Abstract
Objective measures of activity recovery performance in monotonous psychomotor task after brief episodes of daytime sleep were investigated. We used a psychomotor test in which the partially sleep-deprived subjects had to press the button 10 times with alternating left and right hands for 70 min. The task-induced short episodes of sleep, after which subjects had to resume the task. EEG of the anterior and central lead areas was studied when resuming the task after awakening from the first stage of sleep. We compared the situations of resumption of the pattern with left and right hands. The power of beta- and gamma-EEG oscillations during preparation and beginning of psychomotor activity was found to be higher when the subject starts to press the button with the left (nondominant) hand which may be a manifestation of sleep inertia. We attribute this to the fact that in this case the strategy for resuming activity interrupted by the short sleep is suboptimal and energy intensive, requiring greater cognitive activity. We also assume that this situation is preceded by a lower level of consciousness, in which the subject correctly remembers the instruction, but does reliably start with the dominant hand. However, the subject fully recovers and correctly performs the motor and synchronized cognitive account task immediately after awakening. This study contributes to an objective assessment of fluctuations in the human condition in monotonous work, which can lead to loss of concentration, reduced response time, drowsiness and negatively affect work safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The study of cognitive activity in different functional states of the subject, which are determined by the sleep–wake cycle, is of great theoretical and practical importance. The phenomenon of awakening is a good experimental model for studying the recovery of consciousness and activity interrupted by a sleep episode. The speed and accuracy of the subject's motor response are usually used to assess the functional state under these conditions [1]. Neurophysiological studies show a faster recovery of baseline characteristics of bioelectrical activity in motor and sensorimotor cortical areas during awakening [2,3,4]. In other areas, such as the parietal cortex, delta oscillations typical to sleep state may persist [5]. There are few works that study the neural correlates of cognitive performance after awakening. In them, subjects performed the Stroop test [6] or arithmetic calculations (descending subtraction task) [7].
Current neurophysiological studies of activity in a motor task immediately after awakening are virtually non-existent. It has previously been shown that button press parameters objectively assess the transition from sleep to wakefulness [8,9,10]. This behavioral assessment is consistent with the bioelectrical characteristics observed during and immediately after awakening [9]. Based on behavioral and EEG data, right hemisphere (left handed) dominance has been shown when performing the bimanual tapping test during brief nocturnal as well as after final morning awakening [11]. We have previously investigated the neurophysiological correlates of the performance in the unimanual psychomotor test after spontaneous awakenings from daytime sleep. It was shown that complete and correct reproduction of the test tasks is accompanied by a more pronounced and widespread alpha rhythm in the cortex than in cases of incomplete reproduction [12]. These results were obtained when performing the psychomotor test with the right hand only. However, the bimanual performance of psychomotor tests has significant behavioral and electroencephalographic differences from their unimanual versions (with right (dominant) and left (subdominant) hand presses) [13, 14].
The purpose of this study is to identify differences in EEG when resuming bimanual psychomotor test performance with the dominant and non-dominant hand after episodes of daytime sleep. We hypothesize that the onset of test performance by the nondominant hand will impede the onset of activity and will be reflected in its neural correlates. The task of the study was to compare the power characteristics of the EEG during preparation and the beginning of button pressing with the dominant and non-dominant hands.
2 Methods
2.1 Participants
Twenty-seven people (14 women and 13 men), aged 19–40 years (median 24 years) without neurological, psychiatric or sleep disorders were studied. They were not taking any medications. They were instructed not to consume alcohol during the day preceding the study. On the day of the experiment, they were advised to refrain from taking coffee, strong tea, chocolate, energy drinks, etc. All participants in the study received no monetary reward.
All subjects signed an informed consent form and were explained the goals and the course of the study. On the night before the experiment, the subjects slept for 4 h, i.e. they were in a state of partial sleep deprivation. The study complied with the ethical standards of the World Medical Association's Declaration of Helsinki, Ethical Principles for Scientific Medical Research Involving Human Subjects, as amended in 2000. It was approved by the Ethics Committee of the Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Sciences (protocol No. 2 of June 3, 2019).
2.2 Study protocol
Participants completed questionnaires to assess their habitual sleep patterns, sleep quality, sleepiness, and general condition before the start of the experiment, (The Chronotype and Psychological Traits Questionnaire; Wellbeing-Activity-Mood questionnaire; Karolinska Sleepiness Scale; Epworth Sleepiness Scale). All experiments were conducted in the daytime, beginning at 13–15 h. The duration of the experiment was about 3 h. EEG was recorded for 70 min. During the recording, the subject lay on a couch with closed eyes in a darkened, soundproofed and ventilated room.
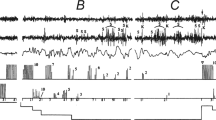
The psychomotor test [15] was used in the study. A button was fixed on the index finger of each hand, which the participant had to press with the thumbs. The subjects were instructed to press the buttons once per second 10 times, alternating series of presses with the right and left hand. Performance of the task continued until a subject spontaneously fell asleep. After sleep followed by spontaneous awakening, subjects were required to immediately resume the psychomotor test. The alternation of episodes of falling asleep and waking up accompanied by psychomotor activity makes it possible to study neural correlates of changes in the functional state and level of human consciousness.
2.3 Data
Heart rate measurement, cutaneous galvanic response, and monopolar EEG (64 Ag/AgCl leads using the 10–10 system) were recorded using an ActiChamp amplifier (Brain Products, Germany). The frequency bandpass was 0.5–70 Hz. The reference electrode was placed on the midline, in the Cz lead. Electrooculogram (EOG) was recorded using bipolar Ag–AgCl electrodes above and below the left eye; mechanograms of button presses were also recorded. The sampling rate for all data was 1000 Hz. Two independent experts staged polysomnograms on 30-s epochs by the American Academy of Sleep Medicine (AASM) criteria [16].
Segments of EEG recordings corresponding to episodes of spontaneous awakening, after which the subject fully recovered psychomotor activity, were selected for analysis. After awakening, the participant had to perform at least once a pattern consisting of 10 presses, first with one and then with the other hand. Subjects who performed this pattern at least once starting with each hand were included in the analysis. There were 11 such subjects (6 women and 5 men), all of them right-handed. In this study, we included awakenings only from stage 1 of sleep, because there were significantly more of them than from other stages. Two-second EEG recordings before and after the onset of button presses were extracted. For analysis, 25 EEG leads in the antero-central cortical regions associated with motor activity and control functions were selected as the region of interest. These leads are shown in Figs. 1 and 2.
Schematic maps of anterior and antero-central regions of EEG leads 2 and 1 s before the resumption of psychomotor test performance after an episode of sleep. Theta, beta and gamma frequency bands are shown. The leads with significant (p < 0.05) predominance of EEG power characteristics in the case when the subjects started to perform the test with their left compared to right hand are highlighted in red
Schematic maps of anterior and antero-central EEG leads 1 and 2 s after resumption of the psychomotor test after an episode of sleep. The designations are as in are as in Fig. 1
2.4 Statistical analysis
To estimate the power characteristics of the bioelectrical activity of the cortex, we performed continuous wavelet transformation based on the “mother” complex Morlet wavelet (Matlab 78.01, parameters for the scripts were taken from [17]. Wavelet transform coefficient modulus (WTC) distribution maps were plotted in the band 0.5–40 Hz with a step of 0.5 Hz and a time resolution of 0.01 s. WTC was averaged over frequency in the theta (4–7.5 Hz), beta (15–19.5 Hz), and gamma (20–40 Hz) frequency bands; it was further averaged over time, in 1-s intervals (2 s before and after the onset of button presses). For each 1-s interval and each spectral range for all selected EEG leads, the characteristics of bioelectrical activity were compared between right- and left-hand button press resumption situations using the paired Student. These characteristics were then averaged across leads in the left and right hemispheres.
The obtained EEG amplitude-power characteristics for each frequency range were analyzed using analysis of variance with repeated measures (ANOVA RM). Factors included in the analysis were: “Hand pressing the button” (two levels—right and left hand), “Psychomotor activity” (two levels—before the resumption of pressing the button and after it), “Hemisphere” (two levels—right and left hemisphere) and “Time” (two levels—two 2-s time segments). Statistical results were obtained using the Greenhouse–Geisser correction. All statistical computations were run in SPSS 13.0.
3 Results
The analysis of variance of the total power characteristics of EEG showed the following. In the theta-band, joint influence of the factors “Hemisphere-Time” (F(1, 10) = 9.88; p = 0.010) and a trend towards the influence of “Situation” factor (F(1, 10) = 4.72; p = 0.054) were found. In the beta range, the interaction of factors “Hand-Situation-Hemisphere” (F(1, 10) = 8.16; p = 0.021) was present. In the gamma range, there is a trend towards the influence of “Hand” factor (F(1, 10) = 3.59; p = 0.087) and “Hand-Situation-Hemisphere” interaction (F(1, 10) = 3.56; p = 0.089). The results of pairwise comparison of amplitude characteristics by paired t-test for the studied EEG leads are shown in Figs. 1 and 2. In all cases, EEG power characteristics were greater in the situation where subjects started pressing the button with their nondominant (left) hand.
4 Discussion
The frontal theta rhythm is associated with the processes of cognitive control [18, 19]. In [19], the theta rhythm increases not only during the performance of psychomotor test tasks but also during the preparation for it. The function of activity control is turned on earlier than the activity itself begins. In our study, this effect is more pronounced when the subjects resume the activity with the left hand rather than the dominant right hand.
We hypothesize that in this case their strategy for resuming a psychomotor test interrupted by a sleep episode may be suboptimal and energy-consuming, requiring more cognitive control in its implementation. Moreover, it is known that the efficiency of activity immediately after awakening is decreased due to sleep inertia. This phenomenon is accompanied by higher power characteristics of low-frequency EEG components (delta- and theta-bands) compared to recordings during activity in the waking state before falling asleep [1, 6, 20]. In our experiment, the effect of sleep inertia is as follows: the subjects correctly recall the instruction (“press-and-count”) but is yet unable to reliably start with a dominant hand. As a result, they sometimes start performing the psychomotor test with a nondominant hand, which may indicate suboptimal conditions for test implementation against the background of a general decrease in the level of consciousness caused by the sleep episode. However, due to the involvement of cognitive control, the subjects fully recover and correctly perform the synchronized motor and cognitive task of counting button presses immediately after awakening. This suggests that not only the current level of consciousness but also nonconscious processes influence the efficiency of recovery from the activity interrupted by a brief daytime sleep.
Previously, the role of beta and gamma rhythms in motor activity and sensorimotor integration has been shown [21, 22]. These rhythms are also associated with cognitive processes such as attention, perception, memory, etc. [23]. A study [24] shows an increase in high-frequency EEG oscillations (beta and gamma) at the end of microsleep before the resumption of activity. This fact, according to the authors, reflects the subconscious brain drive to restore consciousness, to resume the interrupted perceptual connection with the external environment, to re-synchronize attention and memory to return to the performance of the cognitive task [24]. Our findings with respect to beta- and gamma-rhythms may indicate that the recovery of psychomotor activity after an episode of sleep with the non-dominant hand requires a greater cognitive load in the process of preparation for it and during its realization.
5 Limitation
A limitation is the small number of participants and arousals/awakenings in the study. Further work is needed to investigate not only the spectral characteristics but also the spatiotemporal relationships of the EEG.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
L. Trotti, Waking up is the hardest thing I do all day: sleep inertia and sleep drunkenness. Sleep Med. Rev. 35, 76–84 (2017). https://doi.org/10.1016/j.smrv.2016.08.005
P.-J. Tsai, S.C.-J. Chen, C.-Y. Hsu et al., Local awakening: regional reorganizations of brain oscillations after sleep. Neuroimage 102, 894–903 (2014). https://doi.org/10.1016/j.neuroimage.2014.07.032
L. Peter-Derex, M. Magnin, H. Bastuji, Heterogeneity of arousals in human sleep: a stereo-electroencephalographic study. Neuroimage 123, 229–244 (2015). https://doi.org/10.1016/j.neuroimage.2015.07.057
S. Alcaide, J. Sitt, T. Horikawa et al., fMRI lag structure during waking up from early sleep stages. Cortex 142, 94–103 (2021). https://doi.org/10.1016/j.cortex.2021.06.005
M. Terzaghi, I. Sartori, L. Tassi et al., Evidence of dissociated arousal states during nrem parasomnia from an intracerebral neurophysiological study. Sleep 32(3), 409–412 (2009). https://doi.org/10.1093/sleep/32.3.409
P. Tassi, A. Bonnefond, O. Engasser et al., EEG spectral power and cognitive performance during sleep inertia: the effect of normal sleep duration and partial sleep deprivation. Physiol. Behav. 87(1), 177–184 (2006). https://doi.org/10.1016/j.physbeh.2005.09.017
R. Vallat, D. Meunier, A. Nicolas, P. Ruby, Hard to wake up? The cerebral correlates of sleep inertia assessed using combined behavioural, EEG and fMRI measures. Neuroimage 184, 266–278 (2019). https://doi.org/10.1016/j.neuroimage.2018.09.033
A. Anch, J. Salamy, G. McCoy, J. Somerset, Behaviorally signalled awakenings in relationship to duration of alpha activity. Psychophysiology 19, 528–530 (1982). https://doi.org/10.1111/j.1469-8986.1982.tb02580.x
S. Campbell, W. Webb, The perception of wakefulness within sleep. Sleep 4, 177–183 (1981). https://doi.org/10.1093/sleep/4.2.177
D. Redington, F. Perry, E. Gibson, J. Kamiya, Discrimination of early sleep stages: behavioral indicators. Sleep 4, 171–176 (1981). https://doi.org/10.1093/sleep/4.2.171
M. Casagrande, M. Bertini, Night-time right hemisphere superiority and daytime left hemisphere superiority: a repatterning of laterality across wake–sleep–wake states. Biol. Psychol. 77, 337–342 (2008). https://doi.org/10.1016/j.biopsycho.2007.11.007
E. Cheremushkin, N. Petrenko, V. Dorokhov, Sleep and neurophysiological correlates of activation of consciousness on awakening. Neurosci. Behav. Physiol. 52(2), 213–217 (2022). https://doi.org/10.1007/s11055-022-01226-2
D. Serrien, Coordination constraints during bimanual versus unimanual performance conditions. Neuropsychologia 46(2), 419–425 (2008). https://doi.org/10.1016/j.neuropsychologia.2007.08.011
J. Rudisch, S. Fröhlich, N. Pixa et al., Bimanual coupling is associated with left frontocentral network activity in a task-specific way. Eur. J. Neurosci. 58(1), 2315–2338 (2023). https://doi.org/10.1111/ejn.16042
V.B. Dorokhov, Alpha-bursts and K-complex: phasic activation pattern during spontaneous recovery of correct psychomotor performance at difference stages of drowsiness. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 53(4), 503–512 (2003)
R.B. Berry, R. Brooks, C. Gamaldo, S.M. Harding, R.M. Lloyd, S.F. Quan, M.T. Troester, B.V. Vaughn, AASM scoring manual updates for 2017 (Version 2.4). J. Clin. Sleep Med. 13(5), 665–666 (2017). https://doi.org/10.5664/jcsm.6576
C. Tallon-Baudry, O. Bertrand, F. Peronnet et al., Induced gamma band activity during the delay of a visual short term memory task in humans. J. Neurosci. 18(11), 4244–4255 (1998). https://doi.org/10.1523/JNEUROSCI.18-11-04244.1998
J. Cavanagh, M.J. Frank, Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 18(8), 414–421 (2014). https://doi.org/10.1016/j.tics.2014.04.012
P.S. Cooper, F. Karayanidis, M. McKewen et al., Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. Neuroimage 189, 130–140 (2019). https://doi.org/10.1016/j.neuroimage.2019.01.022
E. Cheremushkin, N. Petrenko, V. Dorokhov, EEG characteristics and anxiety levels in subjects with different levels of success in recovering psychomotor activity on waking during daytime sleep. Neurosci. Behav. Physiol. 52(4), 562–567 (2022). https://doi.org/10.1007/s11055-022-01275-7
A. Athanasiou, M.A. Klados, Ch. Styliadis et al., Investigating the role of alpha and beta rhythms in functional motor networks. Neuroscience 378, 54–70 (2018). https://doi.org/10.1016/j.neuroscience.2016.05.044
C. Babiloni, C.D. Percio, F. Vecchio et al., Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin. Neurophysiol. 127, 641–654 (2016). https://doi.org/10.1016/j.clinph.2015.04.068
V.N. Dumenko, The functional role of neocortical activity in the processes of interregional interaction. Neurosci. Behav. Physiol. 45(3), 239–251 (2015). https://doi.org/10.1007/s11055-015-0063-2
M.H. Zaky, R. Shoorangiz, G.R. Poudel et al., Increased cerebral activity during microsleeps reflects an unconscious drive to re-establish consciousness. Int. J. Psychophysiol. 189, 57–65 (2023). https://doi.org/10.1016/j.ijpsycho.2023.05.349
Funding
This study was funded by the Russian Science Foundation (Grant #22-28-01769).
Author information
Authors and Affiliations
Contributions
Conceptualization EACh, VBD. Data curation EACh, NEP. Formal analysis NEP, JNK, GNA, AOT. Funding acquisition VBD. Investigation EACh, NEP. Methodology EACh, VBD. Project administration VBD. Resources JNK, GNA, AOT. Supervision VBD. Visualization EACh, NEP. Writing—original draft NEP, JNK. Writing – review and editing: EACh, VBD, PAN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, and the decision to publish the results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheremushkin, E.A., Petrenko, N.E., Kuznetsova, Y.A. et al. Neural correlates of the efficiency of psychomotor activity recovery following short sleep episodes. Eur. Phys. J. Spec. Top. 233, 601–606 (2024). https://doi.org/10.1140/epjs/s11734-023-01062-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-01062-6