KA, Mishra R, Purohit R, Ravenscroft P, Cronin A, Howarth RJ, Chatterjee A, Talukder T, Lowry D, Houghton S, Chadha DK (2004) Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. ApplGeochem 19:1255–1293
Meharg AA, Jardine L (2003) Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol 157:39–44
Meharg AA, Rahman MM (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37:229–234
Miclean M, Cadar O, Levei EA, Roman R, Ozunu A, Levei L (2019) Metal (Pb, Cu, Cd, and Zn) transfer along food chain and health risk assessment through raw milk consumption from free-range cows. Int J Environ Res Public Health 16(21):4064
Miclean M, Cadar O, Levei L, Senila L, Ozunu A (2018) Metal contents and potential health risk assessment of crops grown in a former mining district (Romania). J Environ Sci Health 53(9):595–601
Muller G (1979) Schwermetalle in den sediments des Rheins- Veran-derungenseitt 1971. Umschan 79:778–783
Muller G (1981) Die Schwermetallbelstung der sedimente des neckars und seiner nebenflusse: eine stand saufnahme. Chem Zeitung 105:157–164
Müller V, Chavez-Capilla T, Feldmann J, Mestrot A (2022) Increasing temperature and flooding enhance arsenic release and biotransformations in Swiss soils. Sci Total Environ 838:156049. https://doi.org/10.1016/j.scitotenv.2022.156049
Murphy T, Phan K, Yumvihoze E, Irvine K, Wilson K, Lean D, Poulain A, Laird B, Chan LHM (2018) Effects of arsenic, iron and fertilizers in soil on rice in Cambodia. J Health Pollut 8(19):180910
China’s Food Safety National Standard for Maximum Levels of Contaminants in Foods (GB 2762-2012). 2014. Peoples Republic of China. USDA Foreign Agricultural Service, Global Agricultural Information Network (GAIN), GAIN Report Number: CH14058, FAIRS Subject Report.
Ngoc NTM, Chuyen NV, Thao NTT, Duc NQ, Trang NTT, Binh NTT, Sa HC, Trans NB, Ba NV, Khai NV, Son HA, Han PV, Wattenbergs EV, Nakamura H, Thuc PV (2020) Chromium, cadmium, lead, and arsenic concentrations in water, vegetables, and seafood consumed in a Coastal Area in Northern Vietnam. Environ Health Insights V 14:1–9. https://doi.org/10.1177/1178630220921410
Norton GJ, Islam MR, Deacon CM, Zhao FJ, Mcgrath SP, Islam S, Jahiruddin M, Feldmann J, Price AH, Meharg AA (2009) Identification of low inorganic and total grain arsenic rice cultivars from Bangladesh. Environ Sci Technol 43:6070–6075. https://doi.org/10.1021/es901121jCCC
Parker GH, Gillie CE, Miller JV, Badger DE, Kreider ML (2022) Human health risk assessment of arsenic, cadmium, lead, and mercury ingestion from baby foods. Toxicol Rep 9:238–249. https://doi.org/10.1016/j.toxrep.2022.02.001
Podgorski J, Berg M (2020) Global threat of arsenic in groundwater. Science 368(80):845–850. https://doi.org/10.1126/science.aba1510
Rafique N, Tariq SR (2016) Distribution and source apportionment studies of heavy metals in soil of cotton/wheat fields. Environ Monit Assess 188:309. https://doi.org/10.1007/s10661-016-5309-0
Rahaman MS, Rahman MM, Mise N, Sikder T, Ichihara G, Uddin MK, Kurasaki M, Ichihara S (2021) Environmental arsenic exposure and its contribution to human diseases, heavyity mechanism and management. Environ Pollut 289:117940. https://doi.org/10.1016/j.envpol.2021.117940
Rahman MA, Hasegawa H, Rahman MM, Rahman MA, Miah MAM (2007) Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution infractions of rice grain. Chemosphere 69:942–948. https://doi.org/10.1016/j.chemosphere.2007.05.044
Rahman MA, Hogan B, Duncan E, Doyle C, Krassoi R, Rahman MM, Naidu R, Lim RP, Maher W, Hassler C (2014) Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35) Ecoheavyol. Environ Saf 106:126–135. https://doi.org/10.1016/j.ecoenv.2014.03.004
Rahman MA, Rahman A, Khan MZK, Renzaho AMN (2018) Human health risks and socio-economic perspectives of arsenic exposure in Bangladesh: a scoping review. Ecoheavyol Environ Saf 150:335–343. https://doi.org/10.1016/j.ecoenv.2017.12.032
Rahman MM, Asaduzzaman M, Naidu R (2013) Consumption of arsenic and other elements from vegetables and drinking waterfrom an arsenic-contaminated area of Bangladesh. J Hazard Mater 262:1056–1063. https://doi.org/10.1016/j.jhazmat.2012.06.045
Rahman MS, Miah MAM, Khaled HM, Islam A, Panaullah GM (2010) Arsenic concentrations in groundwater, soils, and irrigated rice in Southwestern Bangladesh. Commun Soil Sci Plant Anal 41:1889–1895. https://doi.org/10.1080/00103624.2010.495800
Ravenscroft P, Burgess WG, Ahmed KM, Burren M, Perrin J (2005) Arsenic in groundwater of Bengal Basin, Bangladesh: distributions, field relations and hydrogeological setting. Hydrogeol J 13:727–751
Rahman MS, Reza AHMS, Ahsan MA, Siddque MAB (2023) Arsenic in groundwater from Southwest Bangladesh: Sources, water quality, and potential health concern. HydroResearch 6(2023):1–15. https://doi.org/10.1016/j.hydres.2022.12.001
Ravenscroft P, McArthur JM, Hoque BA (2001) Geochemical and palaeohydrological controls on pollution of groundwater by arsenic. In: Chappel WR, Abernathy CO, Calderon R (eds) Arsenic exposure and health effects. Elsevier Science Ltd, Oxford, pp 53–78
Rehman K, Naranmandura H (2012) Arsenic metabolism and thioarsenicals. Metallomics 4(9):881–892
Rehman ZU, Khan S, Qin K, Brusseau ML, Shah MT, Din I (2016) Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Sci Total Environ 550:321–329
Reid MC, Asta MP, Falk L, Maguffin SC, Pham VHC, Le HA, Bernier-Latmani R, Vo PL (2021) Associations between inorganic arsenic in rice and groundwater arsenic in the Mekong Delta. Chemosphere 265:129092
Reza AHMS, Jean JS, Yang H-J, Lee MK, Woodall B, Liu CC, Lee JF, Luo SD (2010) Occurrence of arsenic in core sediments and groundwater in the Chapai-Nawabganj District, Northwestern Bangladesh. Water Res 44:2021–2037
Rokonuzzaman M, Li WC, Wu C, Ye ZH (2022) Human health impact due to arsenic contaminated rice and vegetables consumption in naturally arsenic endemic regions. Environ Pollut 308:119712. https://doi.org/10.1016/j.envpol.2022.119712
Rokonuzzaman M, Ye ZH, Wu C, Li WC (2022) Arsenic accumulation in rice: alternative irrigation regimes produce rice safe from arsenic contamination. Environ Pollut 310:119829. https://doi.org/10.1016/j.envpol.2022.119829
Roychowdhury T (2008) Impact of sedimentary arsenic through irrigated groundwater on soil, plant, crops and human continuum from Bengal delta: special reference to raw and cooked rice. Food Chem Toxicol 46:2856–2864. https://doi.org/10.1016/j.fct.2008.05.019
Roychowdhury T (2010) Groundwater arsenic contamination in one of the 107 arsenic-affected blocks in West Bengal, India: status, distribution, health effects and factors responsible for arsenic poisoning. Int J Hyg Environ Health 213:414–427. https://doi.org/10.1016/j.ijheh.2010.09.003
Sandberg GR, Allen IK (1975) Arsenical pesticides. In: Woolson EA (ed) ACS SymposSer 7. AmerChernSoc, Washington, DC, pp 124–127
Selinus O, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P (2005) Essentials of medical geology: impacts of the natural environment on public health. Elsevier Academic Press, Amsterdam, pp 353–354
Shaji E, Santosh M, Sarath KV, Prakash P, Deepchand V, Divya BV (2021) Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci Front 12:101079. https://doi.org/10.1016/j.gsf.2020.08.015
Shakoor MB, Riaz M, Niazi NK, Ali S, Rizwan M, Arif MS (2019) Recent advances in arsenic accumulation in rice—sciencedirect. Adv Rice Res Abiotic Stress Toler 2019:385–398
Sharma S, Kumar R, Sahoo PK, Mittal S (2020) Geochemical relationship and translocation mechanism of arsenic in rice plants: a case study from health prone south west Punjab, India. Groundw Sustain Dev 10:100333
Siddique MAB, Alam MK, Islam S, Diganta MTM, Akbor MA, Bithi UH, Chowdhury AI, Ullah AKMA (2020) Apportionment of some chemical elements in soils around the coal mining area in northern Bangladesh and associated health risk assessment. Environ Nanotechnol Monit Manage 14:100366. https://doi.org/10.1016/j.enmm.2020.100366
Singh RB (2001). Heavy metals in soils: sources, chemical reactions and forms. Proceedings of the 2nd Australia and New Zealand Conference on Environmental Geotechnics 8th October, 2000, Australian Geochemical Society, pp. 77–93.
Sipter E, Rozsa E, Gruiz K, Tatrai E, Morvai V (2008) Site-specific risk assessment in contaminated vegetable gardens. Chemosphere 71:1301–1307
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. ApplGeochem 17:517–568
Suriyagoda LDB, Dittert K, Lambers H (2018) Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric Ecosyst Environ 253:23–37. https://doi.org/10.1016/j.agee.2017.10.017
Tanhan P, Kruatrachue M, Pokethitiyook P, Chaiyarat R (2007) Uptake and accumulation of cadmium, lead and zinc by Siam weed [Chromolaena odorata (L.) King & Robinson]. Chemosphere 68:323–329
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. GeochimCosmochimActa 28(8):1273–1285
Ullah AKMA, Maksud MA, Khan SR, Lutfa LN, Quraishi SB (2017) Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. J Heavyol Rep. https://doi.org/10.1016/j.toxrep.2017.10.002
Upadhyay MK, Majumdar A, Barla A, Bose S, Srivastava S (2019) An assessment of arsenic hazard in groundwater–soil–rice system in two villages of Nadia district, West Bengal. Environ Geochem Health 41:2381–2395. https://doi.org/10.1007/s10653-019-00289-4
USEPA (1989) Risk assessment guidance for superfund, vol. I: human health evaluation manual. EPA/540/1-89/002. Office of Emergency and Remedial Response, Washington, DC
USEPA (2000) Risk-based concentration table. United States Environmental Protection Agency, Washington DC, Philadelphia
USEPA (2005) Guidelines for carcinogen risk assessment; EPA/630/P-03/001F. Risk Assessment Forum, Washington, DC, USA
USEPA (2007) Integrated risk information system-database’. USEPA, Philadelphia
USEPA (2010) Toxicological Review of Inorganic Arsenic in Support of Summary Information on the Integrated Risk Information System (IRIS) (Draft). EPA/635/R-10/001. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=219111. Accessed Jan 22 2019.
USGS (United States Geological Survey) (2021). Earth explorer, U.S. Geological survey. https://earthexplorer.usgs.gov. Accessed 25 May 2021.
Vahter M (2008) Health effects of early life exposure to arsenic. Basic Clin Pharmacol Heavyol 102:204–211. https://doi.org/10.1111/j.1742-7843.2007.00168.x
Wang C, Ji J, Chen M, Zhong C, Yang Z, Browne P (2017) Atmospheric contribution to boron enrichment in aboveground wheat tissues. Chemosphere 174:655–663. https://doi.org/10.1016/j.chemosphere.2017.01.124
Wang J, Wang Z, Mao H, Zhao H, Huang D (2013) Increasing Se concentration in maize grain with soil- or foliar-applied selenite on the loess plateau in China. Field Crops Res 150:83–90
WHO (1996) Permissible limits of heavy metals in soil and plants, vol 4. WHO, Switzerland, pp 45–67
WHO (2011) Evaluation of certain contaminants in food. Seventy-second report of the joint FOA/WHO Expert Committee on food additives, WHO technical report series No. 959. World Health Organization (WHO), Geneva.
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ Sci Technol 40:4903–4908
Yang PT, Hashimoto Y, Wu WJ, Huang JH, Wang SL (2019) Effects of long-term paddy rice cultivation on soil arsenic speciation. J Environ Manag 254:109768
Zakir HM, Quadir QF, Mollah MZI (2021) Human health risk assessment of heavy metals through the consumption of common foodstuffs collected from two divisional cities of Bangladesh. Expo Health 13:253–268. https://doi.org/10.1007/s12403-020-00380-7
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar Coastal Shelf Sci 54(6):1051–1070
Zheng Y, Stute M, van Geen A, Gavrieli I, Dhar R, Simpson HJ, Schlosser P, Ahmed KM (2004) Redox control of arsenic mobilization in Bangladesh groundwater. ApplGeochem 19:201–214
Zhuang P, Murray B, McBride Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine. South China Sci Total Environ 407(5):1551–1561
Acknowledgements
The authors are grateful to the authority of the Institute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh, for providing laboratory facilities and other logistic support during the research work.
Funding
The authors received no specific funding for this research work.
Author information
Authors and Affiliations
Contributions
MSR: methodology, formal analysis, investigation, writing—original draft preparation. AHMSR: conceptualization, methodology, investigation, data curation, writing—reviewing and editing, supervision. MABS: methodology, formal analysis, investigation, data curation, validation, writing—reviewing and editing, resources, supervision. MAA: resources, writing—reviewing and editing. MH: writing—reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, M.S., Reza, A.H.M.S., Siddique, M.A.B. et al. Accumulation of arsenic and other metals in soil and human consumable foods of Meherpur district, southwestern Bangladesh, and associated health risk assessment. Environ Sci Eur 35, 47 (2023). https://doi.org/10.1186/s12302-023-00751-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00751-2