Abstract
The transport properties of ion-exchange membranes depend on many factors, primarily, on ion-exchange capacity and chemical composition. Therefore, the correlation of transport properties with a single particular parameter is generally not quite strict. In this study, the dependence of transport properties of several perfluorinated sulfonated cation-exchange membranes that differ in side chain length and fraction of fragments containing ether groups on ion-exchange capacity is analyzed. It is shown that, with a decrease in the ion-exchange capacity from 1.35 to 0.66 mg-equiv/g, the proton conductivity of membranes contacting water and their diffusion permeability with respect to a 0.1 M HCl solution decrease by two orders of magnitude. A decrease in relative humidity leads to the most significant decrease in the conductivity of membranes with a low ion-exchange capacity. Thus, at a relative humidity of 32%, the conductivity of the studied membranes decreases more than 600-fold with a decrease in ion-exchange capacity. In general, the oxygen permeability of membranes is characterized by a similar dependence. However, in this set of membranes, it varies as little as threefold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Ion-exchange membranes are used for several practical applications, the most common of which are water treatment and concentrating, food purification, and a number of other technologies associated with separation processes based on ion transfer [1–4]. The membrane selectivity in these processes is primarily determined by the ratio of transfer rates of ions with different signs or charges [5–8]. In most technologies, relatively cheap heterogeneous membranes are most commonly used. It should be noted that, owing to the synthesis method, heterogeneous membranes are characterized by a bimodal pore size distribution and a low selectivity [9–11]. A higher selectivity is exhibited by homogeneous ion-exchange membranes, in particular, perfluorinated sulfonated cation-exchange membranes used in the energy sector, primarily, in fuel cells. In this case, negative processes are primarily associated with the through transport of neutral molecules of a fuel (hydrogen, methanol) or an oxidizer (oxygen) across the membrane, which does not lead to energy generation (crossover) [12].
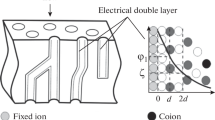
Despite the fundamental differences in the nature of transport processes for coions and neutral molecules, their transfer generally occurs via similar routes. Since the pore walls of cation-exchange membranes have a negative charge, which is determined by the localization of \({\text{-SO}}_{3}^{ - }\) anions on them, a Debye layer is formed near pore walls; most of the cations are localized within this layer [13, 14]. Conversely, the so-called electrically neutral solution is localized in the middle of pores; the composition of this solution is close to that of the solution in contact with the membrane. In the case of using membranes in fuel cells, the composition of this solution is close to that of clean water. It is this solution through which most coions and neutrally charged nonpolar molecules or low-polarity molecules are transferred [13]. Therefore, the mechanisms of their transfer should be similar, and the selectivity values, which are determined from coion transfer and crossover, should correlate with each other.
However, it stands to reason that the selectivity of transport processes is not the only characteristic that determines the properties of membranes. An equally important parameter is ionic conductivity. It should be noted that there is a certain correlation for ion-exchange membranes: the higher the conductivity, the lower the selectivity, and vice versa [15–17]. It is obvious that this correlation is purely statistical. The problem is that the transport properties of ion-exchange membranes depend on many factors, primarily, ion-exchange capacity (IEC) and chemical composition [18–23]. Therefore, their dependence on only one of the parameters is generally not very strict. In addition, to increase the selectivity or conductivity of membranes, various approaches are commonly used; they include bulk or surface modification of membranes [24–28], crosslinking [29–31], and other approaches that also affect the transport process rate [32–34].
In view of the above, the correlation of transport properties and IEC becomes more evident for membranes of a similar nature, for example, in a set of grafted materials with different ratios of the original and grafted polymer [35, 36]. For materials with similar structures, the value of these parameters is primarily determined by the structure of pores and channels. Typically, the higher the concentration of electricity carriers, cations, determined by functional group concentration, the higher the membrane conductivity. At the same time, with an increase in the counterion concentration in the membranes, their water uptake and, consequently, pore size increases [37–39]. In this case, the charge density on the pore walls and the Debye layer thickness vary only slightly; the pore volume increases primarily owing to an increase in the concentration of the electrically neutral solution through which undesirable transport processes occur [40]. Therefore, with an increase in the IEC, the membrane conductivity should increase, while the selectivity should decrease. In this context, it is of interest to study the dependence of ionic conductivity and selectivity of membrane samples having similar structures and different concentrations of functional groups. Perfluorinated sulfonated cation-exchange membranes, which are in high demand in modern alternative power engineering, are of the most interest [41].
The aim of this study was a comparative study of the transport properties (conductivity, diffusion permeability, and gas permeability) of several perfluorinated sulfonated cation-exchange membranes of the Nafion and Aquivion type characterized by different IECs, side chain lengths, and fractions of fragments containing ether groups. It should be noted that the description of the dependence of the proton conductivity of perfluorinated membranes on the IEC varied in a narrower range at room temperature is given in [19, 42].
EXPERIMENTAL
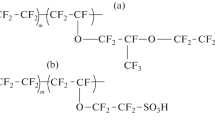
The properties of several perfluorinated sulfonated cation-exchange (PFSA) membranes of Nafion (long side chain, five carbon atoms in the side chain) and Aquivion type (short side chain, two carbon atoms in the side chain) manufactured by various companies were studied; the IEC and water uptake of the membranes vary over a wide range, (Fig. 1, Table 1).
To standardize the experimental conditions, all studied membranes were conditioned in accordance with a standard procedure, which included the sequential exposure to a 3% aqueous solution of H2O2 and a 3% aqueous solution of HCl at 80°С for 2 h at each stage and then to two runs of exposure to large amounts of deionized water at 80°С.
Infrared (IR) spectra were recorded in the attenuated total reflectance mode on a Nicolet iS5 Fourier transform IR spectrometer in a range of 520–4000 cm–1. Prior to recording, the membranes were held at RH = 95%.
The water uptake of the membranes was determined on a Netzsch TG 209 F1 thermal balance in a temperature range of 25–200°C at a heating rate of 10 K/min. The samples were either withdrawn from water and wiped with filter paper immediately before experiments or held in a desiccator at RH = 95%. The IEC of the samples was determined by direct titration. The IEC values were calculated per weight of the anhydrous sample. A ~0.3-g weighed portion of the membrane in the dry state was held in 50 mL of a 0.1 M NaCl solution under constant stirring for 12 h. After that, the salt solution and the membrane were titrated with a 0.01 M NaOH solution.
To study the transport properties of the samples, their ionic conductivity in the hydrogen form, the diffusion permeability with respect to an HCl solution, and oxygen gas permeability were studied. The conductivity of the materials was studied using an Elins Z1500 impedance meter in a frequency range of 10 Hz to 2 MHz in the potentiostatic mode with a sinusoidal signal amplitude of 100 mV in a temperature range of 25–95°С. The studies were conducted both in contact with deionized water and at a controlled humidity. In the latter case, a Binder MKF115 climatic chamber was used (humidity control accuracy of ±2.5%). The electrodes were prepared using graphite paper (surface area of 1 cm2). Ionic conductivity values were found via extrapolating the impedance hodograph to the axis of active resistances.
The diffusion permeability of the samples was measured in a two-compartment cell separated by a membrane along the ion flow generated when the membrane was placed between 0.1 M HCl solutions and deionized water. The pH value was measured using an Econix-Expert-001 pH millivoltmeter. A more detailed description of these experiments can be found in [43].
To measure the gas permeability of the synthesized materials, the sample was fixed in a two-compartment cell between the flows of diffusing oxygen and a carrier gas (argon). The concentration of the passed gas was determined on a Kristallyuks-4000M chromatograph equipped with a thermal conductivity detector (current of 60 mA) and two packed columns (HayeSep T sorbent, 60/80 mesh, 2 m, 150°C, 30 cm3/min, He). The sample was introduced via two heated gas valves with a loop volume of 0.891 and 0.887 mL, respectively, at a temperature of 49°C. The measurements were conducted at an RH of the gases and the membrane of 100% and a temperature of 30°C.
RESULTS AND DISCUSSION
The IR spectra of all the studied membranes are represented by a combination of stretching and bending vibrations of water and vibrations of sulfo groups and the perfluorinated carbon chain; they slightly differ in intensity ratios of individual bands (Fig. 2). This fact confirms that their structures are similar. For membranes with a long side chain, the spectra exhibit a peak splitting in the region of 970 cm–1, which corresponds to the region of the stretching vibrations of the C–O–C groups of the side chains.
Ion-exchange capacity in terms of dry membrane varied almost twofold in a range of 0.66–1.35 mg-equiv/g. This fact makes it possible to observe the dependence of various transport parameters of these membranes on the IEC, which characterizes the concentration of functional groups and charge carriers. The water uptake of the membranes also varies over a wide range: from 16 to 35% in the case of contact of the membranes with water and from 11 to 20% at RH = 95%; it increases in proportion to the increase in the IEC. The water uptake of the membranes also significantly decreases with a decrease in the RH value. Therefore, to characterize the ionic conductivity of the studied membranes, three reference points were selected, namely, in contact with liquid water and at RH = 95 and 32%. Despite the fact that, in the first two cases, the activity of water is almost identical, the water uptake of the membranes changes, on average, is twofold owing to Schroeder’s paradox [44]. This finding is attributed to the fact that some of the ions leave the ion-exchange membrane to pass into the aqueous phase. Therefore, the charge of negatively charged pore walls of the membrane is not fully compensated; this factor leads to the expansion of the pores and an increase in water uptake.
The proton conductivity values of the studied materials in contact with water are quite typical for perfluorinated sulfonated cation-exchange membranes. They increase by two orders of magnitude with an increase in the IEC from 0.66 to 1.35 mmol/g (Fig. 3). Note that the error in the determination of the conductivity of these thin membranes can be up to 8%; this value does not affect the drawn conclusions. In addition, the proton conductivity of the membrane with the highest functional group concentration significantly exceeds the reported conductivity values for these materials. The causes of this dependence are described above: an increase in the IEC leads to an increase in the carrier concentration, pore volume, and water uptake and, along with them, in the conductivity value.
At RH = 95%, this tendency is preserved; however, the lower the IEC, the more pronounced the dependence of conductivity on humidity. Thus, in contact with water, the membrane conductivity changes as little as 100-fold, whereas at RH = 95% it changes 160‑fold (Fig. 4). With a further decrease in RH to 32%, the proton conductivity of the membranes changes more than 600-fold (Fig. 5). In addition, the higher the functional group concentration, the less pronounced the dependence of the conductivity on the IEC; conversely, the maximum decrease in conductivity at a low humidity is achieved for membranes characterized by a minimum IEC (Figs. 3–5). This dependence appears to be quite natural. In fact, the lower the functional group concentration, the smaller the pore size and the larger the distance between the groups. These factors lead to a lower level of pore binding and a violation of percolation for membranes with a low exchange capacity. In addition, owing to an increase in the distance between the functional groups at a low water uptake, the activation energy for proton hopping between them increases.
An important characteristic of ion-exchange membranes is their diffusion permeability. It should be noted that the passage of salts or acids across a cation-exchange membrane requires simultaneous transfer of the cation and anion. Therefore, the salt diffusion rate (permeability) is limited to the transfer of anions, the concentration of which in the pores is significantly lower [45]. Therefore, proton conductivity is determined by proton transfer, whereas diffusion permeability characterizes the undesirable transfer of anions. The ratio of these values can be used to determine the selectivity of transport processes in membranes. It is evident from Fig. 6 that the dependence of the diffusion permeability of perfluorinated sulfonated cation-exchange membranes with respect to an HCl solution on the IEC of the membranes is fairly similar to the analogous dependence for ionic conductivity. Even the value of it for the studied set of membranes changes by almost the same two orders of magnitude (Fig. 6). The analogy in the change in the general tendencies is determined by the increase in the water uptake of the membranes and the size of pores and channels with an increase in the IEC. It is obvious that this factor has a similar effect on all transport processes, although the revealed similarity in the change in the quantities does not appear equally obvious.
In terms of use in fuel cells, another important parameter is gas permeability, which determines the loss of power owing to the transfer of feeding gases across a membrane in the molecular form. In this work, these processes were studied using the example of oxygen permeability of membranes. To standardize the experimental conditions, the water uptake of the membranes was maintained by oxygen saturation via slowly bubbling water vapors through it. In general, the behavior of the recorded dependence of the oxygen permeability of perfluorinated sulfonated cation-exchange membranes on their IEC shows the same tendencies as for the conductivity and HCl diffusion permeability. However, with an increase in the IEC, the last two values for the studied membranes change by two orders of magnitude, whereas the oxygen permeability changes in a significantly narrower range, namely, as little as threefold (Fig. 7). This finding is apparently attributed to the fact that gas permeability across a membrane occurs not only through the system of pores and channels. It is highly probable that this process partly occurs via gas diffusion across the perforated polymer matrix. In fact, the determined values are comparable to the oxygen permeability of many polymer films, in particular, Teflon [46, 47]. However, the presence of a pronounced increasing dependence on the IEC of the membranes suggests that the contribution of oxygen diffusion through the system of pores and channels is also significant. These assumptions are in good agreement with the data of the authors of [48], who studied a “resistor network model” to describe gas diffusion across membranes. In addition, comparison of the data (ratio of proton conductivity and gas permeability) suggests that membranes with a high IEC should exhibit advantages for use in fuel cells.
Comparison of the recorded dependences shows that the transport properties of perfluorinated membranes substantially depend on their IEC. In addition, the dependence of their conductivity on the IEC is most critical at a low humidity. The authors of some reports on studying Aquivion membranes argue that short side chains and the absence of an ester group and tertiary carbon in the side chain provide a better strength of the membranes and an improvement in their transport properties [49]. It has been speculated [42] that this effect is primarily attributed to their lower equivalent weight (this parameter is inversely proportional to the IEC); this assumption is in excellent agreement with the data of this study. At the same time, in this study, it has been speculated that the presence of a short side chain provides a less significant dependence of the membrane conductivity on humidity. The derived data show that, most probably, this effect is also the result of an analogous dependence on the IEC.
CONCLUSIONS
One of the main tasks of membrane science is to increase the transfer rate of the target component, while suppressing undesirable processes. For various fields of application of ion-exchange membranes, this task reduces to increasing the counterion conductivity, while minimizing the coion transport numbers or the amount of transferred gases feeding fuel cells. The dependence of the transport properties of membranes on their IEC is one of the dominant dependences. To minimize the effect of the chemical composition, in this study, the dependence of the transport properties of a number of perfluorinated sulfonated cation-exchange membranes that differ in the fraction of fragments containing ether groups and the number of carbon atoms of the side chains on the IEC has been analyzed. With a decrease in the IEC from 1.35 to 0.66 mg-equiv/g, the proton conductivity of membranes in contact with water and their diffusion permeability with respect to a 0.1 M HCl solution decrease by two orders of magnitude. In addition, with a decrease in the RH of the atmosphere, the conductivity of membranes with a low IEC decreases most significantly. Thus, at RH = 32%, the conductivity of the studied membranes differs more than 600-fold. In general, the oxygen permeability of membranes is characterized by a similar dependence. However, the decrease in the gas permeability with a decrease in the IEC is significantly smaller. This finding shows that membranes with a higher IEC can have substantial advantages for use in fuel cells. These advantages include both a higher ionic conductivity and a much less significant dependence of conductivity on ambient humidity and a higher selectivity, which is evident as a considerably smaller increase in the oxygen permeability of the membranes with an increase in exchange capacity.
REFERENCES
H. Strathmann, A. Grabowski, and G. Eigenberger, Ind. Eng. Chem. Res. 52, 10364 (2013).
J. Ran, L. Wu, Y. He, Z. Yang, and T. Xu, J. Membr. Sci. 522, 267 (2017).
A. Campione, L. Gurreri, M. Ciofalo, G. Micale, A. Tamburini, and A. Cipollina, Desalination 434, 121 (2018).
M. Noguchi, Y. Nakamura, T. Shoji, A. Iizuka, and A. Yamasaki, J. Water Proc. Eng. 23, 299 (2018).
Y. Ji, H. Luo, and G. Geise, J. Membr. Sci. 563, 492 (2018).
F. Roghmans, E. Evdochenko, M. C. Martí-Calatayud, M. Garthe, R. Tiwari, A. Walther, and M. Wessling, J. Membr. Sci. 600, 117854 (2020).
Y. Zhang, L. Wang, W. Sun, Y. Hu, and H. Tang, J. Ind. Eng. Chem. 8125, 7 (2020).
Y. Zhao, Y. Liu, E. Ortega, and B. Van der Bruggen, in Nanocomposite Membranes for Water and Gas Separation Micro and Nano Technologies, Ed. by Sadrzadeh M. and Mohammadi T. (Elsevier Inc., 2020).
N. Kononenko, V. Nikonenko, D. Grande, C. Larchet, L. Dammak, M. Fomenko, and Yu. Volfkovich, Adv. Colloid Interface Sci. 246, 196 (2017).
N. D. Pismenskaya, E. V. Pokhidnia, G. Pourcelly, and V. V. Nikonenko, J. Membr. Sci. 566, 54 (2018).
A. B. Yaroslavtsev, I. A. Stenina, and D. V. Golubenko, Pure Appl. Chem. (2020). https://doi.org/10.1515/pac-2019-1208
M. Ahmed and I. Dincer, Int. J. Energy Res. 35, 1213 (2011).
V. V. Nikonenko, A. B. Yaroslavtsev, and G. Pourcelly, Ion Transfer in and Through Charged Membranes. Structure, Properties, Theory.InIonic Interactions in Natural and Synthetic Macromolecules, Ed. by Ciferri A. and Perico A. (John Wiley & Sons, Inc., Hoboken, New Jersey, 2012).
S. Mondal, I. M. Griffiths, and G. Z. Ramon, J. Membr. Sci. 588, 117166 (2019).
G. M. Geise, M. A. Hickner, and B. E. Logan, ACS Appl. Mater. Interfaces 5, 10294 (2013).
D. H. Cho, K. H. Lee, Y. M. Kim, S. H. Park, W. H. Lee, S. M. Lee, and Y. M. Lee, Chem. Commun. 53, 2323 (2017).
D. V. Golubenko, G. Pourcelly, and A. B. Yaroslavtsev, Sep. Purif. Technol. 207, 329 (2018).
A. Kusoglu, A. M. Karlsson, and M. H. Santare, Polymer 51, 1457 (2010).
E. Moukheiber, G. De Moor, L. Flandin, and C. Bas, J. Membr. Sci. 389, 294 (2012).
S. Shi, A. Z. Weber, and A. Kusoglu, Electrochim. Acta 220, 517 (2016).
S. Shi, A. Z. Weber, and A. Kusoglu, J. Membr. Sci. 516, 123 (2016).
K. Talukdar, P. Gazdzicki, and K. A. Friedrich, J. Power Sources 439, 227078 (2019).
X. Luo, S. Rojas-Carbonell, Y. Yan, and A. Kusoglu, J. Membr. Sci. 598, 117680 (2020).
A. B. Yaroslavtsev and Y. P. Yampolskii, Mendeleev Commun. 24, 319 (2014).
E. Yu. Safronova and A. B. Yaroslavtsev, Petr. Chem. 56, 281 (2016).
K. Oh, O. Kwon, B. Son, D. H. Lee, and S. Shanmugam, J. Membr. Sci. 583, 103 (2019).
M. Rabbani, E. Sadegh, A. Aktij, Z. Dabaghian, M. D. Firouzjaei, A. Rahimpour, J. Eke, I. C. Escobar, M. Abolhassani, L. F. Greenlee, A. R. Esfahani, A. Sadmani, and N. Koutahzadeh, Sep. Purif. Technol. 213, 465 (2019).
M. B. Karimi, F. Mohammadi, and K. Hooshyari, Int. J. Hydrogen Energy 44, 28919 (2019).
M. S. Cha, J. Y. Lee, T.-H. Kim, H. Y. Jeong, H. Y. Shin, S.-G. Oh, and Y. T. Hong, J. Membr. Sci. 530, 73 (2017).
M. M. Hossain, L. Wu, X. Liang, Z. Yang, J. Hou, and T. Xu, J. Power Sources 390, 234 (2018).
T. R. Willson, I. Hamerton, J. R. Varcoe, and R. Bance-Soualhi, Sustain. Energ. Fuels 3, 1682 (2019).
A. D. Khoiruddin and I. G. W. Subagjo, J. Appl. Polym. Sci. 134, 45540 (2017).
E. Yu. Safronova, I. A. Stenina, and A. B. Yaroslavtsev, Petr. Chem. 57, 299 (2017).
S. Pawlowski, J. G. Crespo, and S. Velizarov, Int. J. Mol. Sci. 20, 165 (2019).
M. M. Nasef, S. A. Gursel, D. Karabelli, and O. Guven, Prog. Polym. Sci. 63, 1 (2016).
E. Y. Safronova, D. V. Golubenko, N. V. Shevlyakova, M. G. D’yakova, V. A. Tverskoi, L. Dammak, D. Grande, and A. B. Yaroslavtsev, J. Membr. Sci. 515, 196 (2016).
A. Vishnyakov and A. V. Neimark, J. Phys. Chem. 118, 11353.
N. A. Kononenko, M. A. Fomenko, and Yu. M. Volfko-vich, Adv. Colloid Interface Sci. 222, 425 (2015).
R. Hammer, M. Schonhoff, and M. R. Hansen, J. Phys. Chem. 123, 313.
P. Yu. Apel, O. V. Bobreshova, A. V. Volkov, V. V. Volkov, V. V. Nikonenko, I. A. Stenina, A. N. Filippov, Yu. P. Yampolskii, and A. B. Yaroslavtsev, Membr. Membr. Technol. 1, 45 (2019).
K. A. Mauritz and R. B. Moore, Chem. Rev. 104, 4535 (2004).
E. Yu. Safronova, A. K. Osipov, and A. B. Yaroslavtsev, Petr. Chem. 58, 130 (2018).
P. A. Yurova, I. A. Stenina, A. B. Yaroslavtsev, Pet. Chem. 58, 1144 (2018).
V. I. Roldugin, Kolloidn. Zh. 78, 783 (2016).
I. A. Stenina, E. Yu. Voropaeva, A. A. Ilyina, and A. B. Yaroslavtsev, Polym. Adv. Technol. 20, 566 (2009).
L. M. Robeson, J. Membr. Sci. 320, 390 (2008).
B. Comesana-Gandara, J. Chen, C. G. Bezzu, M. Carta, I. Rose, M.-C. Ferrari, E. Esposito, A. Fuoco, J. C. Jansen, and N. B. McKeown, Energ. Environ. Sci. 12, 2733 (2019).
M. Schalenbach, T. Hoefner, J. T. Gostick, W. Lueke, and D. Stolten, J. Phys. Chem. 119, 25156.
J. Li, M. Pan, and H. Tang, RSC Adv. 4, 3944 (2014).
Funding
The study of the transport properties of membranes was supported by the Russian Science Foundation (project no. 17-79-30054). Membrane characterization was performed under a state task to the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences in the field of basic research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Prikhno, I.A., Safronova, E.Y., Stenina, I.A. et al. Dependence of the Transport Properties of Perfluorinated Sulfonated Cation-Exchange Membranes on Ion-Exchange Capacity. Membr. Membr. Technol. 2, 265–271 (2020). https://doi.org/10.1134/S2517751620040095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751620040095