Abstract
The use of portable oxygen concentrators for preparing an air-based breathing gas can be a good alternative to that of pure oxygen from cylinders in emergency relief. Membrane modules can be used as a gas separation device. In this study, the compressor–vacuum scheme of a portable membrane oxygen concentrator has been analyzed and examined and its choice has been substantiated. An experimental sample with the characteristics of the target stream that meets the requirements of medicine—a flow rate of 6 L/min and an oxygen concentration of 50 vol %—has been developed and tested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In medical practice, therapies using a breathing gas (BG) are widely used. Medical oxygen (MO) is the most significant, being especially important in emergency and urgent forms of assistance in emergency situations or field hospitals [1].
The regulations, in accordance with which medical personnel operate to provide emergency assistance to patients, prescribe the use of BG with different MO contents, depending on the condition of the patient [2, 3]. Moreover, a relative oxygen concentration of no more than 50 vol % in the breathing gas is sufficient in many cases [14].
Recent experience in the use of artificial lung ventilation (ALV) in world medical practice has shown that the invasive method of BG delivery to the lungs has many disadvantages [5], especially in extreme conditions. In this regard, anesthesiologists/resuscitators are developing alternative methods, for example, high-flow nasal oxygenation [6].
Its main differences from mechanical ventilation methods are the delivery of BG through nasal cannulas in a volume exceeding the peak inspiratory flow rate. At the same time, the use of nasal cannulas provides comfortable conditions for the respiratory support of patients [7].
In devices for breathing gas preparation, medical gas cylinders with compressed oxygen (at least 99.5 vol %) are mainly used as an MO source [1]. From a safety point of view, their storage and use is not an optimal option, since MO is generally inapplicable in such a concentration [2].
An alternative option is the on-site preparation of breathing gas from air using oxygen concentrators, which, as a rule, are based on adsorption or membrane technology.
Adsorption oxygen generators are widely represented in the patent and scientific literature [8, 9]. They are also undisputed leaders in the medical equipment market. The main advantage of such devices is the ability to obtain a breathing gas with an oxygen concentration of up to 95 vol %.
Despite all the advantages of adsorption technology as applied to portable oxygen generators, devices based on it have a number of disadvantages: the technological cycle developed by Skarstrom [10] for obtaining oxygen-enriched air implies cyclic flow switching, which can be performed only with the use of automatic valves and an electronic control system. Thus, the need for service and careful handling of the device in home use are not significant disadvantages, but when they become critical and play a decisive role in the choice of technology in the case of using in extreme conditions.
Another disadvantage associated with the operating cycle of adsorption generators is the batch withdrawal of the oxygen-enriched stream, so that a BG accumulating receiver is installed to mitigate this problem.
In view of both the focus on high-flow nasal oxygenation and the disadvantages of adsorption generators, membrane technology for producing an oxygen-enriched gas stream and devices based on it have great potential.
First, membrane devices are characterized by high reliability and simplicity of the hardware design. The breathing gas parameters are regulated with one or two control valves, which can be adjusted both in manual and automatic modes.
Second, the design of the membrane device allows for a continuous flow of oxygen-enriched air. Therefore, there is no need to install a receiver. This means that the flow parameters (concentration) are adjustable in real-time mode.
Third, the specific energy consumption of the production of air enriched with oxygen up to 50–55 vol % for membrane units with relatively low productivity is lower in comparison with adsorption devices [11].
Thus, the use of membrane technology in oxygen generators has the greatest promise in the creation of portable devices for providing emergency care with an oxygen concentration of up to 50 vol %.
The aim of this work is to determine the feasibility of using membrane technology to obtain an oxygen-enriched stream to care in extreme conditions.
THEORETICAL
The membrane technology of air enrichment in oxygen is based on the difference in the rate of transport of air components (nitrogen and oxygen) through the membrane. The classical model of gas transfer through a nonporous membrane is the phenomenological solution–diffusion model [12–16], according to which the coefficient of gas permeability (Λ) through the membrane is determined by the product of the diffusion (D) and sorption (σ) coefficients:
The main characteristic of membranes from the point of view of their gas separation properties is the separation factor (α), which shows how many times permeation of one component through the membrane is better than that of the other and is equal to the ratio of the permeability coefficients of the corresponding components of the gas mixture.
The volumetric gas flow rate through the membrane is directly proportional to the mass transfer driving force, which is equal to the differential partial pressure (Δp) of the gas on both sides of the membrane:
where δ is the thickness of the selective membrane layer.
The ideal membrane separation factor reported in the literature usually differs from the actual separation factor in the module. The corrections are related to the design features of the membrane module, surface defects of the selective layer, and other factors. A more informative value of the separation factor (α) for the module is calculated through the ratio of the flow rates of the components of the feed gas mixture:
In the practice of gas separation, the hollow-fiber design of membrane modules is optimal because of a number of their advantages over other types (plate-and-frame, spiral-wound): high specific membrane area (membrane working area per unit volume of the apparatus, m2/m3), which makes it possible to ensure minimal device dimensions, and low unit costs for their manufacture.
The calculations of separation processes in membrane modules were carried out on the basis of the equations set forth in [17], in a mode close to the cross-flow mode, which is due to the type of both the membranes used (asymmetric hollow fibers) and the design of the module based on them.
EQUIPMENT AND METHODS
In this study, a commercial hollow-fiber membrane module from Ube was investigated and used as a gas separation module, which has relatively small mass and size (Table 1).
The gas separation characteristics of the membrane module were measured on a laboratory bench by determining the flow rate of the gas fed to the pressure channel in a dead-end mode and passed through the membrane. The permeabilities of oxygen and nitrogen were determined. The oxygen flow rate was measured using an LZM-6T gas flow meter. The nitrogen flow rate was measured with an SMC PFM7 flow sensor.
RESULTS AND DISCUSSION
The first step in the design of a portable membrane concentrator is to determine the gas separation characteristics of the membrane module. We have determined the characteristics at various driving forces. The results are shown in Table 2.
Separation factor of the selected membrane module is 5. This value is taken as the separation factor in the system calculations.
Next, it is necessary to choose a process flow diagram.
The driving force of separation in the device can be provided by a compressor, a vacuum pump, or both of them simultaneously. The limiting parameters in designing the concentrator are the power consumption of the device and its dimensions. Since the rate of gas flow through unit membrane area depends on the difference in the partial pressures of the mixture components upstream and downstream of the membrane, it is obvious that the compressor scheme provides a large driving force, and, hence, the flux through the membrane.
On the other hand, the vacuum scheme is more energy efficient, since the main work is done only on the permeate stream, the volume of which is less. However, the required membrane area in a vacuum system is significantly higher compared to the compressor one. In addition, in vacuum schemes, there is a need for additional equipment (air blower, compressor) to create a flow in the space upstream of the membrane. This disadvantage, together with higher capital costs, determines the choice in favor of compressor or compressor–vacuum schemes.
The operating parameters (with unchanged gas separation characteristics of the membrane module), which determine the attainable oxygen concentration in the permeate stream, are the pressure ratio in the feed (p1) and purge channels (p2) (γ = p1/p2) and the separation factor. Assuming that the separation factor of the selected membrane is constant, let us compare various compressor and compressor–vacuum schemes.
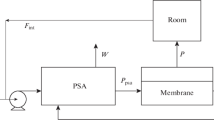
A block diagram of a single-stage membrane unit is shown in Fig. 1.
Diagram of a single-stage membrane unit with permeate recycle, where P1 is the pressure in the feed channel, P2 is the pressure in the permeate channel, C is the oxygen concentration, and Q is the volumetric flow rate; subscripts: 0—feed air, F—stream at the module inlet, P—permeate stream, W—retentate stream, R—recycle stream.
By recycling a part of flow QP back to the membrane module inlet, it is possible to increase the oxygen concentration in the stream fed to the module for separation (QF) and, thereby, to increase the driving force of the process and the oxygen concentration in the QP stream.
The values of CP, CF, and the flow split ratio θ = QP/QF are related to each other by the parameters of the membrane itself (α) and the conditions of the separation process (γ = p1/p2). Using the procedure for calculating membrane modules from [18], we show the relationship between the oxygen concentration in the permeate stream (CP) and the split ratio (θ) at a fixed value of the oxygen concentration at the inlet to the membrane module (CF). To do this, we plot the dependences (Fig. 2) of the oxygen concentration in the permeate stream on the split ratio in the membrane module at various oxygen concentrations at the membrane module inlet.
We define the relationship between CP and θ as follows:
This expression was further used in the calculations of the selected separation schemes.
The calculation (Fig. 2) proves once again that the Ube membrane module we have chosen can be used in the design of a concentrator with a target value of the oxygen concentration of 50 vol % in the permeate stream, but only in the case of an increase in the oxygen concentration in the feed stream by recycling.
At low values of the split ratio (at which it is possible to reach 50 vol % oxygen in the permeate), the single-stage scheme is not optimal because of the high required recycle ratio.
It is possible to reduce the recycle flow rate by using a two-stage scheme (Fig. 3).
As applied to the problem in question, such a scheme has the same drawback as the single-stage one: it is necessary to use two compressors (or a compressor and a vacuum pump) in one device. In addition to this, the use of a vacuum scheme at one of the stages (two stages) will require a significant area in comparison with the compressor scheme; therefore, a two-stage scheme is inappropriate for solving this problem.
An optimal scheme for solving this problem is a two-stage membrane process with a recycling the second-stage permeate to the compressor inlet (Fig. 4) [19].
The feed air passes sequentially along the surface of the membranes in the feed channel of the first and second membrane modules, being depleted in oxygen as the easily permeable component, thereby making it possible to achieve an oxygen-enriched stream in QR and \({{Q}_{{{{P}_{1}}}}}\) flows. The oxygen concentration \(\left( {{{C}_{{{{F}_{1}}}}}} \right)\) in the \({{Q}_{{{{F}_{1}}}}}\) stream is determined by mixing the air stream with the oxygen-enriched QR stream. Since the oxygen concentration \(\left( {{{C}_{{{{W}_{2}}}}}} \right)\) in the retentate stream of the second membrane module \(\left( {{{Q}_{{{{W}_{2}}}}}} \right)\) can be arbitrarily small, the separation mode of the first membrane module is chosen such that the oxygen concentration in its permeate stream \(\left( {{{C}_{{{{P}_{1}}}}}} \right)\) reaches the level required by the conditions of the problem (50 vol %). Due to the right choice of the split ratio and the area of the second membrane module, it is possible to achieve a high recovery and the required oxygen concentration in the QP stream at an insufficiently high separation factor of the existing membrane.
Let us prove mathematically the optimality of using two separation stages. Note that in the case of QP1 = 0, the scheme transforms into a one-stage scheme with recycle; therefore, all the equations will be written for the two-stage scheme.
Material balance equation for the first module:
If QP1/QF1 = θ1, then
wherein
Material balance equation for the second membrane module:
where QP2/QF2 = θ2.
Note that
Balance equation for flow at the compressor inlet:
where θ0 = Q0/QF1, θ = Q/QF1.
Balance equation for flow at the vacuum pump inlet:
Balance equation for the entire unit:
Concentrations, flows, and parameters of membrane modules can be calculated as follows:
Choosing a value for θ1 and an initial approximation for CF1;
Calculating the value of CP1 and CW1 through the function CP1 = f(θ1, CF1 = const) and material balance Eqs. (5) and (6);
Choosing a value for θ2;
Calculating the value of CP2 and CW2 through the function CP2 = f(θ2, CF2 = const) and material balance Eqs. (9) and (10);
Calculating the value of Q by the formula
If CP2 is less than 0.5 (the concentration specified by the conditions of the problem), the problem has no solution with the given parameters.
We calculate Q0 by the formula
We calculate CF1 by the equation, which becomes a new approximation
The calculation continues until the required accuracy is achieved with the given parameters θ1 and θ2 and the desired concentration.
When moving from theoretical calculations and estimates to practical implementation, it is necessary to focus not only on mathematical relationships, but also on the technical characteristics of available equipment. So, for example, compressor equipment is selected not only in terms of capacity and operating pressure, but also in terms of dimensions, weight, and tightness of the inlet chamber (in the case of using the recycle scheme); commercial membrane modules have a membrane area specified by the manufacturer, which cannot be changed during the design of the device.
The calculation according to the algorithm described above has shown that for the selected membrane module at a pressure in the feed channel of p1 = 6 atm, a pressure in the purge channel of p2 = 0.6 atm, am oxygen concentration in the product stream of 50 vol %, and a flow rate of 6 L/min, the split ratio of the first membrane module (θ1) can take values of 0.1–0.2 and that of the second membrane module (θ2) will range within 0.4–0.6, depending on θ1 (Fig. 5).
The prototype of the device was made on the basis of the diagram in Fig. 4. The concentrator operates as follows. The air flow from the atmosphere is fed by the compressor to the first stage of membrane separation. At the compressor inlet, an oxygen-enriched second-stage stream is mixed with air to increase the oxygen concentration and, hence, the driving force of the separation process. After separation in the first stage, the oxygen-enriched stream is supplied to the consumer by a vacuum pump. The gas stream that has not passed through the membrane of the first stage is fed to the second stage. One part of the oxygen-enriched stream after separation in the second stage is sent to the compressor inlet, and the other part is mixed into the first-stage permeate stream to achieve the productivity (6 L/min).
The design of the prototype makes it possible to vary the concentration/productivity with a control valve without changing the operating pressure in the system.
The prototype has been tested. By adjusting the split ratios within the calculated values, we managed to achieve an oxygen concentration of 52 vol % in the product stream. The flow rate of the gas compressed by the compressor was 24 L/min. The retentate stream of the second membrane module had the following characteristics: the flow rate of 11 L/min and the oxygen concentration of 7.5 vol %.
It can be seen from the results obtained that the two-stage compressor–vacuum scheme with recycle allows obtaining the required flow rate and oxygen concentration, which exceeds 50 vol %.
The device prototype weighing 9.5 kg includes a filter; a dryer’ and concentration, pressure, and temperature sensors; and shut-off valves in addition to the membrane modules, the compressor, and the vacuum pump. The total power consumption of the prototype is 350 W/h at a productivity of 6 L/min.
CONCLUSIONS
The available samples of membrane modules have insufficient gas separation characteristics for one-stage production of a stream with an oxygen concentration of 50 vol %. The required performance was achieved by dividing the process into two stages and organizing a recycle stream enriched in oxygen.
A mathematical model of a two-stage membrane unit and a calculation algorithm are presented. Numerical studies have been carried out and the ranges of flow split ratios at two stages are shown, which make it possible to achieve the required characteristics of the product stream.
The experimental prototype assembled according to the developed scheme and adjusted within the calculated values of the split ratios showed the required characteristics of the product stream. The concentrator itself has a relatively small weight and dimensions, which make it portable, and its low power consumption allows the use of rechargeable batteries as a power source.
The development of a number of related areas, such as the production of gas separation membranes and modules based on them, the production of compressor machinery, will make it possible to more efficiently solve the problem of oxygen recovery from it using membrane technology and will render this area more competitive. However, there are already specific problems of oxygen production that can be effectively solved using existing gas separation membranes.
REFERENCES
Yu. V. Miroshnichenko, R. A. Enikeeva, and E. M. Kassu, Vest. Ross. Voen.-Med. Akad. 2, 157 (2016).
Yu. V. Miroshnichenko, et al., Vest. Ross. Voen.-Med. Akad. 1, 203 (2016).
E. I. Sakanyan, et al., Vest. Ross. Voen.-Med. Akad. 3, 162 (2015).
T. S. Omarov, et al., Mezhdunar. Nauch.-Issled. Zhurn, 4, 82 (2019).
V. I. Zagrekov, I. Yu. Aref’ev, and A. P. Frolov, Med. Al’manakh 2, 63 (2020).
A. S. Svistov and S. B. Onikienko, Morsk. Med. 6, 100 (2020).
G. P. Plotnikov, et al., Vysokotekhnol. Med. 6, 12 (2019).
V. V. Shappo, I. B. Ushakov, A. T. Logunov, and V. I. Grishin, RF Patent No. 83924 (2009).
R. A. Enikeeva, Izvest. Ross. Voen.-Med. Akad. 36, 81 (2017).
C. W. Skarstrom, Patent, No. 2944627 (1960).
W. J. Koros and G. K. Fleming, J. Membr. Sci. 83, 1 (1993).
Yu. I. Dytnerskii, V. P. Brykov, and G. G. Kagramanov, Membrane Gas Separation (Khimiya, 1991) [in Russian].
S. T. Hwang, Korean J. Chem. Eng. 28, 1 (2011).
L. M. Robeson, et al., J. Membr. Sci. 453, 71 (2014).
G. G. Kagramanov and E. N. Farnosova, Theor. Found. Chem. Eng. 51, 38 (2017).
R. W. Baker, Membranes for Vapor/Gas Separation (Membrane Technology and Research Inc., 2006).
Y. Shindo, et al., Separ. Sci. Technol. 20, 445 (1985).
G. G. Kagramanov, V. N. Gurkin, and E. N. Farnosova, Membr. Membr. Technol. 2, 244 (2020).
V. K. Ezhov, et al., Teor. Osnovy Khim. Tekhnol. 20, 10 (1986).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Gurkin, V.N., Kagramanov, G.G., Loiko, A.V. et al. Development of a Portable Membrane Oxygen Concentrator. Membr. Membr. Technol. 3, 186–191 (2021). https://doi.org/10.1134/S2517751621030045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751621030045