Abstract
Methods of evaluation of substates of sleep and sleepiness differ in, at least, two respects. Although sleepiness has not been separated from other wake and sleep substates using yes-or-no criteria for sleep scoring, it would be consciously perceived and, therefore, assessed with a questionary. However, such subjective method was challenged by the finding suggesting a disconnect between two most widely used subjective and objective indicators of excessive daytime sleepiness (EDS), a score on the Epworth sleepiness scale (ESS) > 10 and a reduced latency to sleep onset (SOL), respectively. We examined whether these two EDS indicators differ in their association with physiological sleepiness, i.e., the polysomnographic indexes of elevated sleep pressure. In the afternoon hours, polysomnographic recordings were obtained throughout 54 50-min and 56 90-min napping attempts of 27 and 28 university students, respectively. Within some but not all 10-min intervals of the 50- or 90-min naps, each EDS indicator was validated against different objective polysomnographic indexes suggesting an association of EDS with elevated sleep pressure. Significant differences in sleep indexes were found between participants with short and longer SOL, but they disappeared right before the appearance of such differences between participants with higher and lower ESS score (usually at the 4th 10-min interval). This mismatch in timing of appearance of significant differences might be a plausible explanation for the lack of significant association between the ESS and SOL. Therefore, the physiologic underpinnings of the ESS can be uncovered despite such a disconnect between these two EDS indicators.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The concepts from complex systems can be applied to the physiology of sleep–wake regulation, that is the control of the dynamics of alternation between alert and sleepiness substates of wake state as well as between two—non-REM and rapid eye movement (REM)—phases of sleep [1,2,3]. The dominated methodologies for study of such substates within the wake–sleep continuum differ. Unlike sleep substates, sleepiness has not been yet clearly separated from other wake and sleep substates by applying objective polysomnographic criteria, such as yes-or-no criteria for sleep scoring. Therefore, sleep and circadian rhythm researchers agree that, in spite of the extraordinary progress made by this research in the last decades, the measurement of sleepiness remains one of the most challenging problems [4]. On the other hand, sleepiness can be consciously perceived by anybody. Therefore, it can be assessed with a questionary instrument. Since an increase in sleepiness is usually preceding a clock hour chosen for falling asleep [5, 6], subjective sensation of sleepiness might evolve as an adaptation motivating humans to switch from any kind of current daytime activities to sleep-preparatory behaviors.
Research of the subjective concept of sleepiness produced many controversial questions, including such questions as whether the physiological component of subjective feeling of drowsiness can be precisely defined in scientific terms, and what can be its reliable physiological marker [8,9,10]. Given such controversy, the dominating approach to evaluation of sleepiness contrasts with the conventional methods of elaboration of the well-discriminated substates of sleep. For many years, sleepiness has been viewed as a paradoxical vigilance substate for which the consensus on the method of its objective measurement has not been reached so far, despite the universal ability of humans to consciously perceive sleepiness [7].
There is an important practical aspect of the methodology for objective measurement of sleepiness. A method of quick and user-friendly evaluation of sleepiness has obvious implications for medicine and safety–critical occupations and procedures [11]. It was recognized that the well-established methods of objective evaluation of sleepiness, such as the measurements of sleep propensity with the Multiple Sleep Latency Test (MSLT), seem to be impracticable due to the requirement of attendance at a sleep laboratory for many hours [12]. Consequently, standardized questionnaires represent a suitable compromise [13].
The eight-item Epworth Sleepiness Scale (ESS) [14] serves as the most popular questionnaire tool for diagnostic of excessive daytime sleepiness (EDS) in clinical populations [13, 15]. For instance, an umbrella review of the literature on sleepiness as a complex construct revealed that the ESS was the first among 6 most frequently cited sleepiness assessment tools (from 99 tools in total) [16]. However, the physiologic underpinnings of this scale are not well understood [17, 18]. In particular, one of the consistent findings of the studies aimed on validation of the ESS against objective measures of sleepiness is a lack of the expected close association between subjective sleepiness assessed as a score on the ESS and objective sleep propensity measured in MSLT that is mean latency to sleep onset (SOL) [19,20,21,22,23,24,25].
Therefore, the current study sought to validate the ESS against objective measurements of sleep in 60- and 90-min sleeping attempts.
Similar to the mentioned above publications, a close relationship between an ESS score and SOL was not found in two our previous nap studies of university students [26, 27]. One more sample of university students from the same population was currently studied to address the issue of disconnect between these two most popular subjective and objective indicators of EDS. We asked the following main question: what can be a cause of this disconnect.
Depending upon the answer to this question, we additionally asked whether we can trust the result of these nap studies suggesting a very high rate of EDS diagnosed from the ESS self-scorings provided by university students. Finally, in a survey with a much larger number of participants from the same population, we addressed the following question: what are the possible causes of high rate of EDS reported by university students? To answer this question, we included some results of this survey in this paper.
2 Methods
All procedures performed in this nap study of human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments, and in accordance with the ethical standards of the institutional research committee. The Ethics Committee of the Institute approved the experimental protocol in June 2019 (Approval#12402-02-7112). The study participants were informed in detail about all procedures, and informed written consent was obtained from each participant.
2.1 Participants of Nap Study
Unpaid volunteers of the 50- and 90-min nap studies were 27 and 28 (12 and15 female) students with mean age of 19.3 and 20.0 years and standard deviation of 1.3 and 1.1 years, respectively. After the structured interview with a sleep researcher, they were invited to participate in the nap study. The exclusion criteria were: age either younger than 18 or older than 23 years, pregnancy or breastfeeding (for female participants), denying history of mental or sleep disorder, any complains about poor physical condition and functioning, current mild cold and missing classes due to any sickness in 2 previous weeks, involvement in shift or night work, crossing several time zones in the previous month, irregularity of sleep–wake schedule exemplified by more than 1-h difference in weekday bedtimes, frequent sleep reduction exemplified by, at least, one night of partial sleep deprivation in the previous week.
2.2 Study protocol
Each study participant had three either 50-min or 90-min napping attempts in a sleep laboratory. The visits to the sleep laboratory were preceded and followed by the attending classes in the same building. The intervals between visits varied from 3 days to a month. Each visit to the sleep laboratory was scheduled at the same afternoon hour (between 12:30 and 15:30). The first napping attempt was regarded an adaptation nap, while only polysomnographic records of the 2nd and 3rd naps were analyzed.
Prior to each napping attempt, the eight-item ESS [14] was administered, and scores obtained in two last naps were averaged for dividing participants into the groups without and with ESS-based EDS, ESS score < 11 and ESS score > 11 (n = 21 and 17 without vs. 7 and 10 with EDS in the 50- and 90-min naps, respectively). This division was found to be in agreement with a score obtained before the 1st (adaptation) nap, i.e., prior to any polysomnographic recordings.
2.3 Polysomnographic recordings
During the preparation to polysomnographic recordings, a participant was instructed to try to relax and to nap for 50 or 90 min after light off in the 50- or 90-min nap study, respectively. In the 90-min study, the recordings were performed with a Neurovisor BMM-36 (Medical Computer Systems LLC, Moscow), the MCScap Sleep electrode helmet, and the NeoRec 1.4 software. The electrodes were applied to record the EEG signals from 19 channels connected by a monopolar 10–20 scheme with two reference electrodes on the mastoid bones. Other recorded polysomnographic signals included two electrooculogram channels, one electromyogram channel, and one electrocardiogram channel. The recorded signals were conditioned by the high-pass, low-pass and notch filters (frequencies of 0.5 Hz, 35 Hz, and 50 Hz, respectively). The sampling frequency of the signal was 1000 Hz.
The 50-min nap study differed from the 90-min nap study in using another (16-channel) wireless system (“Neuropolygraph 24”, Neurotech, Taganrog, Russia) for the polysomnographic recordings, the EEG signal recordings from 13 derivations, and their sampling frequency rate of 500 Hz.
2.4 Sleep scoring
In accord with the conventional scoring procedure [28], visual scoring on 30-s epochs of each 50- or 90-min record was performed. The records were independently scored by two experienced scorers. In order to finally produce consensus scores, the scorers reexamined together all intervals with discrepant scores. They were uninformed about names of participants and orders of their napping attempts. The 30-s epochs were classified into five stages, namely, wake stage named W, three stages of NREM sleep named N1, N2, N3, and REM sleep named R (Table 1, Fig. 1, and Supplementary Figures S2, S3, and S8. Latencies to N1 (SOL) and N2 were also estimated (Table 1 and Fig. 4).
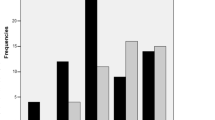
Time courses and correlations of amounts of stages across 50- and 90-min intervals. a, b Comparison of amounts of stages calculated as mean amounts for each of 5 or 9 10-min intervals of napping attempts in subsamples obtained by the division of the sample of 55 or 28 university students, respectively, in accord with subjective and objective Excessive Daytime Sleepiness (EDS) indicators (assessed with “ESS”, score < 11 and score > 11, and measured as “SOL”, < 6 min and ≥ 6 min, respectively). Mean amounts and their SEM (standard error of mean) were obtained by averaging over 55 or 28 participants. c Pearson correlations (r) of each of sleepiness indexes with amounts of stages obtained by averaging within each of 5 or 9 10-min interval of napping attempt. The borderline between significant and non-significant coefficients (p ≥ 0.05) is depicted as two horizontal lines (in parallel with X-axis), i.e., between r > − 0.374 and r < 0.374 and between r > − 0.266 and r < 0.266 for samples of 28 and 55 study participants, respectively. The axis for SOL was inverted (Y-axis on the right) to provide comparison with the ESS (Y-axis on the left). See also statistical results in Tables 1 and 2
SOL was averaged over two naps and used to divide participants in the groups without and with SOL-based EDS, SOL ≥ 6 min and SOL < 6 min (n = 13 and 15, respectively).
2.5 Analysis of the EEG signals
The EEG spectral power densities were calculated using the EEG signals recorded in any nap study from electrodes placed at five derivations (Fz, F4, Cz, Pz and O2 referenced to the ear mastoid sites, M1/M2). The records of the signals from these derivations were visually inspected on 1-s epochs to remove all epochs containing artifacts from further analysis. Spectral power densities for the artifact-free epochs were computed using the fastest Fourier transform in the west (FFTW) package [29] (see http://www.fftw.org for more detail). In the process of calculation of absolute spectral power densities (µV2), Hamming window taper was used on 1-s epochs. Further analysis was performed on 16 single-Hz frequency bandwidths, between 1 and 16 Hz (i.e., 0.50–1.49 Hz for 1 Hz, 1.50–2.49 Hz for 2 Hz, 2.50–3.49 Hz for 3 Hz, …, 15.50–16.49 Hz for 16 Hz). These sets consisting of 16 single-Hz power densities were averaged within 30-s intervals of EEG records and ln-transformed.
For purposes of statistical analyses, the spectral powers of each participant (180 per each derivation of each napping attempt) were further averaged, e.g., over derivations (Tables 1, 2, and Figs. 2, 3, S4–S7, S9, and S10), within and over each of two napping attempts (Tables 1 and 2, and Supplementary Figure S4–S7, S9, and S10), and within 4 4-Hz frequency ranges of delta (1–4 Hz), theta (5–8 Hz), alpha (9–12 Hz), and sigma (13–16 Hz) powers (Tables 1, 2, Figs. 2, 3, S4, S5, and S9).
Time courses and correlations of spectral powers and principal component scores across 50- and 90-min intervals. a, b Comparison of amounts of spectral powers and principal component (PC) scores calculated as mean values for each of 5 or 9 10-min intervals of napping attempts in subsamples obtained by the division of the sample of 55 or 28 university students, respectively, in accord with subjective and objective indicators of excessive sleepiness (assessed with “ESS”, score < 11 and score > 11, and measured as “SOL”, < 6 min and ≥ 6 min, respectively). c Pearson correlations (r) of each of two EDS indexes with spectral powers and scores on principal components obtained by averaging within each of 5 or 9 10-min interval of napping attempt. See statistical results in Tables 1 and 2
Averaged amounts of stages, sleep latencies, spectral powers, and principal component scores on the 50-min intervals of 50- and 90-min naps. a Comparison of averaged amounts of stages, latencies, and ESS score, b comparison of spectral powers and principal component scores. The subsamples were obtained by dividing the whole sample of 55 university students in accord with either subjective or objective indicator of excessive daytime sleepiness (EDS). Results of three-way ANOVAs with independent factors “EDS group” (Grouping on either “ESS”, score < 11 and > 11, or “SOL”, < 6 min and ≥ 6 min), “Sex” (male and female students) and “Nap study” (either 50-min nap or the first 50 min of 90 min nap); See also results on correlations and other statistical results in Tables 1 and 2
2.6 Loadings and scores on the 1st and 2nd principal components of the EEG spectrum
The SPSS23.0 statistical software package (IBM, Armonk, NY, USA) was used for statistical analysis. Principal component analysis was performed on the sets of 16 ln-transformed single-Hz power densities (1–16 Hz) from each of 5 derivations, and scores on the 1st and 2nd principal components of variation in the EEG power spectra were calculated (Tables 1 and 2).
2.7 Participants of the preceding online survey
Online survey of the same student population preceded nap studies. The survey questions about sleepiness were used to compare the rates of EDS calculated for small samples of two (50- and 90-min) nap studies (n = 55) with the rate in a much larger sample of this survey (n = 633). Moreover, the survey questions about night sleep timing and time course of sleepiness provided a possibility to evaluate which of sleep characteristics might be associated with high rate of EDS in this population of university students.
2.8 Questionnaires of the online survey
When attending classes, students were invited by the lectures to voluntarily participate in this survey. The students were asked to anonymously respond from their smartphones to the questions about their sleep and sleepiness. To collect their responses, the web page was designed (https://docs.google.com/forms/d/e/1FAIpQLSdIEeg00XFqmoULmKjXMqGI9rtMwpPD4HVwv5ZqYtH-BDMd3A/viewform). Demographic information on the collected sample (n = 633) and subsamples with different sleepiness levels is reported in Tables S1 and S2. The questionnaire battery (Fig. 4 and Tables 3, and S1–S4) included: (1) the Munich ChronoType Questionnaire (MCTQ) for self-reporting sleep onset latency and times of night sleep onset and offset for free and weekdays [30], (2) the Pittsburgh Sleep Quality Index (PSQI) for self-reporting monthly averaged sleep onset latency and sleep times, hours slept, sleep efficiency, and subjective sleep quality score [31], (3) the 8-item Epworth Sleepiness Scale (ESS) for the determination of level of daytime sleepiness [14], and (4) the 19-item Visuo-verbal Judgment Task (VJT) for reporting sleepiness levels expected for 19 clock times on 1.5-day time interval [32].
Time courses of KSS score and correlation of this score with ESS score. a Time course of KSS score in participants without and with excessive daytime sleepiness from the results of three-way rANOVA with within subjects’ factor “Clock h” (KSS suggested for 19 time points by responding to the VJT) and independent factors “Gender” (male vs. female) and “ESS score” (≤ 10 and > 11, respectively). b Time course of correlation between KSS and ESS scores. Two horizontal lines depict the range of significant Spearman’s correlation coefficients (p < 0.05); h: clock hour
The MCTQ [30] was used to collect responses to the questions on sleep onset latency at night, sleep onset time (bedtime + this latency) and sleep offset time on weekdays and free days. Additional calculations provided some other estimates, such as sleep duration and free-weekday difference in each of sleep times. Previously, we demonstrated that, after averaging over many samples, such sleep times are almost identical to the times predicted by a sleep–wake regulation model for free and weekdays [33, 34]. The results of these simulations suggested a possibility to estimate weekday sleep loss from self-reports on weekday and weekend sleep times, an indicator of the extend of reduction in sleep duration due to early wakeups on weekdays [34]. Such a sleep loss (%) is calculated as
The responses to several questions (1–4 and 9) of the PSQI [31] were used to obtain such self-reports as monthly averaged SOLN and times of sleep onset and offset, as well as some other subjective indexes of sleep, such as its percentage (sleep efficiency), subjective quality, and hours slept (Tables 3, S1 and S3).
The ESS [14] quantifies the likelihood to fall asleep in each of 8 different daily life situations with a scale ranging from 0 to 3, where 0 corresponds to none and 3 to the situation when dozing off is the most likely. The total score ranges from 0 to 24. Values above 10 are considered to be indicative for significant (excessive) sleepiness. The psychometric properties of the ESS have been investigated on multiple occasions (e.g., [35]). Its internal consistency (Cronbach’s alpha) varies between 0.73 and 0.90 [36].
In the samples collected in this survey (n = 633), Cronbach's Alpha attained the value of 0.698. In one of two our previous nap studies [26], the Spearman’s rank correlation coefficient between scores obtained prior to two naps of this study (usually divided by a 1-week time) attained the value of 0.921 (p < 0.001, 23 participants). Test–retest reliability of the ESS score in the present 90-min nap study attained the value of 0.707 (p < 0.001, 28 participants).
For additional comparison of the daily variation in sleepiness, the VJT was applied. It was designed to evaluate how sleepy survey participants thought they would be at different randomly presented times after having habitual night sleep terminated at approximately 7:30, either by a waking up signal or due to spontaneous awakening [31]. The proposed setting further evoked a pictured sleepiness-neutral situation (i.e., ‘‘sitting and reading’’). The time cues from 8 a.m. to midday and from 8 p.m. to midnight were presented with 1-h intervals, while time cues between midday and 8 p.m. and after midnight were presented with 2-h intervals (Figure S1). Moreover, the participants would see at the screen a visual aid that consisted of clock times along a scale illustrating the daily variation in the outdoor illumination level and indicating the duration of the waking period (see [31] for these illustrations and other details). In the survey of these university students, the test was slightly modified by using the ten-item Karolinska Sleepiness Scale (KSS) [37] instead of VAS (Visual Analog Scale) [38] of the original version for reporting sleepiness (see also the caption to Figure S1).
2.9 Statistical analysis
Pearson correlation coefficient (r) was calculated to check significance of association of the EDS indicators with sleep indexes obtained in naps (Table 1 and S8–S10). Three- and four-way repeated measure ANOVAs (rANOVAs) were run to test significance of main effect of independent factor “EDS group” (without and with EDS diagnosed with either ESS score or SOL), independent factor “Sex” (male and female students), and repeated measure “Time” (5 or 9 10-min intervals of nap). In four-way rANOVAs, an additional factor was “Nape duration” (the 1st 5 10-min intervals of 50- and 90-min naps). Mauchly’s test was conducted to assess the sphericity and, if necessary, the Greenhouse-Geiser correction was used to adjust the degrees of freedom, but the original degrees of freedom are reported in Table 2. The results rANOVAs are also illustrated in Figs. 1ab and 2ab) and Supplementary Figures S2–S7 illustrate the results four- and five-way rANOVAs with an additional repeated measure “Nap”.
For analysis of data of the survey, two-way ANOVAs and ANCOVAs were applied (Tables 3, S1 and S2, and Tables S3 and S4, respectively). Three- and four-way repeated measure ANOVA (rANOVA) was run to analyze daily variation in sleepiness assessed with the VJT (Figs. 4, S1). Finally, Spearman's coefficient of correlation was calculated to determine significance of association between sleepiness assessed with the ESS and KSS (Fig. 4b).
3 Results
3.1 Patterns of correlation of two EDS indicators with sleep indexes
Coefficient of correlation between EDS indicators, ESS scores and SOL, was found to be close to zero (Table 1). The patterns of correlations of these two EDS indicators with sleep indexes obtained on the 50-min nap interval were different (Table 1, left). Only few correlations were significant for the ESS score, while most of correlations were significant for SOL (Table 1).
3.2 Difference between sleepiness indicators in results of rANOVAs
Results of rANOVAs of sleep indexes suggested non-significant interactions of independent factor “Grouping” with “Sex” or “Nap”, and their triple interaction was also non-significant. Therefore, the differences between the groups with normal and excessive levels of daytime sleepiness in sleep indexes were replicable over two sexes and two naps. Such replicability is illustrated in Figures S2–S10.
These rANOVAs of sleep indexes revealed most general difference between divisions obtained by applying two criteria of EDS (Table 2). This difference suggests that, if two groups with high and lower ESS score were often significantly different in the level of sleep index (i.e., in the main effects of the factor “EDS group”), the two groups with short and longer interval of SOL were often significantly different in interaction of this factor with “Time” (i.e., in the time courses of sleep indexes). If the diagnosis of EDS was based on ESS, a higher ESS score was associated with smaller amount of W, larger amounts of N2, higher power density in delta range, lower PC2 score, and higher difference between PC1 and PC2 scores. Such significant differences can be interpreted as the signs of intensification of sleep process and they pointed at an elevated level of sleep pressure during napping attempt. As for the difference between two groups obtained in accord with SOL, it was non-significant in analysis of the 9 10-min intervals (Table 2). This is mainly because significant differences in the beginning of nap disappeared in the middle of 90-min nap. Due to such time course of this difference, this EDS diagnosis was also associated with interaction between the EDS group and time (a sequence of 9 10-min intervals). This interaction was significant for most of sleep indexes (Table 2). This implies that all events occurring in the first sleep cycle, not only the event of sleep onset, were advanced in participants with short SOL as compared to participants with longer SOL, i.e., the entrances in stages N2 and N3, a rise of spectral powers in slow frequency ranges, etc. (Table 2). Such acceleration of sleep process pointed at an elevated level of sleep pressure during napping attempts of participants with EDS based on SOL. In contrast, this acceleration was not seen in data on participants with EDS based on an ESS score. Figures 1ab and 2ab illustrate such most remarkable difference between two EDS criteria used for subdivision of study participants in the groups with normal and excessive levels of daytime sleepiness.
3.3 Difference between sleepiness indicators in results on time course of correlation
Moreover, Figs. 1c and 2c illustrate the supporting evidence for such a difference between two criteria of EDS provided by the time course of correlation coefficient between a sleepiness indicator (based either on the ESS or SOL) and a sleep index. It shows that correlations of SOL with almost any sleep index reached the level of significance already during the first three-four 10-min intervals of napping attempt. In contrast, the correlations with ESS score reached the level of significance only after these three-four 10-min intervals, i.e., when a correlation with SOL turned to become non-significant (Figs. 1c, 2c).
3.4 Examples of difference between sleepiness indicators in time course of correlation
For instance, Fig. 2c shows that correlations of SOL with spectral powers in delta and alpha frequency ranges were significant (positive and negative, respectively), but the time period for significant correlations was limited to the 1st three 10-min intervals. This implies that an advance shift of sleep process characterized the participants with SOL-based EDS as compared to the participants without this EDS. During the 4th 10-min interval, these coefficients reached the borderline of significance, and, thereafter (during the next three 10-min intervals), the coefficients of correlations between ESS score and spectral powers in low-frequency range became significant. This indicated the intensification of sleep process in the participants with the ESS-based EDS compared to the participants without this EDS. Therefore, it seems that the 50-min interval was not long enough to reveal this intensification statistically (Tables 1, 2).
In particular, this pattern of difference between the divisions based on ESS and SOL can be clearly seen in results on delta powers shown in the left graphs of Fig. 2c. They pointed at the advancing shift of appearance of significant difference between the SOL-based divisions relative to the appearance of significant difference between the ESS-based division. Moreover, similar pattern of correlations can be seen in the graphs on the left side of Fig. 2c that shows the time course of correlation with such spectral EEG index as the difference between scores on the 1st and 2nd principal components (Fig. 2c, compare the graph on the left and right). This similarity of the patterns of correlation can be explained by a close resemblance between delta power and the difference between the 1st and 2nd principal component scores. For instance, the difference mostly becomes positive with increasing delta powers and negative with their decreasing.
3.5 Difference between sleepiness indicators in results on averaged sleep indexes
Overall, the results of analysis of amounts of sleep stages suggested that the ESS-based sleepiness was associated with lower amounts of W and N1 and higher amounts of N2 and N3, and that the SOL-based sleepiness was associated with lower amount of W and higher amounts of NREM sleep stages (Fig. 3a, left, and Table 2). The results of analysis of spectral powers indicated that the ESS-based sleepiness was significantly associated with higher delta power, the indicator of intensity of reduction of sleep pressure during NREM sleep, whereas the SOL-based sleepiness was mostly associated with lower alpha power, the indicator of reduced amount of relax wakefulness during napping attempt and higher amount of sleep (Table 2, left, and Fig. 3b, left). Moreover, both EDS indicators were associated with a larger difference between principal component scores. However, two indicators differed in major contributor to the elevation of the score. Either the 1st or the 2nd principal component score were the major contributors to the elevated difference between scores in either subjectively or objectively sleepy participants, respectively (Table 2, left, and Fig. 3b, right).
3.6 Sleepiness and its causes in results of the online survey
Results of the online survey with participation of 633 university students supported the results of the nap studies indicating a high prevalence of ESS score > 10 in this population of university students (Tables 3, S1–S5). The results also indicated that university students with such high score suffered more than other university students from loss of sleep on weekdays caused by early wakeups on weekday mornings (Tables 3, S1, S3). Therefore, they also suffered from higher levels of sleepiness during the day (Figs. 4, S1). The results allowed the conclusion that waking up too early on weekdays can be a major cause of chronic sleep deprivation that, in turn, significantly contribute to the elevation of sleep pressure in the afternoon in the university students with high ESS scores.
4 Discussion
People are capable to consciously perceive sleepiness. Therefore, they are also capable to report EDS by responding to several questions of questionnaire, such as the eight-item ESS. However, the attempts to validate this scale against such objective measure of sleepiness as SOL were unsuccessful. Here, we demonstrated a possibility to validate self-reported EDS by tracing time course of various sleep indexes throughout 90-min napping attempt. We showed that both subjective and objective indicators of excessive sleepiness, a high ESS score and a short time interval of SOL, have physiological underpinnings and that these underpinnings can be associated with elevated levels of sleep pressure. However, if the SOL-based EDS was associated with the signs of acceleration of sleep process in the 1st sleep cycle, such an acceleration was revealed neither for the ESS-based EDS nor for any of two groups without EDS. Although sleep process was developing with the same rate in participants without EDS and with ESS-based EDS, this process was intensified in the middle of nap in the participants with ESS-based EDS, i.e., at the peak of NREM sleep that is associated with dropping sleep pressure in the middle of the 1st sleep cycle.
This mismatch in time for appearance of the signs of elevated sleep pressure in the participants with EDS based on the ESS and SOL might be an explanation of the lack of significant association between these two most popular indicators of EDS. For instance, the correlation analysis revealed only few significant correlations between sleep indexes and ESS score on the 50-min interval of napping attempts. However, rANOVAs of 9 90-min intervals revealed significant difference in several sleep indexes between the groups with low and high ESS score. On the other hand, these analyses found that the differences did not reach the level of significance in the 1st three-four 10-min intervals. Therefore, when a shorter (50-min) interval of nap was analyzed, these groups were rare separated on sleep indexes.
Overall, the results of nap studies indicated that, despite a disconnect between two sleepiness indicators, physiologic underpinnings of the ESS can be uncovered. Since these signs of elevated sleep pressure appeared in participants with a high ESS score latter than in participants with short SOL, these underpinning can be revealed in the analysis of polysomnographic records obtained throughout the whole 1st sleep cycle. This comes as no surprise. Similar pattern was revealed for recovery night sleep in the night sleep deprivation experiments. Similar to the ESS-based EDS, it is difficult to recognize the polysomnographic signs of high sleep pressure in the beginning of sleep. The best indicator of elevated sleep pressure is the builds up of delta power during deep sleep (i.e., N3) to a significantly higher level during a recovery sleep compared to the baseline night sleep [39, 40]. Moreover, it remains unknown whether there is a reliable EEG indicator of such a buildup of sleep pressure during the preceding wakefulness.
Our survey and nap studies pointed at relatively high prevalence of EDS determined with the ESS in students of this university. However, it is unlikely that most of these students are pathologically sleepy individuals. The results of the survey pointed at chronic sleep deprivation on weekdays as a significant contributor to an elevated ESS score. These results are in line with findings previously reported for other populations. For instance, self-reported frequency of not getting enough sleep was found to be most important predictor of ESS score (e.g., 10.3–13.9% of the variance in score) [7]. Therefore, although the studies suggested that individual variation in ESS score showed the expected impact of genetic component (i.e., [41]), the results of questionnaire studies indicated that an ESS score > 10 observed on elevated level in the population of university students seems to reflect, to the most extent, the insufficiency of sleep on weekdays rather than an abnormally high prevalence of predisposition to pathological ESD in this population. It seems that high rates of diagnosis of EDS obtained from self-scorings can be trusted not only due to similarity of the results of nap study and survey, but also due to the results on physiological indexes pointing at the elevated sleep pressure in afternoon naps of study participants with an ESS score > 11.
There are several limitations of this study. Due to a relatively small sample size of nap study, its results require replication. Moreover, using a sample of participants denying the episodes of sleep disorders does not allow the generalization of our results to the clinical populations with EDS. Finally, the limitations also include the absence of any objective information on health and previous sleep episodes of the participants of our studies.
5 Conclusions
In the study of napping attempts of university students, EDS diagnosed as an ESS score > 11 was validated against objective sleep indexes obtained on 10-min intervals of naps. We showed that the disconnect between the ESS and SOL can be explained by the difference between the EDS participants with a high ESS score and with a very short SOL in timing in appearance of the signs of elevated sleep pressure. Since these signs appeared later in the participants with a high ESS score, most of these signs cannot be recognized in polysomnographic records obtained during short napping attempts. High rates of the ESS-based EDS in population of university students can be trusted, because they seem to be chronically sleep deprived during the days of attending classes and because they demonstrated the signs of elevated sleep pressure during afternoon nap.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
T.L. Baker, Introduction to sleep and sleep disorders. Med. Clin. N. Am. 69, 1123–1152 (1985)
C. Metzner, A. Schilling, M. Traxdorf, H. Schulze, P. Krauss, Sleep as a random walk: a super-statistical analysis of EEG data across sleep stages. Commun. Biol. 4(1), 1385 (2021). https://doi.org/10.1038/s42003-021-02912-6
E. Olbrich, P. Achermann, T. Wennekers, The sleeping brain as a complex system. Philos. Trans. A Math. Phys. Eng. Sci. 2011(369), 3697–3707 (1952). https://doi.org/10.1098/rsta.2011.0199
J.M. Mullington, C.A. Czeisler, N. Goel, J.M. Krueger, T.J. Balkin, M. Johns, P.J. Shaw, Panel discussion: current status of measuring sleepiness. J. Clin. Sleep Med. 7, S22-25 (2011)
J. Axelsson, M. Ingre, G. Kecklund, M. Lekander, K.P. Wright, T. Sundelin, Sleepiness as motivation: a potential mechanism for how sleep deprivation affects behavior. Sleep (2019). https://doi.org/10.1093/sleep/zsz291
T. Shochat, N. Santhi, P. Herer, D.J. Dijk, A.C. Skeldon, Sleepiness is a signal to go to bed: data and model simulations. Sleep 44(10), zsab123 (2021). https://doi.org/10.1093/sleep/zsab123
A.A. Putilov, O.G. Donskaya, Calibration of an objective alertness scale. Int. J. Psychophysiol. 94(1), 69–75 (2014). https://doi.org/10.1016/j.ijpsycho.2014.07.010
R. Cluydts, E. De Valck, E. Verstraeten, P. Theys, Daytime sleepiness and its evaluation. Sleep Med. Rev. 6, 83–96 (2002)
D.F. Dinges, Sleep debt and scientific evidence. Sleep 27, 1050–1052 (2004)
J. Horne, Is there a sleep debt? Sleep 27, 1047–1049 (2004)
J.A. Horne, C.V. Burley, We know when we are sleepy: subjective versus objective measurements of moderate sleepiness in healthy adults. Biol Psychol. 83, 266–268 (2010)
P.K. Hitchcott, D. Menicucci, S. Frumento, A. Zaccaro, A. Gemignani, The neurophysiological basis of excessive daytime sleepiness: suggestions of an altered state of consciousness. Sleep Breath. 24, 15–23 (2020)
A.J. Campbell, A.M. Neill, D.A.R. Scott, Clinical reproducibility of the Epworth Sleepiness Scale for patients with suspected sleep apnea. J. Clin. Sleep Med. 14(5), 791–795 (2018). https://doi.org/10.5664/jcsm.7108
M.W. Johns, A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545 (1991)
A. van der Heide, M.K. van Schie, G.J. Lammers, Y. Dauvilliers, I. Arnulf, G. Mayer, C.L. Bassetti, C.L. Ding, P. Lehert, J.G. van Dijk, Comparing treatment effect measurements in narcolepsy: The Sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 38(7), 1051–1058 (2015). https://doi.org/10.5665/sleep.4810
V.P. Martin, R. Lopez, Y. Dauvilliers, J.-L. Rouas, P. Philip, J.-A. Micoulaud-Franchi, Sleepiness in adults: an umbrella review of a complex construct. Sleep Med. Rev. 67, 101718 (2023). https://doi.org/10.1016/j.smrv.2022.101718
D.J. Buysse, M.L. Hall, P.J. Strollo, T.W. Kamarck, J. Owens, L. Lee, S.E. Reis, K.A. Matthews, Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J. Clin. Sleep Med. 4(6), 563–571 (2008)
R. Lok, J.M. Zeitzer, Physiological correlates of the Epworth Sleepiness Scale reveal different dimensions of daytime sleepiness. Sleep Adv. 2(1), zpab008 (2021). https://doi.org/10.1093/sleepadvances/zpab008
R.N. Aurora, B. Caffo, C. Crainiceanu, N.M. Punjabi, Correlating subjective and objective sleepiness: revisiting the association using survival analysis. Sleep 34, 1707–1714 (2011)
S.R. Benbadis, E. Mascha, M.C. Perry, B.R. Wolgamuth, L.A. Smolley, D.S. Dinner, Association between the Epworth sleepiness scale and the multiple sleep latency test in a clinical population. Ann. Intern. Med. 130(4 Pt 1), 289–292 (1999)
R.D. Chervin, M.S. Aldrich, The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 52, 125–131 (1999)
M.W. Johns, Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standard. J. Sleep Res. 9, 5–11 (2000)
R. Mehra, L. Wang, N. Andrews, W.H.W. Tang, J.B. Young, S. Javaheri, N. Foldvary-Schaefer, Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J. Clin. Sleep Med. 13(12), 1411–1422 (2017)
L.G. Olson, M.F. Cole, A. Ambrogetti, Correlations among Epworth Sleepiness Scale scores, multiple sleep latency tests and psychological symptoms. J. Sleep Res. 7, 248–253 (1998)
O. Munkhjargal, Y. Oka, S. Tanno, H. Shimizu, Y. Fujino, T. Kira, A. Ooe, M. Eguchi, T. Higaki, Discrepancy between subjective and objective sleepiness in adolescents. Sleep Med. 96, 1–7 (2022). https://doi.org/10.1016/j.sleep.2022.04.025
V.B. Dorokhov, A.I. Taranov, A.M. Narbut, D.S. Sakharov, S.S. Gruzdeva, O.N. Tkachenko, G.N. Arsen’ev, I.S. Blochin, A.A. Putilov, Effects of exposure to a weak extremely low frequency electromagnetic field on daytime sleep architecture and length. Sleep Med. Res. 10, 97–102 (2019)
V.B. Dorokhov, D.S. Sveshnikov, A.N. Puchkova, A.O. Taranov, D.S. Sakharov, S.S. Gruzdeva, O.N. Tkachenko, G.N. Arsen’ev, N.V. Ligun, V.V. Dementienko, Z.B. Bakaeva, E.B. Yakunina, Y.P. Starshinov, V.I. Torshin, O.G. Donskaya, A.A. Putilov, Differential relationship of two measures of sleepiness with the drives for sleep and wake. Sleep Breath 25, 2179–2187 (2021)
Iber C, Ancoli-Israel S, Chesson A, & Quan SF, eds. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, 1st ed. Westchester, IL: American Academy of Sleep Medicine, 2007.
M. Frigo, S.G. Johnson, The design and implementation of FFTW3. Proc. IEEE 93, 216–231 (2005)
T. Roenneberg, A. Wirz-Justice, M. Merrow, Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms 18, 80–90 (2003)
D.J. Buysse, C.F. Reynolds, T.H. Monk, S.R. Berman, D.J. Kupfer, The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989)
N. Marcoen, M. Vandekerckhove, D. Neu, N. Pattyn, O. Mairesse, Individual differences in subjective circadian flexibility. Chronobiol. Int. 32, 1246–1253 (2015)
A.A. Putilov, V.B. Dorokhov, M.G. Poluektov, Evening chronotype, late weekend sleep times and social jetlag as possible causes of sleep curtailment after maintaining perennial DST: ain’t they as black as they are painted? Chronobiol. Int. 37, 82–100 (2020)
A.A. Putilov, E.G. Verevkin, Simulation of the ontogeny of social jet lag: a shift in just one of the parameters of a model of sleep-wake regulating process accounts for the delay of sleep phase across adolescence. Front. Physiol. 9(1529), 1–11 (2018). https://doi.org/10.3389/fphys.2018.01529
T.B. Kendzerska, P.M. Smith, R. Brignardello-Petersen, R.S. Leung, G.A. Tomlinson, Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med. Rev. 18, 321–331 (2014)
O. Mairesse, D. Neu, Tired of blunt tools? Sharpening the clinical assessment of fatigue and sleepiness. Psychiatry Res. 238, 100–108 (2016)
T. Åkerstedt, M. Gillberg, Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 52, 29–37 (1990)
T.H. Monk, A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 27, 89–99 (1989)
A.A. Borbély, F. Baumann, D. Brandeis, I. Strauch, D. Lehmann, Sleep deprivation; effect on sleep stages and EEG power density in man. Electroenceph. Clin. Neurophysiol. 51, 483–493 (1981)
A.A. Borbély, S. Daan, A. Wirz-Justice, T. Deboer, The two-process model of sleep regulation: a reappraisal. J. Sleep Res. 25, 131–143 (2016)
D. Carmelli, D.L. Bliwise, G.E. Swan, T. Reed, A genetic analysis of the Epworth Sleepiness Scale in 1560 World War II male veteran twins in the NAS-NRC Twin Registry. J. Sleep Res. 10(1), 53–58 (2001). https://doi.org/10.1046/j.1365-2869.2001.00241.x
Acknowledgements
The authors are very grateful to Dr. Dmitry S. Sveshnikov, Dr. Zarina V. Bakaeva, Dr. Olga V. Mankaeva, and Prof. Vladimir I. Torshin from the Medical Institute at the Peoples' Friendship University for help in organization of the survey and polysomnographic recordings of their students. The North-Caucasus Federal University provided technical and other similar support to AAP.
Funding
The Russian Science Foundation funded RAE (Grant #22-72-10061).
Author information
Authors and Affiliations
Contributions
Conceptualization AAP; funding acquisition EBY, and VBD; data curation VBD, EBY, ANP, DES, EOG, AOT, ONT, NVL, GNA, AER, AEM, VVD, and AAP, resources AAP, DSS, and VBD; project administration AAP and VBD; supervision AAP; software DES, ANP, and AAP; investigation VBD, ANP, and AAP; methodology AAP, VBD, and ANP; sleep scoring: ANP and EOG; spectra calculation: DES; validation AAP; visualization AAP; writing—review and editing AAP, VBD, EBY, ANP, DES, EOG, AOT, ONT, NVL, GNA, AER, AEM, and VVD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, and the decision to publish the results.
Additional information
Brain Physiology Meets Complex Systems. Guest editors: Thomas Penzel, Teemu Myllylä, Oxana V. Semyachkina-Glushkovskaya, Alexey Pavlov, Anatoly Karavaev.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dorokhov, V.B., Yakunina, E.B., Puchkova, A.N. et al. Can physiological sleepiness underlie consciously perceived sleepiness assessed with the Epworth sleepiness scale?. Eur. Phys. J. Spec. Top. 232, 569–582 (2023). https://doi.org/10.1140/epjs/s11734-023-00771-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-00771-2