Abstract
Purpose
Since disagreement has been found between an objective sleep propensity measured by sleep onset latency (SOL) and subjective sleepiness assessment measured by the Epworth sleepiness scale (ESS) score, distinct underlying causes and consequences were suggested for these two sleepiness measures. We addressed the issue of validation of the ESS against objective sleepiness and sleep indexes by examining the hypothesis that these two sleepiness measures are disconnected due to their differential relationship with the antagonistic drives for sleep and wake.
Methods
The polysomnographic records of 50-min napping attempts were collected from 27 university students on three occasions. Scores on the first and second principal components of the electroencephalographic (EEG) spectrum were calculated to measure the sleep and wake drives, respectively. Self-assessments of subjective sleepiness and sleep were additionally collected in online survey of 633 students at the same university.
Results
An ESS score was disconnected with the polysomnographic and self-assessed SOL in the nap study and online survey, respectively. An ESS score but not SOL was significantly linked to the spectral EEG measure of the sleep drive, while SOL but not ESS showed a significant association with the spectral EEG measure of the opposing wake drive.
Conclusions
Each of two sleepiness measures was validated against objective indicators of the opposing sleep-wake regulating processes, but different underlying causes were identified for two distinct aspects of sleepiness. A stronger sleep drive and a weaker opposing drive for wake seem to contribute to a higher ESS score and to a shorter SOL, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive sleepiness occurs frequently in population and negatively impacts both individuals and society in general. The aversive effects of excessive sleepiness on various aspects of health include disability, morbidity, and mortality. Beyond its health consequences, excessive sleepiness is a major determinant of road traffic accidents and implicated in poor academic and workplace performance [1]. Therefore, the ability to evaluate sleepiness has obvious implications for medicine and safety-critical occupations and procedures [2]. Since objective assessment of sleepiness, such as measurements of sleep propensity with the multiple sleep latency test (MSLT), requires attendance at a sleep laboratory, this methodology is impracticable in this respect. Consequently, standardized questionnaires represent a suitable compromise, with the 8-item Epworth sleepiness scale (ESS) [3] being the most popular [1].
The ESS scores subjective sleepiness defined as the propensity to fall asleep in 8 different daily life situations [3]. However, one of the consistent findings of the studies on validation of the ESS against objective measures of sleepiness is a lack of the expected connection between subjective sleepiness, as measured by the ESS, and objective sleep propensity, as measured by mean sleep onset latency (SOL) on MSLT [4,5,6,7,8,9]. It was concluded that the ESS and SOL could not perfectly correlate one to another because they are measuring related but distinct aspects of sleepiness and they may have distinct underlying causes and consequences [7,8,9,10].
Application of a computerized analysis to recordings of the human electroencephalographic (EEG) signal provides a possibility to associate certain quantitative changes in spectral composition of the EEG with changes in the underlying processes of sleep regulation [11, 12]. The classic example is the conceptualization of the brain activity in delta range of the EEG spectrum (1–4 Hz) as an objective marker of the homeostatic process of sleep regulation. It was suggested that the power density in this frequency range of the EEG spectrum can serve as a marker of the strength of the drive for sleep [13]. We previously concluded that the brain response to sleep loss might affect a much wider range of frequencies of the EEG signal instead of affecting just one or two of several traditionally recognized frequency ranges (delta, theta, alpha, sigma, etc.). Consequently, a methodology was developed for calculation of objective (spectral EEG) sleepiness score that accounts for the contribution of single-Hz powers of four 4-Hz frequency rangers (1–16 Hz) to the response of the sleepy brain to sleep deprivation [14].
Moreover, we suggested that the conceptualization of delta activity as a marker of sleep drive does not take into account a possible influence of the opposing wake drive on the spectral powers in this frequency range [15, 16]. The EEG changes in this and other frequency ranges might reflect the underlying influence (sometimes in the opposite directions) of, at least, two antagonistic sleep-wake regulating processes, the drives for sleep and wake. Scores on the first and second principal components of the EEG spectrum were shown to reflect the influence of sleep and wake drive, respectively, and the calculation of these scores allows the separation of the contributions of these two antagonistic drives to each of single-Hz powers [15,16,17].
To our knowledge, nobody has tested yet the associations of the ESS score and SOL with such spectral EEG markers of sleepiness and of drives for sleep and wake. Significance of the associations for the ESS might be regarded as evidence for its validity against objective indexes of sleep-wake regulatory processes. Moreover, such associations might allow the consideration of the drives for sleep and wake as the underpinnings of sleepiness measured with the ESS and SOL. Given that the ESS and SOL seem to measure distinct aspects of sleepiness and that they may have different underlying causes and consequences (e.g., [7,8,9,10]), we hypothesized a differential contribution of the antagonistic drives for sleep and wake to these two distinct aspects of sleepiness as a possible explanation for the disconnect between the ESS and SOL.
Consequently, the following possibilities were tested:
-
1. A self-reported assessment of sleepiness, ESS score, might be related to the strength of sleep dive or to the strength of wake drive or to both.
-
2. An objective measure of sleep propensity, the SOL, obtained in three afternoon naps might be related to the sleep drive strength or to the wake drive strength or to both.
-
3. The disconnect between such sleepiness measures as ESS and SOL might be explained by their differential relationship with the sleep and wake drives as well as with an objective (spectral EEG) sleepiness score.
Methods
Participants of nap study
Volunteers of the present nap study were 15 male and 12 female students of the Medical Institute at the Peoples’ Friendship University of Russia (Moscow). They were invited to participate in the study by their lecturers and were included in the study after the structured interview when denied history of mental or sleep disorder, pregnancy or breastfeeding, poor physical condition and functioning, current mild colds, missing classes due to any sickness in the previous two weeks, involvement in shift or night work or crossing several time zones during the previous month, irregular sleep-wake schedule (i.e., more than 1-h difference in bedtimes on weekdays), or frequent sleep deprivation (i.e., at least, 2 days of, at least, partial sleep deprivation in the previous 7 days). Other exclusion criteria were ages, either younger than 18 or older than 22 years.
Nap study protocol and polysomnographic recordings
The participants visited the sleep laboratory three times for 50-min napping attempt. The intervals between these three attempts varied from 3 days to 1 month. Each visit lasted for less than 2 hours (between 1 p.m. and 3 p.m.). Prior to the first napping attempt, the 8-item Epworth sleepiness scale (ESS) [3] was administered during a pre-nap interview (see Supplementary for more details on this questionnaire).
The electrodes for polysomnographic recordings were applied when a participant was lying in bed in the sleep laboratory under dim light (approximately 10 lux). He/she was instructed to try to nap after light off for the following 50 min. The polysomnographic recordings were performed via a 16-channel wireless system (“Neuropolygraph 24,” Neurotech, Taganrog, Russia). A standard monitoring montage was used for polysomnographic recordings (i.e., 13 EEG channels, one chin electromyogram channel and two electrooculogram channels). All electrodes were placed in accord with the International 10–20 system of electrode placement. The recorded EEG signals were conditioned by the high-pass, low-pass, and notch filters (frequencies of 0.5, 35, and 50 Hz, respectively). The signals were sampled and stored on a hard disc with a frequency of 500 Hz.
Initially, conventional scoring procedure [18] was performed visually and independently by two experienced scorers on 30-sec epochs of each 50-min record. Thereafter, they reexamined together all intervals with discrepant scores in order to produce consensus scores. The epochs were classified into stages including wakefulness stage (W), REM (rapid eye movement) sleep (R), and three stages of NREM (non-REM) sleep (stage 1 sleep or N1, stage 2 sleep or N2, and slow-wave sleep or N3). Latencies to N1 (SOL) and N2 (stage N2 onset latency) were determined (Tables 1, 2, and S1).
Analysis of the EEG signal
The EEG signals from electrodes placed at 5 derivations (Fz, F4, Cz, Pz, and O2 referenced to the ear mastoid sites, M1/M2) were used for calculating the EEG power density spectra (Fig. 1b, c, and e). The EEG records were visually inspected on 1-s epochs for removing all epochs containing artifacts from further analysis. The FFTW (fastest Fourier transform in the West) package [19] was applied to compute power spectra densities for the artifact-free epochs (see www.fftw.org for more detail). Hamming window taper was used on 1-s epochs to calculate absolute spectral power densities (μV2) for each of the first 16 single-Hz frequency bandwidth (i.e., 0.50–1.49, 1.50–2.49, 2.50–3.49, ..., 15.50–16.49 Hz). These 16 single-Hz power densities were averaged on each of 30-s intervals of EEG records (i.e., the interval of stage scoring) and ln-transformed. The number of averaged 1-s epochs ranged between 5 and 30. For statistical analyses, the individual ln-transformed powers were further averaged, e.g., over derivations (5 in total), over 10-min intervals of the records (5 in total), over the first 5 1-min intervals (Fig. 1e and 1f), over the whole 16-Hz range (Fig. 2d), and over 4 4-Hz frequency ranges, delta, theta, alpha, and sigma (Fig. 2e and 2f).
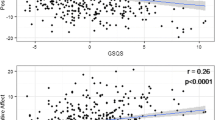
Loading spectra and correlation spectra. a Loading spectra; the two largest (first and second) principal components were extracted by means of principal component analysis; (b–f) correlation spectra; correlations of power spectra with two sleepiness measures (ESS score and SOL averaged over three naps); ρ Spearman’s rank correlation coefficient
Comparison of subsamples with low and high sleepiness on single spectral EEG indexes. See statistical results of rANOVAs in Table 2.
Using these 16 ln-transformed single-Hz power densities (Fig. 1), three single spectral EEG scores were calculated: objective (spectral EEG) sleepiness score (Fig. 2a) and scores on the first and second principal components (Fig. 2b and 2c, respectively). To obtain such a score, these 16 power densities were weighted and summed:
where pi is a power density for a i-th frequency bin and wi is a weight for this i-th frequency bin (i = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, and 16 Hz). Sixteen weights for calculating objective (spectral EEG) sleepiness score (1) were taken from the previous publication [14]: −0.195, 0.006, 0.217, 0.335, 0.165, 0.237, 0.049, −0.117, −0.439, −0.738, −0.216, −0.166, −0.166, −0.129, −0.239, and −0.038, respectively (Fig. 2a). We previously demonstrated that the correlation coefficient between the time course of such an objective (spectral EEG) score and the time course of the 9-step Karolinska sleepiness scale [20] score attained the value of 0.98 [14]. The SPSS23.0 statistical software package (IBM, Armonk, NY, USA) was used for calculations of principal component scores (Fig. 2b and 2c) and for testing significance of the association of sleepiness measures with other variables (Tables 1, 2, and S2). Figure 1a illustrates 16 weights obtained with the SPSS software for the calculation of scores on the first and second principal components (1) by means of principal component analysis of spectral powers from each of 5 deviations (Fig. 2b and c, respectively).
Online survey of a larger sample
Self-assessments of sleep and subjective sleepiness, including ESS and SOL, were additionally collected in online survey of 633 students at the same university. The methods and results of this questionnaire study are described in the Supplementary.
Statistical analysis
The Spearman’s rank correlation coefficient (ρ) was applied to evaluate the associations between sleepiness measures and other variables (Tables 1, S1, and S2). The significance of main effect of independent factor “sleepiness” (a high or low ESS score or a short or long SOL) on a sequence of measurements (e.g., spectral EEG scores) was tested with repeated measure ANOVAs (rANOVAs). A repeated measure (see Fig. 2) was “minute” (5 10-min or the first 5 1-min intervals) and/or “derivation” (Fz, F4, Cz, Pz, and O2) and/or “nap” (the first, second, and third). An additional repeated measure (see Fig. 1) was “single-Hz” (16 spectral powers from 1 to 16 Hz). Mauchly’s test was conducted to assess the sphericity and, if necessary, the Greenhouse-Geisser correction was used to adjust the degrees of freedom, but the original degrees of freedom are reported in Table 2.
Results
Relationships between sleepiness measures in nap study
Mean age ± standard deviation (SD) for 27 study participants (15 males and 12 females) was 19.33 ± 1.33. Mean self-reported durations of night sleep prior to the first, second, and third visit to the laboratory ± SD were 6.03 ± 2.45, 6.12 ± 2.27, and 5.75 ± 2.66 hours, respectively. Mean ESS score ± SD was 8.33 ± 3.27, and mean SOL ± SD were 9.72 ± 9.74, 11.54 ± 12.67, and 13.07 ± 14.79 min in the first, second, and third napping attempts, respectively. Self-reported excessive sleepiness was defined by an ESS score above 10, and objectively measured excessive sleepiness was defined as a nap-averaged SOL below 8 minutes. The criteria for excessive sleepiness (either subjective, ESS score < 11, or objective, SOL > 8 min) were not met by only 10 of 27 students (6 of 15 males and 4 of 12 females). There were 5 excessively sleepy students (2 males and 3 females) meeting both criteria. Eight students (3 males and 5 females) met the first criterion for excessive sleepiness and 14 students (8 males and 6 females) met the second criterion (i.e., near one third and more than a half of study participants, respectively). The correlation between ESS and SOL was nonsignificant (highlighted in Table 1, the first line).
Since SOL is a measure of sleep propensity linked to the transitional state of falling asleep (i.e., between wake and sleep states, W and N1), whereas sleepiness is a substate of wake state preceding such a transitional state, we also tested the association of an objective (spectral EEG) sleepiness score with other sleepiness measures, a ESS score and SOL in each of three napping attempts (Tables 1 and S2). The results pointed at the expected close association of an objective sleepiness score with SOL in any of naps, but not with an ESS score (Fig. 1e and f and Tables 1, 2, S1, and S2).
Relationships of sleepiness measures with spectral EEG indexes
Figure 1 illustrates a close similarity between the loading spectra of the first and second principal components (Fig. 1a) and the patterns of correlations of spectral powers with the ESS score and SOL, respectively (Fig. 1b–d). Therefore, the scores on the first principal component were the correlates of an ESS score but not SOL, while the scores on second principal component were the correlates of a SOL in any of the napping attempts but not ESS (Table 1 and S2). Since frequency in a separate range (delta or theta or alpha or sigma) might contribute to both scores (sometimes in the opposite directions), they were also found to be the correlates of the ESS and SOL (Fig. 1 and Tables 1 and S2). Similarly, such correlates were also found among those sleep stages that are characterized by a predominance of these powers (Tables 1 and S1). All these results of correlation analysis were fully confirmed by the results of rANOVAs of the subgroups of study participants with high and low sleepiness (Fig. 2 and Table 2).
In overall, the result indicated that the wake drive represented by such its marker as the second principal component score underlies an objective (spectral EEG) measure of sleepiness and SOL, while, in contrast, the sleep drive represented by such its marker as the first principal component score underlies the subjective measure of sleepiness, the ESS score (Figs. 1a and 2 and Tables 1, 2, S1, and S2). Importantly, such a clear differential relationship to two sleepiness measures, the ESS score and SOL, was found only for these two markers of the sleep and wake drives. The associations shown by the power densities in conventional frequency ranges of the EEG spectrum were more complex because they can be associated with both ESS and SOL. For instance, although higher delta power was associated with a higher ESS score, it was additionally associated with shorter SOL (Tables 1 and S2). Similarly, the significant main effects of both ESS and SOL were found for alpha power (Table 2). The associations of the ESS and SOL were even more overlapping in the case of any conventional sleep stage, such as the amounts of wake after sleep onset and N3 (Table 1).
Relationships between sleepiness and sleep assessments in online survey
The results of online survey of 633 students supported the results of the nap study in that an ESS score showed significant association with none of three SOL, in spite of a much larger size of the sample of this online survey as compared to the sample of the nap study (Tables S3–S6).
When the excessive sleepiness was self-reported (i.e., an ESS score > 10), the students also suffered from early morning wakeups on weekdays. For instance, their larger sleep loss was evidenced from a larger difference in sleep offset on free and weekdays. In contrast, when excessive sleepiness was indicated by the self-reported nighttime SOL of 10 min or less, these students were also characterized by an earlier circadian timing of their sleep-wake cycle (Tables S3–S6). Therefore, the survey results further supported the result of the nap study suggesting that the two measures of sleepiness are linked to different external and underlying causes of excessive sleepiness.
Discussion
Due to our ability to perceive sleepiness, we can detect and report excessive sleepiness easily and quickly, e.g., by means of a questionnaire, such as the ESS. However, the attempts to validate this scale against objective sleepiness measures, such as SOL, were unsuccessful. Here, we validated self-reports of excessive sleepiness against objective sleep and sleepiness indexes and tested the hypothesis that the disconnect between the ESS and SOL can be explained by their differential relationship with the antagonistic drives for sleep and wake. The following possibilities were supported by the study results:
-
1. Such a measure of subjective sleepiness as ESS score was significantly related to the EEG markers of the sleep dive strength but not to the EEG markers of the wake dive strength.
-
2. Such an objective measure of sleep propensity as SOL in three afternoon naps was significantly related to the EEG markers of the wake drive strength but not to the EEG markers of the sleep dive strength.
-
3. The disconnect between the ESS and SOL might be explained by such differential relationship with the sleep and wake drives. Moreover, we also found that only SOL was significantly related to an objective (spectral EEG) sleepiness score that, in turn, was significantly related to the EEG markers of the wake drive strength but not to the EEG markers of the sleep dive strength.
The analyses of the associations of power densities in conventional frequency ranges (e.g., delta and alpha) also provided significant results on their link to both measures of sleepiness, the ESS and SOL. However, it has to be emphasized that such links were not as strong and as clearly differential as were the links uncovered by the analysis of the relationships of the ESS and SIOL with scores on two principal components of the EEG spectrum representing the antagonistic drives for sleep and wake.
Such results are in line with the previous research in a general theoretic framework suggesting that the transitions between different substates of the sleep-wake continuum are governed by complex neurobiological mechanisms that, ultimately, might be delineated as alternations between opposing processes, i.e., those promoting arousal and inhibiting sleep and those promoting sleep and inhibiting arousal (e.g., [21, 22]). In particular, although spectral power density in delta range is regarded the classical marker of the sleep drive [13], the present and previous findings supported an interpretation of this index as representing mutual influence of the drives for wake and sleep, i.e., an increase/decrease of power density in delta rage might be explained by either a weakening/strengthening of the former or a strengthening/weakening of the latter or both [15,16,17].
Definitely, these findings should be considered exploratory. In practical terms, the results of the study, if replicated, might be applied for the development of the procedures for verification of self-reports of sleepiness by simple measurement of two spectral EEG markers of the sleep and wake drives in afternoon naps. People would not only prove their subjective perception of excessive sleepiness but also benefit from this nap regarded a potent behavioral strategy minimizing sleepiness, fatigue, and impairments of cognitive and physical functioning [23].
Conclusions
We examined whether an ESS score and SOL might be linked to such two distinct sleep-wake regulatory processes as the drives for sleep and wake. Each of these two sleepiness measures was validated against objective indicators of the opposing sleep-wake regulating processes. Different underlying causes were identified for two distinct aspects of sleepiness. When a stronger sleep drive might be responsible for a higher ESS score, a weaker opposing drive for wake might underlie a shorter SOL.
Availability of data and material
On reasonable request to the corresponding author.
References
Hitchcott PK, Menicucci D, Frumento S, Zaccaro A, Gemignani A (2020) The neurophysiological basis of excessive daytime sleepiness: suggestions of an altered state of consciousness. Sleep Breath 24:15–23
Horne JA, Burley CV (2010) We know when we are sleepy: subjective versus objective measurements of moderate sleepiness in healthy adults. Biol Psychol 83:266–268
Johns MW (1991) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14:540–545
Aurora RN, Caffo B, Crainiceanu C, Punjabi NM (2011) Correlating subjective and objective sleepiness: revisiting the association using survival analysis. Sleep 34(12):1707–1714
Benbadis SR, Mascha E, Perry MC, Wolgamuth BR, Smolley LA, Dinner DS (1999) Association between the Epworth sleepiness scale and the multiple sleep latency test in a clinical population. Ann Intern Med 130(4 Pt 1):289–292
Chervin RD, Aldrich MS (1999) The Epworth sleepiness scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 52:125–131
Johns MW (2000) Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res 9:5–11
Mehra R, Wang L, Andrews N, Tang WHW, Young JB, Javaheri S, Foldvary-Schaefer N (2017) Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 13(12):1411–1422
Olson LG, Cole MF, Ambrogetti A (1998) Correlations among Epworth Sleepiness Scale scores, multiple sleep latency tests and psychological symptoms. J Sleep Res 7(4):248–253
Trotti LM (2017) Characterizing sleepiness: are we drawing the right line in the sand? J Clin Sleep Med 13(12):1369–1370
Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D (1981) Sleep deprivation; effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 51:483–493
Dijk DJ, Beersma DGM, Daan S (1987) EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythm 2:207–219
Borbély AA (1982) A two process model of sleep regulation. Hum Neurobiol 1:195–204
Putilov AA, Donskaya OG, Verevkin EG (2019) Can we feel like being neither alert nor sleepy? The electroencephalographic signature of this subjective sub-state of wake state yields an accurate measure of objective sleepiness level. Int J Psychophysiol 135:33–43
Putilov AA (2011) Principal components of electroencephalographic spectrum as markers of opponent processes underlying ultradian sleep cycles. Chronobiol Int 28(4):287–299
Putilov AA, Münch MY, Cajochen C (2013) Principal component structuring of the non-REM sleep EEG spectrum in older adults yields age-related changes in the sleep and wake drives. Cur Aging Sci 6(3):280–293
Putilov AA (2015) Rapid changes in scores on principal components of the EEG spectrum do not occur in the course of “drowsy” sleep of varying length. Clin EEG Neurosci 46:147–152
Iber C, Ancoli-Israel S, Chesson A, Quan SF (eds) (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, 1st edn. American Academy of Sleep Medicine, Westchester
Frigo M, Johnson SG (2005) The design and implementation of FFTW3. Proc IEEE 93:216–231
Åkerstedt T, Gillberg M (1981) The circadian variation of experimentally displaced sleep. Sleep 4:159–169
Boutrel B, Koob GF (2004) What keeps us awake: the neuropharmacology of stimulants and wake-promoting medications. Sleep 27:1181–1194
Saper CB, Chou TC, Scammell TE (2001) The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 24:726–731
Milner CE, Cote KA (2009) Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res 18:272–281
Acknowledgments
The authors are very grateful to Ekaterina V. Tiunova and Irina A. Piletskaya (the Moscow State Pedagogical University, Moscow, Russia) for their help in conducting the nap study and to the unpaid volunteers for their participation in the nap study and online survey.
Funding
This study was funded by the Russian Foundation for Basic Research (VBD, grant number 20-013-00683; ANP, grant number 19-013-00747; DSS, grant number 20-013-00603; ONT, grant number 19-29-06071; and OGD and AAP, grant number19-013-00424).
Author information
Authors and Affiliations
Contributions
VBD designed and VBD, DSS, AIT, SSG, ONT, GNA, and ZBB performed the nap study; AAP and DSS designed and AAP, DSS, ANP, ZBB, EBY, YPS, and VIT performed the survey. VBD, AAP, DSS, OGD, AIT, SSG, ONT, GNA, ZBB, ANP, EBY, YPS, and VIT equally contributed to the analysis of data, and AAP wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in the studies were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocols of the studies were approved by the Ethics Committees of the Institutes (#2 from 03.06.2019 and #5 from 15.12.2019).
Informed consent
Each participant of the experimental study was informed in detail about all procedures and gave his/her written consent. In the questionnaire study, informed consent was obtained from each individual participant by choosing the answer “yes” prior to the first survey question.
Additional information
Comments
This article is focused on the development of easy performed and effective approach for evaluation of sleep quality using the ESS and SOL. Authors demonstrate that the ESS score reflects individual variation in the strength of sleep drive, while SOL is more informative for assessment of opposing drive for wake. The results might be applied for the development of technology for verification of self-reports of sleepiness by simple measurement of two spectral EEG markers of the sleep and wake drives in afternoon naps. People would not only prove their subjective perception of excessive sleepiness but also benefit from this nap regarded a potent behavioral strategy minimizing sleepiness, fatigue, and impairments of cognitive and physical functioning.
Oxana Glushkovskaya
Saratov, Russian Federation
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 54 kb)
Rights and permissions
About this article
Cite this article
Dorokhov, V.B., Sveshnikov, D.S., Puchkova, A.N. et al. Differential relationship of two measures of sleepiness with the drives for sleep and wake. Sleep Breath 25, 2179–2187 (2021). https://doi.org/10.1007/s11325-020-02269-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02269-w