Abstract
Our study aims to clarify the mechanisms of successful microsleep tolerance that accompanies the monotonous solving of simple cognitive tasks. On the example of a homogeneous pool of participants with such stability, we demonstrated objectively calculated characteristics of psychometric and neural correlates that were statistically different in two identified subgroups for the process of long-term monotonous recognition of bistable images (Necker cubes). We hypothesized that the observed differences between the two types of monotony resistance were due to different personal characteristics of different subgroups, viz.: type I observers were characterized by an innate resistance to the occurrence of monotony caused by the organization of the brain and psyche, in particular, by pronounced left-sided frontal electroencephalogram (EEG) asymmetry; type II observers did not have such powerful innate quality albeit implemented a powerful mechanism of self-motivation and/or self-discipline. For subgroup I, it was demonstrated that significant areas of scalp spatial zones had well-formed event-related potentials (ERP), while in subgroup II, the number of spatial zones with well-formed ERP was significantly lower. At the same time, subjects in subgroup II made fewer errors in solving cognitive tasks than those in subgroup I. Use of the proposed simple psychometric characteristics (reaction time \(\textrm{Tr}\) distributions) could be adapted to implement training with biofeedback for exercising attention control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A systematic study of monotony as a mental phenomenon emerged at the end of the nineteenth century, primarily in connection with ongoing industrialization and predominance of simple monotonous activities directly associated with it [1, 2]. Monotony, occurring during monotonous repetitive activity in a sensory-impoverished environment, is considered a subjective feeling of boredom, accompanied by emergence of vivid memories and internal images that distract from routine tasks [3, 4]. Studies of monotonous activity are still relevant [5,6,7], which is primarily associated with further development and importance of various types of monotonous activity, such as control of technical devices [4, 7, 8]. In the tasks of operator activity, the occurrence of monotony could lead to a catastrophic loss of control over the situation, an increase in the response time to its changes, and even to the occurrence of episodes of sleep/microsleep in the subjects [3, 9, 10]. However, the activity of present-day people becomes monotonous not only during long-term automated driving or control over the process management at a nuclear power plant, but also in the course of performing various work-related and educational functions. In particular, highly specialized occupations, along with further improvement of relevant qualifications, require a long monotonous training.
Numerous studies of human psychological reactions to the monotonous presentation of stimuli have established that it can cause a loss of vigilance [11, 12], emotional stress, boredom in the sense of lipophrenia [13], apathy, unwillingness to continue monotonous activities, as well as professional deformations of the personality, including emotional-personal and motivational disorders [14,15,16,17]. Numerous psychological and psychophysiological studies prove that the effective ability to endure monotony (without strong psychological and production losses) is more pronounced in individuals possessing a number of psychological qualities developed as a result of a long experience of activity in conditions of monotony [15]. At the same time, early studies demonstrate relationship between monotonic resistance and nervous system strength [18,19,20], correlated with an equilibrium balance of excitation and inhibition processes [21, 22].
A long-term study of psychological reactions to monotony has led to the emergence of a whole series of works aimed at rapid psycho-correction of the functional state for each specific operator/user, based on brain–computer interfaces systems [23, 24], or psychotherapeutic approaches to learning self-regulation processes of mental states [25, 26]. At the same time, the development of means to combat monotony faces the problem of its individual tolerance/intolerance, as well as the problem of “turning on” self-regulation to counter it. From the standpoint of objective observation of psychophysiological phenomena, the development of monotony during cognitive activity is studied as a phenomenon activating various functional networks of the human brain [27, 28]; as a process transforming visual attention, thereby reducing the ability to track and analyze various changes [29, 30]; as a change in the characteristics of evoked potentials [31, 32]; and also as the detection and description of short episodes of light sleep and deep sleep [33, 34], etc. Studying the predecessors of short periods of unconscious sleep is especially relevant from the applied standpoint due to its propinquity to urgent tasks, for example, the task of long-term control of the automated process of driving a car [4, 35]. Despite considerable applied significance of such studies, the task of psychophysiological description of a person’s successful adaptation to the process of monotonous/boring cognitive activity is by no means fulfilled by them. Attention to such studies, based on combinations of objective methods for measuring the parameters of the psyche, brain activity, and physiological systems, is associated with a fundamental interest in the evolutionary aspects of the emergence of higher cognitive functions (CF) in humans [36], age-related changes in CF during the processes of body maturation and aging [5, 37], as well as the fundamental need to understand the structure of our consciousness and the corresponding organization of its physical basis represented by the brain. At the same time, the applied relevance of such studies is directly associated with the possibility of early diagnosis/prevention of all these disorders, along with the help in controlling hyperactivity syndrome [38, 39] and overcoming the phenomenon of clip thinking [40], attention disorders, and other CF in psychiatric diseases, including those with unfavorable psychoemotional background and/or post-traumatic stress disorders [41,42,43], etc. The ability of a person to successfully engage in monotonous cognitive tasks for a long time is, apparently, a combination of complex psychophysiological features, based both on the level of attention and short-term memory and on the ability to develop self-motivation and discipline. In many ways, professional success, career development and high-quality social and cultural life of a person are associated with the presence of such developed mechanism of self-control. Furthermore, in the contemporary environment, rich in distracting bright stimuli of advertising, social networks, online games, etc., the formation of self-control seems somewhat more complicated and may even require special assistance to compensate for some personality traits. We assume that people initially have different levels of ability to monotony tolerance occurring during monotonous activity, but reasonable help, both medicinal and physiotherapeutic, could reduce the impact of innate personality traits, including the conditions that are borderline with attention deficit disorder (ADD). Hence, we should better understand the issue of resistance to monotonous work to create publicly available training technology for this personality trait, including neurofeedback therapy. In this paper, we present the results of our study of monotonous visual perception of bistable images in a group of subjects capable of resistance to the occurrence of monotony. The study was performed using conventional cognitive methods (measuring reaction time, warm-up time and number of errors) and neural strategies based on evaluating event-related potentials (ERP). Currently, ERP assessment technology is a generally accepted method for evaluating relevant information about an individual’s workload, in particular, via clearly reflecting the dynamics of attentional and working memory processes [44, 45]. The most conventionally established P300 registration technique is the oddball paradigm [46]: observers are presented with two different types of stimuli, of which one (the oddball stimulus) occurs infrequently. Typically, the observer is asked to pay special attention to the oddball, such as counting their number or marking their appearance with a specific response. For such paradigm, P300 ERP component is pronounced to the maximum for the stimulus, on which the observer fixes attention. That phenomenon is actively used in the P300-based brain–computer interface (BCI) technologies [47]. At the same time, there is evidence of generating P300 even in the absence of dividing the stimuli into target (oddball) and non-target categories [48]. In this article, just as in our previous publication [49], the stimuli presented to observers were familiar bistable images of Necker cubes with the ability to control the level of their bistable perception. However, in our paradigm, we suggested that observers should exclude their subjective perception of a bistable object, focusing on the “real” or, in other words, “objective” prevalence of one side of this visual object due to the color intensity of the corresponding cube edges. Thus, the aim of this study is to compare the behavioral scenarios of monotony tolerance and the electrical activity of the cerebral cortex of test subjects during the monotonic visual presentation of bistable stimuli. The obtained scientific knowledge will form the basis for improving the means of counteracting monotony and their use in order to externally regulate the modes of human operator activity in order to reduce his fatigue and maintain the health of the body’s functional systems.
2 Materials and methods
2.1 Experimental procedure
Our experimental work was carried out in compliance with all required ethical standards (World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects [50]) and was approved by the Ethics Committee of Saratov State Medical University, Saratov, Russia. All experiments were performed in accordance with relevant guidelines and regulations. All subjects participated in the experiment on a voluntary and gratuitous basis. They have signed an informed medical consent to participate in the experimental work, including their agreement for further publication of the results, and received all necessary explanations about the procedures. Collected experimental data were processed with respect to confidentiality and anonymity of research subjects.
The recruitment of study participants was organized on the basis of higher educational institutions in Saratov (Saratov State University and Saratov State Medical University). The inclusion criteria for the study were: age from 20 to 30 years, no complaints about physical health, emotional background and night sleep, as well as excellent academic performance in the process of obtaining higher education. The exclusion criteria were as follows: (i) chronic diseases; (ii) deviation from the age-specific norm of the cognitive status; presence of (iii) emotional disorders and (iv) sleep problems. To identify disorders of cognitive and emotional status, anamneses for neurological disorders and neuropsychological testing were used, based on Montreal cognitive assessment [51] and Beck Depression Inventory [52]. Before conducting experimental testing, participants kept a sleep diary for two weeks. The performed analysis of the sleep diary guaranteed the absence of sleep problems.
Our experimental work involved 33 practically healthy subjects, of which 14 were women and 19 were men. Their average age was \(24.1\pm 2.4\) years. Differences between women and men in terms of their age were not statistically significant (\(p > 0.05\)). All subjects were year 5 or 6 university students, or have already completed higher education and continued their studies in graduate school or clinical residency. Their vision was normal or corrected to normal (using contact lenses). All study participants were instructed to have at least 8 h of sleep prior to their involvement in the neuropsychological study, and to maintain their normal sleep and wake patterns for at least a week before the experiment. Their last meal was 3–4 h before neuropsychological monitoring, after which they were allowed solely drinking pure water.
During all experiments, the multichannel electroencephalogram (EEG) data were obtained using the EEG amplifier NVX24 (Medical Computer Systems, Russia, https://mks.ru/en/products/nvx/). The data were recorded at a sampling rate of 500 Hz using the conventional monopolar recording technique with two reference electrodes and multiple recording electrodes (\(N = 19\)). EEG signals were obtained by means of special headcaps with prewired Ag/AgCl adhesive electrodes. Two reference electrodes, A1 and A2, were located on mastoid processes, and the ground electrode, N, was placed above the forehead. EEG signals were filtered via a bandpass filter with cutoff frequencies of 0.5 Hz (HP) and 30 Hz (LP), and a notch filter of 50 Hz.
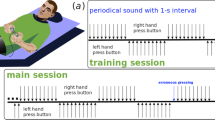
(a) Experimental design: the periods of demonstration of stimulus images (S) are shown in gray, white intervals between them correspond to the pauses between them (P), vertical dotted lines indicate the moment the subject presses the remote control, \(\textrm{Tr}\) is the reaction time, that is, the time interval from the beginning of the stimulus presentation to the moment the subject presses the remote control; (b) determining the ambiguity parameter \(\mathrm {A_p}\) for the bistable image of the Necker cube; all necessary explanations are presented in the text; (c) illustration of Necker cubes as the ambiguity parameter \(\mathrm {A_p}\) increases
The experiments were carried out in the early afternoon hours at a specially equipped laboratory. During the experiment, the volunteer was placed in a comfortable chair at a distance of about 0.6 m from the display screen in a darkened soundproof room. During the monitoring, the subjects were in a comfortable reclining position in a chair with support for their neck and the back of the head in order to avoid the occurrence of artifacts associated with muscle tension in these areas. A stimulus image was presented on the screen in front of the subject for a short time (\(0.55-0.9\) s). Between two presentations of the stimulus, there was a pause (\(3.6-6\) s), during which a noise image was presented on the screen without pronounced centers of attracting visual attention. The scheme of experimental work is shown in Fig. 1a.
As a stimulus image, we used an image of a Necker cube with different edge color intensity ratios. By parameter \(\mathrm {A_p}\), we denoted the color intensity of three edges converging in the left inner corner and marked in orange in Fig. 1b. The intensity of three opposite edges converging in the right inner corner and indicated by a dotted line in Fig. 1b as \((1-\mathrm {A_p})\). In this case, \(\mathrm {A_p} = 1\) corresponded to the maximum intensity (black color), \(\mathrm {A_p} = 0\) corresponded to the minimum intensity (i. e., transparent color), while intermediate values changed the color to different shades of gray, as shown in Fig. 1c. Such stimulus was represented by an image of a three-dimensional cube, the front face of which, depending on the value of the control parameter \(\mathrm {A_p}\), was located in the lower left corner (\(\mathrm {A_p} < 0.5\): left-oriented cube) or in the upper right corner (\(\mathrm {A_p} > 0.5\): right-oriented cube). The Necker cube with edges of the same intensity is a well-known bistable image widely employed as a stimulus in neuropsychological studies of visual perception and attention retention [42, 49, 53].
During the experiment, the subjects had to consciously evaluate the outline of the given object at each stimulus presentation, ignoring their own subjective perception of the bistable image. For example, a cube with an ambiguity parameter \(\mathrm {A_p} = 0.78\) can be subjectively perceived by an observer both as left oriented and right oriented (Fig. 1c). However, in our experiment, the subject had to give an objective answer about the orientation of this object based on a quantitative assessment of the intensity of the cube edges, i.e., to mark this stimulus unambiguously as right. Marking such stimulus as left would mean an error. In connection with this design, cubes with the most ambiguous parameters were not used as stimulus material in the experimental work: \(\mathrm {A_p} \in [0.45; 0.55]\).
During the experiment, the subject’s hands were placed on the table, holding a remote control with two buttons located on the left and right. By pressing the thumbs of the left/right hand on the corresponding button, the subject evaluated each presented stimulus. All presentations of stimuli and responses of the subject were automatically recorded in the form of text protocols.
2.2 Base processing of time reactions
For all protocols of experimental work, the reaction times \(\textrm{Tr}\) were estimated. The type of reaction times \(\textrm{Tr}\) for some participants of experimental work is shown in Fig. 2. The dynamics of \(\textrm{Tr}\) for various subjects who participated in the experimental work can be characterized as purely individual. For example, for Fig. 2a, long warm-up time is characteristic, marked with a gray background, after which the reaction time is, on average, decreasing. Figure 2b displays a significant scatter of \(\textrm{Tr}\) values for different events, while Figs. 2c, d demonstrate the presence of fluctuations around relatively pronounced mean \(\textrm{Tr}\) value. At the same time, a pronounced increase in the reaction time \(\textrm{Tr}\) by the end of the experiment, i.e., in the state of fatigue, does not occur when analyzing the dynamics of the reaction time \(\textrm{Tr}\).
In the psychophysiological studies, the test subjects performed simple routine actions to recognize each presented visual stimulus. The monotonous perception of bistable images did not require significant mental effort. At the same time, each subject must continuously maintain a level of attention high enough to correctly recognize the bistable image. For each subject, we calculated mean reaction time \(\left<\textrm{Tr}\right>\):
Here, mean reaction time \(\left<\textrm{Tr}\right>\) (1) of the participant is not related to the number of presented stimuli, or to the total duration \(T_E\) of the experiment. All parameters of each experiment (number of presented stimuli \(N_s\), mean pause between stimula time \(\left<\textrm{Tp}\right>\), mean reaction time \(\left<\textrm{Tr}\right>\)) are presented in Table 1.
2.3 Base processing of brain activity
Based on the results of recording the cerebral cortex biopotentials in study participants, calculations of cognitive ERP, detected directly upon presentation of visual stimuli in each EEG recording channel, were performed. The procedure of ERP calculation was performed according to the method described in Zhuravlev, (2023) [54]. For each ERP, we calculated its compliance with statistical averages of latencies of the main peaks in a healthy population, established according to available reviews [55,56,57,58,59]. For a numerical comparison of each registered ERP with the reference value, the following estimate \(\chi\) was conducted:
where subscript “\(_{ch}\)” denotes EEG recording channels; N is the number of allocated components; \(M_i\) is the magnitude of the component from among N; \(g_i\) is the rank coefficient of this component chosen for a specific task, experimental design and stimulus type; \(L_i\) is the latency of this component from among N; \(\overline{L_i}\) and \(\Delta L_i\) are the mean and standard deviation for the latency of a given component according to Table 2. The calculation formula for the magnitude \(M_i\) is as follows:
where \(A_i\) is the amplitude of estimated component; \(A_{i-1}\) and \(A_{i+1}\) are the amplitudes of neighboring components. If the neighboring components are not defined, then their amplitudes are defined as \(A_0=0\). Latency value, mean latency, and latency standard deviation are estimated in signal counts (e.g., in this experiment, the EEG signal rate was 500 Hz, i.e., one signal count was equal to 2 ms), and thereby the difference \((L_i-\overline{L_i})\) is always an integer. If the latency of the selected component coincides with the mean, then \(\Vert L_i - \overline{L_i}\Vert\) is assumed equal to 1, which gives a limit to the maximum of the parameter \(\epsilon\), taking into account the magnitude \(M_i\) and rank coefficient \(g_i\) of the given component.
2.4 Statistical data processing
Mean, median, and standard deviation were used in descriptive statistics of collected data. The Mann–Whitney U test for independent samples was performed for the comparison of quantitative data. Calculation and graphing of distributions of \(\textrm{Tr}\) coefficients made in OriginLab version 6.1. The results with a \(\textit{p}\) value \(\le 0.001\) were assumed statistically significant. Statistical analyses were conducted by SPSS version 22.0 software for Windows (IBM, Armonk, NY, USA).
3 Results
3.1 Behavioral data analysis
For each subject, we assessed the distribution of reaction time \(\textrm{Tr}\) to visual stimuli of the Necker cubes. Figure 3a, b demonstrates typical distributions of N characterized by a pronounced global maximum for the reaction time \(\textrm{Tr}^*\), which does not coincide with the mean reaction time \(\left<\textrm{Tr}\right>\) (1) due to asymmetry of the distribution.
The characteristic probability distributions of the reaction time to a stimulus for different subjects are presented on a logarithmic scale. The squares show experimental data, and the lines denote approximate theoretical functions; (a) and (b) correspond to subjects #2, #1, respectively; (c) and (d) relate to the subjects #7, #16, respectively. Points represent experimental data; lines denote approximating theoretical functions (4) and (5) in figures (a), (b) and (c), (d), accordingly. Figure (a) and (b) depicts the distribution width \(\delta\), whereas (c) and (d) show the slope \(\varphi\) of the approximating exponent
All probability distributions could be described by two different types based on the nature of the observed dependence; in other words, on the presence or absence of symmetry with respect to the \(\textrm{Tr}^*\) value. For the first type, the probability distribution is relatively close to normal and can be approximated in standard form as:
where the distribution parameters \(C_0\), C, \(\omega\), and \(\textrm{Tr}_0\) are calculated for each subject from experimental data and are presented in Table 3.
The second type of experimental data does not follow a normal distribution and can be described using exponential functions of the form:
and the parameters A and B calculated from the experimental data for each subject are also given in Table 3.
Typical results of \(N^{1,2}(\textrm{Tr})\) approximations, described by (4) and (5), are shown in Fig. 3 with lines. It is clearly seen that there is a good agreement between the experimental and theoretical signals. Table 2 presents the values of the standard deviation \(\sigma _S\) of experimental data and approximating functions. For such distributions, the main parameters are the width \(\delta\) of the normal distribution \(P_d^1(\textrm{Tr})\) and the slope \(\varphi\) of the exponential distribution \(P_d^2(\textrm{Tr})\). The width of the \(P_d^1(\textrm{Tr})\) distribution characterizes the average level of the scatter in the reaction time \(\textrm{Tr}\), which allows estimating how long it took the subject, on average, to evaluate the presented stimulus during the experiment.
The value of the slope \(\varphi\) of the \(P_d^2(\textrm{Tr})\) distribution is associated with an increase in the individual reaction time \(\textrm{Tr}\) of the subject; in other words, an increase in the slope \(\varphi\) is caused by an increase in the frequency of the reaction time \(\textrm{Tr}\), which is long for this particular subject. The duration of the reaction time \(\textrm{Tr}\) for the subject in Fig. 3c is higher over the entire duration of the experiment, compared with the subject in Fig. 3d. However, the mean and maximum reaction times \(\langle \textrm{Tr}\rangle\) and \(\textrm{Tr}^*\) for both subjects in Fig. 3c, d virtually coincide and cannot serve significant characteristics of the observed process. In other words, \(\langle \textrm{Tr}\rangle\) and \(\textrm{Tr}^*\) are instantaneous characteristics that describe a capability of a person to react solely at a given moment.
(a), (b), (c): Reaction time distributions \(\textrm{Tr}\) of subject #2 for various time stages of experimental work: initial, \(\left[ 0, 1000\right]\) s, intermediate, \(\left( 1000, 2000\right]\) s, and final, \(\left( 2000, 3000\right]\) s, respectively. (d): Reaction time distributions for stimulus for various stages of the experimental work for subject #28, the solid line 1 shows the beginning of the experimental work \(\left[ 0, 1000\right]\) s, the dash-dotted line 2 shows the middle of the experiment \(\left( 1000, 2000\right]\) s, the dotted line indicates the end of the experiment \(\left( 2000, 3000\right]\) s. The local maximum, present in the experimental data, is denoted as \(\textrm{Tr}^{**}\); the theoretical approximation \(P_d^2\) for the reaction time \(\textrm{Tr} \ge \textrm{Tr}^{**}\) is presented by the black solid line
The nature of the reaction time distribution is stable with respect to the duration of the experimental work. Figure 4 demonstrates the results of estimating the distributions of \(N(\textrm{Tr})\) performed for three stages of the experiment (the beginning, the middle, and the end of the experimental work). It is worth noting that for three consecutive stages of the experiment, the subject in Fig. 4a, b, c demonstrates the narrowing of the width \(\delta\) of the \(P_d^1(\textrm{Tr})\) distribution, accompanied by a decrease in \(\textrm{Tr}^*\), which can be explained by the process of the subject-specific warm-up time (i.e., time required to be ready for the task). However, the mean reaction time \(\langle \textrm{Tr}\rangle\) virtually does not change throughout the experiment. In Fig. 4d, it is necessary to note the slope dynamics of the theoretical distribution \(P_d^2(\textrm{Tr})\) (5), where it is possible to observe a slight decrease in the slope angle at the second stage of the experimental work, which, apparently, is also associated with the warm-up time of the subject. The final stage of the experiment is associated with a significant increase in the reaction time \(\textrm{Tr}\) of the subject, which allows observing the second local maximum \(\textrm{Tr}^{**}\). The distribution of the reaction time \(\textrm{Tr}\) exceeding the value of the given maximum \(\textrm{Tr}^{**}\) (\(\textrm{Tr} \ge \textrm{Tr}^{**}\)) could also be described by the exponential distribution \(P_d^2(\textrm{Tr})\) (5).
Let us denote the subgroups of test subjects, whose time \(\textrm{Tr}\) of reaction to stimuli is described by the normal \(P_d^1(\textrm{Tr})\) and exponential \(P_d^2(\textrm{Tr})\) distribution, as I and II groups.
3.2 Brain activity analysis
For each subject, using the registered 19 EEG channels, we calculated 19 ERP arising from the perception of a visual stimulus. Further, the obtained ERP were compared with reference values accordingly the method described in Sect. 2. All values of comparative quality \(\chi\) were recalculated into relative values according to the maximum value observed for the subject, after which group comparison became possible, since all \(\chi\) values were within [0; 1]. For each subject, we constructed probability distributions \(p(\chi )\) for \(\chi\) (Fig. 5a, b). A small number of EEG channels commonly used in neurology causes a jagged appearance of \(p(\chi )\) distribution.
Further, out of the entire array of 19 ERP, only the most significant \(\chi\) were selected for further analysis, in accordance with their extreme values, i.e., the most pronounced ERP (close to reference values) and virtually absent. In the case of the maximum possible approximation for a given individual to the reference values of ERP components, \(\chi\) takes values around 1, whereas in the case of nonstandard ERP dynamics (up to its absence), \(\chi\) tends to zero. However, for different subjects, the duration of the ranges of extreme \(\chi\) values could vary substantially. The procedure for estimating the magnitude of this range was adjusted to individual differences in brain activity, as shown in Fig. 5a, b. First of all, the range \(p\updownarrow\) of the distribution \(p(\chi )\) was estimated, indicated by a vertical arrow in the figures; then one-fifth of this range was calculated, which corresponded to 20 % percent (also indicated in the figure). Then, zones of EEG channels with ERP values approaching reference values were identified, the \(\chi\) characteristic of which exceeded the individually determined value \((1-0.2\cdot p\updownarrow )\). Scalp zones with the least pronounced ERP, for which the value of \(\chi\) did not exceed \((0.2\cdot p\updownarrow )\), were similarly detected.
(a), (b): Statistical distributions of \(\chi _{ch}\) values assessing the compliance of ERP with the standard value for all EEG electrodes, shown for test subjects #6 and #19, respectively. Dashed horizontal lines demonstrate the maximum and minimum probability for a given subject. The vertical double-pointed arrows correspond to the magnitude of the probability range, \(p\updownarrow\). Transparent-blue rectangles show the calculation of the \(0.2\cdot p\updownarrow\); (c): the diagrams of \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) depict the following statistical characteristics of numerical parameters; (d): data legend: interquartile range (\(25-75\) %, inside the box); the median and the mean (transverse line and point inside the box, respectively); 1.5 interquartile range (shown by whiskers); and outliers represented by asterisks. The allocation to groups was performed according to the asymmetric (orange) and symmetrical (gray) types of reaction time distribution of the subjects. The corresponding subject numbers are depicted for ease of interpretation within orange and gray backgrounds
Further, the assessment of the ERP sums with extreme dynamics for selected spatial zones was carried out as follows:
where i and j are a numbers of the EEG recording channel, for the calculated ERP in which the one condition \(\chi _i \in [1-0.2\cdot p\updownarrow ; 1.0]\) or \(\chi _j \in [0.0; 0.2\cdot p\updownarrow ]\) is satisfied. The number of \(C_1\) and \(C_2\) in the general case may be different for the subject, \(C_1 \ne C_2\). Besides, for each subject, a relative value was calculated that characterized the general level of proximity of the observed ERP to reference values, viz.:
The results of calculations, according to formulas (6)–(7), of \(\sum \chi _{min}\), \(\sum \chi _{max}\), and \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) are given in the Table 4 for each test subject.
As shown in Fig. 5c, the numerical assessment, \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\), of the relative ERP severity magnitude in I and II subgroups of volunteers, previously divided by the nature of reaction time distribution \(\textrm{Tr}\), exhibited significant differences. Use of the Wilcoxon test demonstrated that the calculation of \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) allowed reliably separating these groups of test subjects, \(p < 0.05\).
3.3 Comparison of the results
Thus, the processing of behavioral reactions and electrical activity of the cerebral cortex in the presented paradigm of experimental work allowed confidently dividing the participants into two subgroups, specifically: (i) subgroup I of subjects with a normal distribution of \(\textrm{Tr}\) and reduced values of \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\); (ii) subgroup II with an exponential distribution and a significantly increased level of \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) (7).
At the same time, participants in subgroup I exhibited a random deviation of the reaction times \(\textrm{Tr}\) from the maximum reaction time \(\textrm{Tr}^*\). We believe that such behavior of the reaction time \(\textrm{Tr}\) can be attributed to the fact that subjects maintained one stable psychophysiological state during the experiment. We hypothesize that the subjects allocated to subgroup I did not experience a significant change in their working condition during the entire experiment, apparently possessing an innate resistance to monotony.
For the members of subgroup II, the distribution of \(\textrm{Tr}\) no longer corresponded to the noise process. It seems that experimental subgroup II subjects had time to experience the impact of monotonous activity in the perception of visual stimuli, which led to a change in their psychophysiological state and, as we believe, a short-term onset of a state of monotony. Moreover, a number of subjects from subgroup II developed a second local maximum \(\textrm{Tr}^{**}\) on the \(P_d^2(\textrm{Tr})\) distributions. Figure 6 presents the results of a secondary approximation of long reaction times \(\textrm{Tr}\), \((\textrm{Tr}\ge \textrm{Tr}^{**})\) using the exponential law. The appearance of such local maximum \(\textrm{Tr}^{**}\) occurs at the final stage of the experimental process, as shown in Fig. 2d. The existence of the \(\textrm{Tr}^{**}\) maximum could be caused by the occurrence of episodes of intermittent switching of two different psychophysiological states of the subject during the accumulation of a monotonous stimulus. These different states could alternately replace each other during the experiment, and long stages of the conditionally laminar dynamics of uniform stimulus perception could be disturbed by short turbulent phases of fatigue. As previously shown in [60,61,62], similar patterns of intermittent dynamics were observed in dynamic systems of very different nature.
It is worth noting that the division of subjects into subgroups I and II, according to the nature of \(\textrm{Tr}\) distribution associated with different possibilities for resistance to the occurrence of monotony, contributes to identifying differences between the participants in other objective parameters. For example, average number of errors over the entire time of the experiment was \(0.238 \pm 0.154\) % for subgroup I and \(0.156 \pm 0.144\) % for subgroup II. Thus, subgroup II displayed 65% fewer errors than subgroup I. Still, mean reaction time in subgroups I and II was (\(0.621 \pm 0.5\)) s and (\(0.946 \pm 0.268\)) s, respectively. In other words, the reaction time of the first subgroup was lower by 65.62 %. Hence, in subgroup I, we observe virtually instantaneous reaction of the subjects, especially in comparison with the significantly longer and more uniform reaction time in subgroup II.
However, significant differences in the dynamics of ERP \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) of participants in subgroups I and II are complemented by changes in the spatial zones of brain activity. A reduction in \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) in subgroup I (shown in gray in Fig. 5c corresponds to an increase in the area of scalp spatial zones with minimum values of ERP and a decrease in ERP in this area: i.e., with overall, minimization of \(\sum \chi _{min}\) value, as seen, for instance, in test subject #24. Nevertheless, a more common scenario is a parallel increase in the area of scalp spatial zones with ERP values most closely approaching the reference values—in other words, a simultaneous decrease of \(\sum \chi _{min}\) with an increase in \(\sum \chi _{max}\), as observed in test subjects ##5, 10, 29, and others. Thus, overall, subgroup textbfI is characterized by a significant scalp space captured by well-formed ERP, albeit it is worth highlighting an atypical study participant (#32), whose \(\sum \chi _{max}\) growth is very large but the characteristic \(\sum \chi _{min}\) is large, too (\(\approx 1.3\)). The latter ratio of different activity zones is typical for large values of \(\overleftrightarrow {\hspace{-1.66656pt}\chi }\) in subgroup II, as is observed, for example, in test subjects ##3, 27, 28, 30, etc. Overall, for subgroup II, shown in Fig. 5 in red, an increase in \(\sum \chi _{min}\) and a slight decrease in the values of \(\sum \chi _{max}\) are typical. Hence, subgroup II is generally characterized by a less pronounced formation of ERP and the presence of extensive zones, in which ERP are virtually not observed. All in all, mean values of \(\sum \chi _{max}\) for subgroups I and II were 3.62 and 2.51, and mean values of \(\sum \chi _{min}\) were 0.43 and 1.22, respectively.
4 Discussion
The use of a nonstandard experimental paradigm allowed us to observing certain patterns of collective neural activity on the basis of ERP in study subjects. Attempts to use the conventional oddball technology for the task (for example, requests to mark cubes with the maximum level of bistability, or those turned only in a certain direction) excluded the onset of monotony in the subjects of this class and did not contribute to detecting any statistically significant differences among the calculated ERP.
As a hypothesis explaining the observed multilevel differentiation of very homogeneous participants in the experiment into two subgroups, we propose to consider the possibility of two conceivable behavioral scenarios for resisting monotony offered to the subjects. Subgroup I may have had a strong psychophysiological basis, which allowed them concentrating on solving simple monotonous tasks throughout the entire experimental period without pronounced signs of fatigue. Simultaneously, subgroup II, having no similar innate psychophysiological basis, acquired resistance to monotony, based on a developed strategy of self-control and/or self-motivation. Accordingly, a large variability in the considered ERP characteristics within subgroup II may have been associated precisely with the difference in the use of one or another individual strategy. Perhaps, via such interpretation, we could touch on the well-known problem of innate qualities vs. environmental impact. Furthermore, such skillful inner work with the emerging state of monotony could be associated to a certain extent with the high degree of emotional intelligence development in the participants of subgroup II. These ideas correlate well with the results presented by Oh and Roh (2022) [63] who demonstrated the relationship between intrinsic motivation for work and empathy of individuals.
To consider additional arguments in favor of such explanation, let us turn to numerous studies that demonstrated stable interhemispheric differences correlating with personal and physiological characteristics. Mikolajczak, et al (2010) [64] convincingly demonstrated the differential frontal activation seen in highly emotionally intelligent adults, while Santesso, et al (2006) [65] demonstrated similar results in adolescents. In our paradigm, a simple comparison of the activity of the left and right hemispheres did not yield any statistical difference, since the interhemispheric difference, calculated as:
took the values of \(\Delta \chi _{\textrm{RLH}} = 0.800 \pm 0.224\) for subgroup I and \(\Delta \chi _{\textrm{RLH}} = 0.780 \pm 0.249\) for subgroup II. However, the focus on the frontal lobes led to a fundamentally different result. Analysis of the interhemispheric difference in the frontal leads relative to the background activity observed in the central lead \(\textrm{Fz}\), according to
taking the values of \(\Delta \chi _{\textrm{RLFH}} = 4.533 \pm 4.507\) for subgroup I and \(\Delta \chi _{\textrm{RLFH}} = 3.705 \pm 9.815\) for subgroup II. It is fascinating to note that the first subgroup was more homogeneous in the analysis of activity in the frontal leads. Simultaneously, the second subgroup significantly prevailed regarding the maximum spread. Further, having paid attention to the ERP of the left-sided leads \({\textrm{T5}}\) and \({\textrm{F7}}\), the dynamics of which correlated with the control of the attention function [66, 67] and was associated with the occurrence of disorders in ADHD [68, 69], it was possible to observe a fairly significant intergroup difference, viz.: the left frontal zone prevailed at a level of \(0.44 \pm 0.36\) in subgroup I and a level of \(0.19 \pm 0.12\) in subgroup II. Thus, in the first subgroup, as we assume, congenital features were expressed in increased activation of the left temporal zone, which allowed maintaining a consistently high concentration of attention on the task and nearly instantaneous reaction to each event. At the same time, subgroup II had a significantly lower activation of the left frontal area and was characterized by a high degree of heterogeneity in terms of reaction time to events. However, its participants demonstrated significantly higher accuracy in their reported responses to stimulus events. Perhaps the need for constant personal self-inclusion in the monotonous process of cognitive problem solving ultimately leads to an increase in the quality of performed work.
In conclusion, we point out that the brain activity observed in subgroup I, caused by cognitive stimuli, is localized over a larger area and in a more powerful way, compared with subgroup II, which is in good agreement with the data, previously obtained by Makarov et al. (2018) [70], on the formation of the shortest paths and compressed areas of powerful brain activity during the conscious successful implementation of cognitive tasks.
All participants in this study demonstrated a strong capability to resist the onset of monotony and microsleep episodes, but they appeared to achieve this in different ways. The results of our study give us hope that to assess the conditionally innate resistance to a monotonous load, it would be enough to pass a simple test for \(15-17\) min, because the nature of the distribution of reaction times to visual stimuli appears very stable. Besides, we find interesting a novel standpoint on the possibility of training to continuously maintain the attention of the subjects on any monotonous process merely via quickly assessing the distributions of their reaction times and highlighting the distinct onset of intermittent dynamics, which, as we assume, corresponds to switching between different psychophysiological states of the subjects. In this case, biofeedback can be employed without involving measurements of biomedical signals in the subject (EEG, cardiogram, eye movements, etc.), which could often lead to a significant increase in the technology cost and to complicating the use of such methods in wide practice. The analysis of the response of the training participant provides ample opportunities for embedding such technology, for instance, in mobile devices and network technologies, including the adaptation of the gameplay to the personal characteristics of the player.
The generalizability of these results is subject to certain limitations. The complicated procedure for setting up highly homogeneous group in this study could lead to a sample characterized by specific personality traits. In the same time, despite the established absence of psychoemotional disorders, the social and psychological profiles of test subjects were undescribed. In other words, the question of the correlation of certain personality traits and/or social status with one or another identified scenario of resistance to monotony remains open. Besides, the volunteers participating in the study belonged to one population, located in same area. The described neurophysiological scenarios for monotony tolerance may have obvious population limitations, correlated, for example, with patterns of parent–child relationships or the educational technologies. Further studies are required to analyze the degree of sustainability and universality of these scenarios. A particularly interesting topic is the study of impact of additional motivation for participants initially without a significant control over own attention unlike volunteers in this work. For example, in earlier paper Maksimenko, Runnova, Zhuravlev, et al [71], we demonstrated that the additional motivation (small monetary payment) for test subjects changed patterns of the oscillatory EEG activity of alpha, delta and beta bands in the occipital brain region. We can assume that the addition of similar “external” motivation in the experimental paradigm proposed in this work would provide a chance to investigate new facets of the self-control development during monotous perception.
Thus, the estimation of variabilities degree of different scenarios for controlling monotony in various population groups, differing in their social status, and/or characteristics of attention and other cognitive functions, can lead to interesting findings in the field of establishing different scenarios for controlling monotony. We plan to continue collecting experimental materials in this direction to math model the stability of attention during monotonous actions and further BCI-development trained self-control of microsleep tolerance.
Data availibility statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
E.N. Henderson, A study of memory: for connected trains of thought. Psychol. Rev. 5(6), (1903)
J. B. Miner, M., visual and applied rhythms. An experimental study and a revised explanation. The Psychol. Rev. 5(4), (1903)
P. Thiffault, J. Bergeron, Fatigue and individual differences in monotonous simulated driving. Personal. Individual Differ. 34(1), 159–176 (2003)
P. Thiffault, J. Bergeron, Monotony of road environment and driver fatigue: a simulator study. Accident Anal. Prevent. 35(3), 381–391 (2003)
S. Halloway, M.E. Schoeny, J. Wilbur, L.L. Barnes, Interactive effects of physical activity and cognitive activity on cognition in older adults without mild cognitive impairment or dementia. J. Aging Health 32(9), 1008–1016 (2020)
M. Sallinen, M. Härmä, R. Akila, A. Holm, R. Luukkonen, H. Mikola, K. Müller, J. Virkkala, The effects of sleep debt and monotonous work on sleepiness and performance during a 12-h dayshift. J. Sleep Res. 13(4), 285–294 (2004)
E.A. Schmidt, M. Schrauf, M. Simon, M. Fritzsche, A. Buchner, W.E. Kincses, Drivers’ misjudgement of vigilance state during prolonged monotonous daytime driving. Accident Anal. Prevent. 41(5), 1087–1093 (2009)
B.T. Jap, S. Lal, P. Fischer, Comparing combinations of EEG activity in train drivers during monotonous driving. Expert Syst. Appl. 38(1), 996–1003 (2011)
V. Kiroi, E. Aslanyan, General laws for the formation of the state of monotony. Neurosci. Behav. Physiol. 36, 921–928 (2006)
P.N. Prudkov, Mental effort and fatigue as consequences of monotony. Behav. Brain Sci. 36, 702–703 (2013)
M.E. Funke, J.S. Warm, G. Matthews, G.J. Funke, P.Y. Chiu, T.H. Shaw, E.T. Greenlee, The neuroergonomics of vigilance: effects of spatial uncertainty on cerebral blood flow velocity and oculomotor fatigue. Hum. Factors 59(1), 62–75 (2017)
R.W. Wohleber, G. Matthews, J. Lin, J.L. Szalma, G.L. Calhoun, G.J. Funke, C.-Y.P. Chiu, H.A. Ruff, Vigilance and automation dependence in operation of multiple unmanned aerial systems (UAS): a simulation study. Hum. Factors 61(3), 488–505 (2019)
P. Toohey, Boredom: a Lively History. Yale University Press, (2011)
I. Deimen, J. Wirtz, Control, cost, and confidence: perseverance and procrastination in the face of failure. Games Econ. Behav. 134, 52–74 (2022)
G. Johansson, Job demands and stress reactions in repetitive and uneventful monotony at work. Int. J. Health Services 19(2), 365–377 (1989)
O. Polyakova, Category “professional deformation’’ in psychology. Proc.-Soc. Behav. Sci. 146, 279–282 (2014)
O.N. Rodina, Personal deformations in the development of a state of chronic fatigue. Lomonosov Moscow State Univ. Bull. Ser. 14, 137–140 (2019)
H. Baumann, R. Baumann, C. Gurk, F. Wolter, Electrophysiological studies of central nervous performance during monotony. Electroencephalography Clin. Neurophysiol. 24(3), 259–273 (1968)
I. Levochkina, The after-effect of an inhibitory functional state induced by monotonous work and properties of the nervous system. Voprosy Psychologii (1976)
V. Rozhdestvenskaya, The effect of the strength of the nervous system and of activation level on performance in a monotonous task. Voprosy Psychologii 19(5), 49–57 (1973)
K.D. Harris, T.D. Mrsic-Flogel, Cortical connectivity and sensory coding. Nature 503(7474), 51–58 (2013)
M. Okun, I. Lampl, Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 11(5), 535–537 (2008)
C.J. de Naurois, C. Bourdin, A. Stratulat, E. Diaz, J.-L. Vercher, Detection and prediction of driver drowsiness using artificial neural network models. Accident Anal. Prevent. 126, 95–104 (2019)
C.-H. Ting, M. Mahfouf, A. Nassef, D.A. Linkens, G. Panoutsos, P. Nickel, A.C. Roberts, G.R.J. Hockey, Real-time adaptive automation system based on identification of operator functional state in simulated process control operations. IEEE Trans. Syst. Man Cybernet.-Part A 40(2), 251–262 (2009)
S. Love, L. Kannis-Dymand, J. Davey, J. Freeman, Risky driving and lapses on the road: An exploration on self-regulatory dysfunction in Australian drivers. Trans. Res. Part F 88, 25–36 (2022)
S. Love, V. Truelove, B. Rowland, L. Kannis-Dymand, Metacognition and self-regulation on the road: a qualitative approach to driver attention and distraction. Appl. Cognit. Psychol. 36(6), 1312–1324 (2022)
T.M. Baran, Z. Zhang, A.J. Anderson, K. McDermott, F. Lin, Brain structural connectomes indicate shared neural circuitry involved in subjective experience of cognitive and physical fatigue in older adults. Brain Imaging Behav. 14, 2488–2499 (2020)
C. Han, X. Sun, Y. Yang, Y. Che, Y. Qin, Brain complex network characteristic analysis of fatigue during simulated driving based on electroencephalogram signals. Entropy 21(4), 353 (2019)
Z. Guo, R. Chen, K. Zhang, Y. Pan, J. Wu, The impairing effect of mental fatigue on visual sustained attention under monotonous multi-object visual attention task in long durations: an event-related potential based study. PloS one 11(9), 0163360 (2016)
M. Körber, A. Cingel, M. Zimmermann, K. Bengler, Vigilance decrement and passive fatigue caused by monotony in automated driving. Proc. Manufacturing 3, 2403–2409 (2015)
Z. Guo, R. Chen, X. Liu, G. Zhao, Y. Zheng, M. Gong, J. Zhang, The impairing effects of mental fatigue on response inhibition: an ERP study. PloS One 13(6), 0198206 (2018)
T. Möckel, C. Beste, E. Wascher, The effects of time on task in response selection—an ERP study of mental fatigue. Sci. Rep. 5(1), 10113 (2015)
E. Cheremushkin, N. Petrenko, M. Gendzhalieva, D. Malakhov, V. Dorokhov, Eeg characteristics during short-term spontaneous waking periods of different durations with changes in psychomotor activity induced by falling asleep. Neurosci. Behav. Physiol. 50, 1232–1238 (2020)
V.B. Dorokhov, E.B. Yakunina, A.N. Puchkova, D.E. Shumov, E.O. Gandina, A.O. Taranov, O.N. Tkachenko, N.V. Ligun, G.N. Arseniev, A.E. Runnova, et al.: Can physiological sleepiness underlie consciously perceived sleepiness assessed with the Epworth sleepiness scale? The European Physical Journal Special Topics, 1–14 (2023)
L. Bier, P. Wolf, H. Hilsenbek, B. Abendroth, How to measure monotony-related fatigue? A systematic review of fatigue measurement methods for use on driving tests. Theoretical Issues Ergonomics Sci. 21(1), 22–55 (2020)
D. Stout, The evolution of cognitive control. Topics Cognit. Sci. 2(4), 614–630 (2010)
G. Sajeev, J. Weuve, J.W. Jackson, T.J. VanderWeele, D.A. Bennett, F. Grodstein, D. Blacker, Late-life cognitive activity and dementia: a systematic review and bias analysis. Epidemiology (Cambridge, Mass.) 27(5), 732 (2016)
C.-F. Hsu, J.D. Eastwood, M.E. Toplak, J.-C. Liang, S.-L. Hwang-Gu, V.C.-H. Chen, Trait and state boredom: associations with attention failure in children with Attention-Deficit/Hyperactivity Disorder. Psychiatry Res 286(112861), 10–1016 (2020)
B. Reimer, L.A. D’Ambrosio, J.F. Coughlin, R. Fried, J. Biederman, Task-induced fatigue and collisions in adult drivers with attention deficit hyperactivity disorder. Traffic Injury Prevent. 8(3), 290–299 (2007)
I.P. Berezovskaya, O.D. Shipunova, Reverse side of multimedia pedagogics: clip thinking. Mediterranean J. Soc. Sci. 6(6 S1), 277 (2015)
M. Butler, E. McCreedy, V.A. Nelson, P. Desai, E. Ratner, H.A. Fink, L.S. Hemmy, J.R. McCarten, T.R. Barclay, M. Brasure, H. Davila, R.L. Kane, Does cognitive training prevent cognitive decline? A systematic review. Ann. Internal Med. 168(1), 63–68 (2018). https://doi.org/10.7326/M17-1531. (PMID: 29255842)
V.A. Maksimenko, A.E. Hramov, N.S. Frolov, A. Lüttjohann, V.O. Nedaivozov, V.V. Grubov, A.E. Runnova, V.V. Makarov, J. Kurths, A.N. Pisarchik, Increasing human performance by sharing cognitive load using brain-to-brain interface. Front. Neurosci 12, 949 (2018)
B.A. Van Der Kolk, H. Hodgdon, M. Gapen, R. Musicaro, M.K. Suvak, E. Hamlin, J. Spinazzola, A randomized controlled study of neurofeedback for chronic PTSD. PloS One 11(12), 0166752 (2016)
A.-M. Brouwer, M.A. Hogervorst, J.B. Van Erp, T. Heffelaar, P.H. Zimmerman, R. Oostenveld, Estimating workload using EEG spectral power and ERPs in the n-back task. J. Neural Eng. 9(4), 045008 (2012)
J. Polich, Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 118(10), 2128–2148 (2007)
J. Polich, A. Kok, Cognitive and biological determinants of P300: an integrative review. Biol. Psychol. 41(2), 103–146 (1995)
J.T. Philip, S.T. George, Visual P300 mind-speller brain-computer interfaces: a walk through the recent developments with special focus on classification algorithms. Clin. EEG Neurosci. 51(1), 19–33 (2020)
B.Z. Allison, J. Polich, Workload assessment of computer gaming using a single-stimulus event-related potential paradigm. Biol. Psychol. 77(3), 277–283 (2008)
A.E. Runnova, A.E. Hramov, V.V. Grubov, A.A. Koronovskii, M.K. Kurovskaya, A.N. Pisarchik, Theoretical background and experimental measurements of human brain noise intensity in perception of ambiguous images. Chaos Solitons Fractals 93, 201–206 (2016)
World Medical Association, et al.: World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310(20), 2191–2194 (2013)
S. Freitas, M.R. Simões, J. Marôco, L. Alves, I. Santana, Construct validity of the montreal cognitive assessment (MoCA). J. Int. Neuropsychol. Soc. 18(2), 242–250 (2012)
D. Gallagher, G. Nies, L.W. Thompson, Reliability of the Beck Depression Inventory with older adults. J. Consulting Clin. Psychol. 50(1), 152 (1982)
A.E. Hramov, V.A. Maksimenko, S.V. Pchelintseva, A.E. Runnova, V.V. Grubov, V.Y. Musatov, M.O. Zhuravlev, A.A. Koronovskii, A.N. Pisarchik, Classifying the perceptual interpretations of a bistable image using EEG and artificial neural networks. Front. Neurosci. 11, 674 (2017)
M. Zhuravlev, M. Novikov, R. Parsamyan, A. Selskii, A. Runnova, The objective assessment of event-related potentials: An influence of chronic pain on erp parameters. Neurosci. Bull. 1–12 (2023)
C.C. Duncan, R.J. Barry, J.F. Connolly, C. Fischer, P.T. Michie, R. Näätänen, J. Polich, I. Reinvang, C. Van Petten, Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 120(11), 1883–1908 (2009)
R.F. Helfrich, R.T. Knight, Cognitive neurophysiology: Event-related potentials. Handbook Clin. Neurol. 160, 543–558 (2019)
I. Iturrate, R. Chavarriaga, L. Montesano, J. Minguez, J. Millán, Latency correction of event-related potentials between different experimental protocols. J. Neural Eng. 11(3), 036005 (2014)
S.H. Patel, P.N. Azzam, Characterization of N200 and P300: selected studies of the event-related potential. Int. J. Med. Sci. 2(4), 147 (2005)
E.M. Sokhadze, M.F. Casanova, E.L. Casanova, E. Lamina, D.P. Kelly, I. Khachidze, Event-related potentials (ERP) in cognitive neuroscience research and applications. NeuroRegulation 4(1), 14–14 (2017)
A.A. Koronovskii, A.E. Hramov, V.V. Grubov, O.I. Moskalenko, E. Sitnikova, A.N. Pavlov, Coexistence of intermittencies in the neuronal network of the epileptic brain. Phys. Rev. E 93(3), 032220 (2016)
O.I. Moskalenko, A.A. Koronovskii, A.E. Hramov, M.O. Zhuravlev, Y.I. Levin, Cooperation of deterministic and stochastic mechanisms resulting in the intermittent behavior. Chaos Solitons Fractals 68, 58–64 (2014)
O. Moskalenko, M. Zhuravlev, A. Koronovskii, A. Hramov, Manifestations of intermittency in unidirectionally coupled Pierce diodes on different time scales. Nonlinear Dyn. 83, 433–439 (2016)
S. Oh, S.-C. Roh, Intrinsic motivation for work activities is associated with empathy: Investigating the indirect relationship between intrinsic motivation for work activities and social support through empathy and prosocial behavior. Personality Individual Differ. 189, 111487 (2022)
M. Mikolajczak, K. Bodarwé, O. Laloyaux, M. Hansenne, D. Nelis, Association between frontal EEG asymmetries and emotional intelligence among adults. Personality Individual Differ. 48(2), 177–181 (2010)
L. Diane Santesso, L. Reker Dana, L.A. Schmidt, S.J. Segalowitz, Frontal electroencephalogram activation asymmetry, emotional intelligence, and externalizing behaviors in 10-year-old children. Child Psychiatry Hum. Dev. 36, 311–328 (2006)
T. Harmony, T. Fernández, J. Silva, J. Bernal, L. Díaz-Comas, A. Reyes, E. Marosi, M. Rodríguez, M. Rodríguez, EEG delta activity: an indicator of attention to internal processing during performance of mental tasks. Int. J. Psychophysiol. 24(1–2), 161–171 (1996)
A. Yoto, T. Katsuura, K. Iwanaga, Y. Shimomura, Effects of object color stimuli on human brain activities in perception and attention referred to EEG alpha band response. J. Physiol. Anthropol. 26(3), 373–379 (2007)
R. Aldemir, E. Demirci, H. Per, M. Canpolat, S. Özmen, M. Tokmakçı, Investigation of attention deficit hyperactivity disorder (ADHD) sub-types in children via EEG frequency domain analysis. Int. J. Neurosci. 128(4), 349–360 (2018)
W. Chatthong, S. Khemthong, Y. Wongsawat, A design thinking model based on quantitative electroencephalography in social emotional learning for attention deficit hyperactivity disorder. Mind Brain Educ. 14(2), 104–113 (2020)
V.V. Makarov, M.O. Zhuravlev, A.E. Runnova, P. Protasov, V.A. Maksimenko, N.S. Frolov, A.N. Pisarchik, A.E. Hramov, Betweenness centrality in multiplex brain network during mental task evaluation. Phys. Rev. E 98(6), 062413 (2018)
V.A. Maksimenko, A.E. Runnova, M.O. Zhuravlev, V.V. Makarov, V. Nedayvozov, V.V. Grubov, S.V. Pchelintceva, A.E. Hramov, A.N. Pisarchik, Visual perception affected by motivation and alertness controlled by a noninvasive brain-computer interface. PloS One 12(12), 0188700 (2017)
Acknowledgements
The study was carried out within the framework of the state task of the Russian Federation’s Ministry of Health #056-00030-21-01 dated 02052021 “Theoretical and experimental study of the integrative activity of various physiological systems of patient under stress” (the State registration number # 121030900357-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Runnova, A., Suetenkova, D., Shamionov, R. et al. Perception of monotonic load: different types of microsleep tolerance. Eur. Phys. J. Spec. Top. 233, 543–558 (2024). https://doi.org/10.1140/epjs/s11734-023-01065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-01065-3