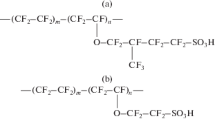Abstract
The free solvent transport number in an MF-4SK perfluorinated membrane in solutions of alkaline metal chlorides and hydrochloric acid is for the first time calculated within the framework of a capillary model based on the data of standard contact porosimetry and membrane conductometry. The reasons for the change in the structural characteristics and specific conductivity upon varying the nature of the counterion are discussed. The portion of through mesopores in MF-4SK homogeneous and MK-40 heterogeneous sulfonated cation-exchange membranes is estimated using the experimental data on the water transport numbers in solutions of electrolytes of different natures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, increasing attention is paid to the problems of creation of closed water cycles and development of zero liquid discharge (ZLD) technologies that include both the traditional concentration methods of waste water such as evaporation of the solution and reverse osmosis and dynamically developing membrane methods, e.g., membrane distillation, forward osmosis, and electrodialysis [1], which find their application in the composition of hybrid and multistage units [2, 3]. The wide use of electrodialysis for the concentration of solutions of electrolytes is first of all limited by the prime cost of the technology, the application of which is justified in the case of extraction of valuable components. In addition, special requirements are imposed to the membranes for electrodialysis. In the case of processing of solutions with complex compositions, the key role in the efficiency of the process belongs to the specific selectivity of the membranes relative to a specific kind of ions. For obtaining concentrates from solutions of individual substances, the main negative factor is the solvent transport through ion-exchange membranes. The solvent flow is composed of electroosmotic and osmotic flows, and the influence of the latter is negligibly small [4–6]. A dynamic ion hydration number [7, 8] or a water transport number [9] which includes bound water present in the composition of the primary and secondary hydrate shells as well as free water transported by the pumping mechanism [10, 11] is used as the quantitative characteristic of the electroosmotic transport through a membrane.
Most works present the results of determination of the electroosmotic solvent transport for a membrane pair directly from the experiments on electrodialysis [5]. In such a case, the measured value of the water transport number or dynamic hydration number is a sum of the individual characteristics of the membranes on the assumption that the coion transport number tends to zero, and there is no electroosmotic flow with the coion. At the same time, the determination of the electroosmotic characteristics for individual membranes opens up a possibility of optimizing the structure of an individual membrane at the stage of its preparation as well as predicting the characteristics of an electrodialysis apparatus upon its development. However, in this case, the water transport with the coion is also generally neglected. The experimental determination of the electroosmotic permeability of individual materials consists in the determination of the volume of water (height of the liquid level, weight, etc.) transported upon the passage of a certain quantity of electricity through the system [12, 13]. The specified experiments are labor-intensive, because of which the theoretical calculation of the electroosmotic permeability of individual ion-exchange membranes is important [14–19]. For this, an effective tool is nonequilibrium thermodynamics that describes the interrelation between flows and forces inducing them via the parameters of a continuous conducting medium [14–16]. However, the consideration of ion-exchange membranes as microinhomogeneous materials makes it possible to find out the peculiarities of the influence of structural organization on the transport properties of membranes [18, 19], which opens up a possibility to a directed variation of the properties of the material. In addition, it is well known that the structure of a membrane has a determining influence on the value of the electroosmotic flow.
The formation of pores and channels in an ion-exchange membrane is determined by the hydration of ionogenic groups, in which connection the model description of their geometry is challenging. One of the common methods of consideration of the complex geometry of a system is the use of a pore tortuosity factor that has been for the first time proposed in [20]. In such a case, a swelled membrane can be represented in the form of a system of through capillaries, some of which are dead-ended (inaccessible for transport) and branched. Thus, the water transport numbers in ion-exchange membranes can be calculated with the use of a capillary model based on simpler experimental data. The key role of the structural type of the membrane was earlier shown upon describing the water transport with a sodium ion in solutions of sodium chloride with a concentration above 1 M within the framework of a capillary model, which made it possible to find out the interrelation between the pore tortuosity and specific water content of the polymer [21]. Also, the effect of the nature of an alkaline metal cation on the electroosmotic transport in an MK-40 heterogeneous cation-exchange membrane was studied with the use of a capillary model. This opened up a possibility of calculating the water transport number based on the known value of specific water content. However, the interrelation between the value of the tortuosity factor and nature of the polymer matrix and electrolyte has not been fully studied so far.
The aim of this work was the study of the influence of the nature of a cation on the electroosmotic free solvent transport in MF-4SK and MK-40 cation-exchange membranes by way of example of alkaline metal ions and a proton with the use of a capillary model. The goal of the work included the investigation of the influence of the structural type of the membrane and nature of the counterion on the effective pore radius distribution of water and specific conductivity as well as the comparison of the results of the calculation of free solvent transport numbers within the framework of a capillary model with the experimental data.
THEORY
The structure of a swelled membrane can be presented as an isoporous capillary system that is schematically depicted in Fig. 1.
It can be assumed that electroosmotic transport is predominantly implemented in mesopores because the radius of micropores is small, while macropores are structural defects. In such pores filled with a solution with a concentration above 1 M, the thickness of the electrical double layer (EDL) and Debye length are much smaller than the curvature radius of a pore, and the electroosmotic flow can be calculated using the Helmholtz–Smoluchowski equation
where veo is the linear electroosmotic velocity of the solvent, l is the length of the pore equal to the thickness of the membrane, η is the dynamic viscosity of the solution, ζ is the electrokinetic potential, Δφ is the difference of potentials on the membrane, and ε and \({{\varepsilon }_{0}}\) are the dielectric permeabilities of water and vacuum of 81 and 8.85 × 10−12 F/m, respectively. The potential in the Helmholtz plane can be calculated based on the Stern theory for a flat EDL by the equation
where \(q\) is the current density at the internal interfacial surface, \({{\varphi }_{1}}\) is the electrostatic potential in the Helmholtz plane, d is the coordinate of the Helmholtz plane, C is the concentration of the electrolyte in depth of the solution, \(A = \sqrt {2\varepsilon {{\varepsilon }_{0}}RT} ,\) F is the Faraday number, R is the universal gas constant, and T is the absolute temperature. It is taken that the distance d is equal to a sum of the diameter of water molecules (0.28 nm) and radius of the counterion and T = 293 K.
The value of the electrokinetic potential \(\zeta \) that is a potential in a point of the diffusion part of the EDL spaced away from the Helmholtz plane at a distance equal to the radius of a hydrated ion which is further used for the calculation of the solvent transport number is calculated within the framework of the Gouy–Chapman theory for the diffusion part by the formula
where \(\xi = \frac{d}{{\sqrt {\frac{{RT\varepsilon {{\varepsilon }_{0}}}}{{2{{F}^{2}}c}}} }},\) \(y = \frac{{F\zeta }}{{RT}},\) and \({{y}_{1}} = \frac{{F{{\varphi }_{1}}}}{{RT}}\) are dimensionless parameters and d is the coordinate of the slip plane numerically equal to a sum of the diameter of a water molecule and radius of the counterion.
Assuming that the water transport number tw is composed of the free water transport number βw and primary ion hydration number h,
and βw can be calculated using Eq. (1) by the formula [21]
where κm is the specific conductivity of the membranes; θ is the proportionality coefficient characterizing the fraction of pores accessible for transport, i.e., directed along the transport axis taking into account their tortuosity as is shown in Fig. 1; ΔV is the volume of water present in the mesopores; ρm is the density of the swelled membrane; and Cw is the number of moles of water in 1 L. The charge density at the internal interfacial surface q under the condition of its uniform distribution throughout the volume of the material can be calculated by the equation [22]
based on the values of the ion-exchange capacity of the membrane Q and specific internal surface area S.
EXPERIMENTAL
Russian-made commercial sulfonated cation-exchange membranes with different polymer matrices, namely, an MK-40 heterogeneous membrane (OAO Shchekinoazot, Shchekino) and an MF-4SK homogeneous perfluorinated membrane (OOO Plastpolimer, St. Petersburg), were used as the study objects.
Prior to the study, the membranes were subjected to chemical conditioning (oxidative thermal pretreatment for MF-4SK and salt pretreatment for MK-40) and then successively transformed to the ionic form under study. Prior to measuring the transport characteristics, the samples were equilibrated with the working solutions of LiCl, NaCl, KCl, CsCl, or HCl; prior to studying the porous structure, the membranes were washed out with distilled water with the control of its resistance.
The electroosmotic permeability of the membranes which was used for comparison with the results of the calculation by a capillary model and estimation of the value of the parameter characterizing the fraction of the pores accessible for transport taking into account their tortuosity was determined by a volumetric method in a two-chamber cell with polarizing silver/silver chloride electrodes and horizontally positioned measuring capillaries [23]. The water transport number that is the number of moles of water transported through the membrane upon the passage of 1F of electricity was calculated by the formula
where V is the volume of transported water, i is the current density, Vw is the molar volume of water, Sm is the area of the membrane, and τ is the time.
The value of specific conductivity of the membranes was determined based on their active resistance measured by an alternating current mercury contact method by the formula
where l is the thickness of the membrane and Rm is the measured resistance.
The distribution of water with respect to the bond energies and pore radii was determined by standard contact porosimetry described in detail in [24]. The specific internal surface area and volume of water in the mesopores were calculated by the formulae presented in [24] based on the experimentally obtained porosimetry curves.
All the experiments were performed at 25°C, the error of the experiments did not exceed 5%.
RESULTS AND DISCUSSION
To calculate the electroosmotic permeability of the membranes within the framework of a capillary model, information about the structural characteristics of the membranes in different ionic forms and data on the specific conductivity in solutions of alkaline metal chlorides and hydrochloric acid is required. Table 1 presents the main physicochemical characteristics of the studied cations in an aqueous solution: primary hydration number h, crystallographic radius rcr and radius in the hydrated state rs, and mobility λ0.
Structural Characteristics of Membranes in Form of Alkaline Metal Cations and a Proton
The results of the experimental study of the distribution of water with respect to the bond energies and effective pore radii in an MF-4SK membrane in different ionic forms are presented in Fig. 2a. For comparison, the same figure presents the porosimetry curve for an MF-4SK membrane in the H+ form. Similar curves for an MK-40 heterogeneous membrane in the form of alkaline metal cations have been adopted from [29] and are supplemented with the curve for the H+ form (Fig. 2b).
From the porosimetry curves, the total volume of the pores filled with water V0 was determined and the specific internal surface area S, distance between the fixed ions L, and charge density at the internal interfacial surface \(q\) were calculated. The obtained structural characteristics of the membranes are presented in Table 2. The table also presents the values of the specific water content of the membranes nm calculated from the data of standard contact porosimetry taking into account the exchange capacity of the membranes.
As is seen from Table 2, the parameter V0 is almost twofold higher for the heterogeneous membrane than for the homogeneous membrane. Analyzing the influence of the nature of the counterion on the characteristics of the porous structure of the membranes, it can be noted that an expected decrease in the total porosity is observed for both membranes in the series of alkaline metals upon the transition from the Li+ form to the Cs+ form, which is associated with the reduction of the hydrate shell of the counterion. For an MK-40 membrane, the transition from the Li+ form to the K+ form is also accompanied by a decrease in the specific internal surface area and distance between the fixed ions; however, there is no such regularity for an MF-4SK membrane. The high water content of the perfluorinated membrane in the H+ form stands out, which was also noted by the authors of [30]. It has been shown in [31] that the swelling of a perfluorinated membrane in some alcohols decreases in the series H+ > Li+ > Na+ > K+ ≈ Cs+.
As is seen from Table 2, the charge density at the internal interfacial surface of an MK-40 heterogeneous membrane varies in a range of 0.44 up to 0.56 C/m2 for different ionic forms, and for an MF-4SK membrane, from 0.48 up to 0.51 C/m2. The obtained values of the parameter q were used for the calculation of tw through the membranes within the framework of a capillary model.
Conducting Properties of Membranes in Solutions of Alkaline Metal Chlorides and Hydrochloric Acid
The concentration dependences of the specific conductivity of MF-4SK and MK-40 cation-exchange membranes in solutions of LiCl, NaCl, KCl, CsCl, and HCl in a range of concentrations of 0.1–3 M which are presented in Fig. 3 were studied for the calculation of βw.
As is seen from Fig. 3b, for an MK-40 heterogeneous membrane, the conductivity in the series of studied counterions decreases in the series H+ > K+ > Na+ > Li+ and correlates with the values of the mobility of these ions in the solutions of electrolytes presented in Table 1. At the same time, in the case of an MF-4SK perfluorinated membrane, the order of the concentration dependences is not in agreement with the series of mobility of the cations in a solution presented in Table 1. As is seen from Fig. 3a, the conductivity of an MF-4SK membrane decreases in the series H+ > Na+ > Li+ > K+ > Cs+ at the concentration of the solutions of electrolytes above 0.5 M. A similar effect was earlier observed by the authors of [32, 33]. The work [34] also noted an abnormally high conductivity of homogeneous membranes in solutions of lithium chloride, and it was shown that a lithium ion occupies a position between hydrogen and sodium ions with respect to the ionic conductivity. In the authors’ opinion, the transport of lithium cation through the channels of a homogeneous membrane is facilitated due to the large hydrate shell and lower energy of interaction of the counterion with the fixed group. At the same time, less hydrated potassium and cesium ions are characterized by higher values of the energy of activation of conductivity, which results in lower conductivity of an MF-4SK membrane in the form of potassium and cesium cations.
The fact that the transport in homogeneous membranes is mainly executed through micro- and mesopores, while the ion transport in heterogeneous membranes occurs through the macropores filled with the equilibrium solution as well, may also be not unimportant.
Calculation of Dynamic Hydrate Characteristics of Alkaline Metal Ions and Proton in Membrane
To estimate βw in an MF-4SK ion-exchange membrane, the dependences of φ1 and ζ on the concentration of the solutions of the studied electrolytes were calculated taking into account that the coordinates of the Helmholtz plane and slip plane in the case of H+, Li+, Na+, K+, and Cs+ ions are 0.334, 0.34, 0.375, 0.413, and 0.445 nm, respectively. These values were obtained by summing up the radius of a water molecule of 0.28 nm and crystallographic radius of the ion rcr. In addition, the values of the internal specific surface area and charge density at the internal interfacial surface presented in Table 2 were used for the calculation.
Figure 4 shows the concentration dependences of the electrokinetic potentials for the studied membranes in solutions of LiCl, NaCl, KCl, CsCl, and HCl. As is seen from the figure, a sharp drop in the electrokinetic potential is observed with the increase in the concentration of the solution of the electrolyte in a range of 0 up to 0.5 mol/L. This is determined by the decrease in the thickness of the EDL in the case of an increase in the concentration of the equilibrium solution. Upon further increasing the concentration of the solutions of electrolytes, the values of the potentials reach a plateau. Analyzing the obtained dependences, we see that, in the case of the membrane in the Li+ form, the value of the electrokinetic potential will be somewhat higher in comparison with the membranes in the K+ or Cs+ form. This is explained by the fact that a Li+ ion has the smallest crystallographic radius in comparison with other singly charged alkaline metal ions, which allow it nearer approaching the pore wall. It should be noted that the calculated values of the potentials of the Helmholtz plane and potential in the slip plane for an MF-4SK membrane and an MK-40 heterogeneous membrane have similar values, which is determined by the comparable values of the charge density at the internal interfacial surface.
To estimate the values of the fraction of the pores accessible for transport and subsequently calculate βw by formula (5), the concentration dependences of tw presented in Fig. 5 were measured for the membranes in the solutions of LiCl, NaCl, KCl, CsCl, and HCl. As is seen from the figure, the curves are ranged as LiCl > NaCl > CsCl > KCl > HCl, which corresponds to the values of the primary cation hydration numbers in a solution except for a cesium cation. The higher value of tw for a cesium cation is associated with the pumping effect similarly to how water is transported with hydrophobic organic ions [11]. It is also seen that, in concentrated solutions, the electroosmotic transport is reduced to the water transport in the composition of the primary hydrate shell of the ion.
For an MF-4SK membrane, the highest values of tw are observed in a solution of lithium chloride, which should decelerate the motion of this ion in an electric field. Despite this, the conductivity of the perfluorinated membrane in the Li+ form is higher than in the K+ or Cs+ form. Because of this, the most probable reason for the abnormally high conductivity of the membrane in a solution of lithium chloride is the deformation of the hydrate shell of a lithium cation upon its motion along the narrow channels of the perfluorinated membrane. At the same time, the hydrate shell of potassium and cesium cations is rigid due to its small radius, in connection with which the mobility of these cations in the perfluorinated membrane decreases.
It was earlier shown [21] that the calculation of βw by a capillary model gives an overestimated value, which is determined by the complex structure of the system of transport channels in ion-exchange materials. Because of this, the authors introduced a formal parameter θ characterizing the fraction of through mesopores and their accessibility for transport taking into account the tortuosity. In this work, experimental concentration dependences of tw presented in Fig. 5 were used for the estimation of the specified parameter. The calculations of the parameter θ were performed by the minimization of the mean square deviation of the experimental and calculated by a capillary model values of βw in a range of concentrations of solutions of LiCl, NaCl, KCl, and CsCl from 1 up to 3 M. The chosen range of concentrations has important practical significance for the concentration of solutions of electrolytes by electrodialysis. Figure 6 presents the concentration dependences of βw for an MF-4SK membrane calculated by a capillary model using formula (5) (curves) and by formula (4) taking into account the experimental values of the water transport numbers (points). As is seen from the figure, the calculated by a capillary model βw in an MF-4SK membrane in solutions of electrolytes with a concentration above 0.75 M are in good agreement with the experimental values. Similar curves for an MK-40 heterogeneous membrane in solutions of LiCl, NaCl, and KCl were earlier obtained and are presented in [35].
The results of the estimation of the value of θ for an MF-4SK perfluorinated membrane are presented in Fig. 7. This same figure presents by way of comparison the dependence of the fraction of through mesopores for an MK-40 heterogeneous membrane in the form of alkaline metal cations from the work [21].
The results of the calculation showed that the values of the parameter θ for the perfluorinated membrane were substantially higher than for an MK-40 heterogeneous membrane. This is first of all explained by the difference in the structural organization of the specified membranes. Perfluorinated membranes are a noncrosslinked gel structure with no pores in the dry state. The formation of these pores that mostly are through occurs particularly due to the hydration of the ionogenic groups. Here, in accordance with the theory of semielasticity of a perfluorinated matrix [36], a more hydrated lithium ion more intensively pulls apart the polymer chains, which formally leads to an increase in the fraction of through mesopores in an MF-4SK membrane upon the transition from the Cs+ form to the Li+ form.
Heterogeneous ion-exchange membranes have a nonuniform structure due to the presence of a polymer binding agent (polyethylene) in them which often forms dead-ended pores in them. As is seen from Fig. 7, in a perfluorinated membrane in solutions of alkaline metals chlorides, an increase in the fraction of through mesopores on both the specific water content of the membrane and radius of the hydrated cation is observed. This is associated with the increase in the volume of the pores upon the retention of their total number in view of the cluster structure of this polymer. This results in an increase in the accessibility of the pores for the transport of hydrated ions.
The investigation of the water transport with a proton is of special interest. As is known, the proton transport in hydrated ion-exchange membranes is described by two main mechanisms: hopping (or Grotthuss mechanism) and migration (or vehicle mechanism) [37]. The hopping mechanism is the reason for the high conductivity of both membranes in the H+ form in comparison with the salt forms of these same membranes (Fig. 3). However, we observe water transport with a proton that moves by the migration mechanism only in the electroosmotic experiments. In the physical sense, tw shows the number of water molecules transported with one counterion. Therefore, it is seen from Fig. 5 that tw with a proton changes from 4 down to 2 mol of H2O/F. This indicates the proton transport in the composition of various hydrate complexes for an MF-4SK membrane: the Eigen ion [H9O4]+ in dilute solutions and the Tsundel ion [H5O2]+ in concentrated solutions, which is in agreement with the earlier obtained results [23]. At the same time, almost no change in tw on the concentration of HCl is observed for an MK-40 heterogeneous membrane and, hence, the water transport is executed by a hydrate complex [H5O2]+.
Using an assumption that, in an electric field, water is transported only with a proton moving by the migration mechanism, the fraction of the migration mechanism in the total proton transport can be estimated based on the analysis of the static (nm) and dynamic (tw) hydrate characteristics of the membranes in hydrochloric acid. It has been found that the fraction of water transported in the composition of hydrate complexes in an electric field of its total content in both types of membranes is 15–20%, which is in agreement with the earlier obtained results for an MF-4SK membrane [23]. The values of the parameter θ were calculated for both membranes in a solution of HCl which turned out to be 0.44 and 0.11 for MF-4SK and MK-40, respectively. These values do not to the full extent correspond to the fraction of through mesopores in these polymers in view of the specified reasons.
CONCLUSIONS
The effect of the nature of counterions on the distribution of water with respect to the bond energies and pore radii, conductivity, and electroosmotic permeability of MF-4SK and MK-40 sulfonated cation-exchange membranes in solutions of LiCl, NaCl, KCl, CsCl, and HCl has been experimentally studied. The analysis of the characteristics of the porous structure of the membranes on perfluorinated and polystyrene matrices has shown that an expected decrease in the total porosity, specific internal surface area, and distance between the fixed ions is observed for an MK-40 membrane upon the transition from the Li+ form to the K+ form, while for an MF-4SK membrane, the regularity is only observed in the change in the total porosity.
The characteristic features of the effect of the nature of the alkaline metal on the conductivity of the homogeneous and heterogeneous sulfonated cation-exchange membranes have been found. It has been found based on the analysis of the concentration dependences of the specific electrical conductivity of the membranes in solutions of alkaline metal chlorides and hydrochloric acid that the electrical conductivity of an MK-40 heterogeneous membrane correlates with the behavior of the studied counterions in a solution, while the electrical conductivity of an MF-4SK perfluorinated membrane decreases in the series H+ > Na+ > Li+ > K+ > Cs+. The reasons for the high conductivity of an MF-4SK membrane in a solution of lithium chloride have been analyzed, the most probable of which is the deformation of the hydrate shell of the lithium cation in the structure of the perfluorinated membrane.
The charge density at the internal interfacial surface has been calculated for the sulfonated cation-exchange membranes from the porosimetry curves which has been used for the estimation of free solvent transport numbers through the membranes within the framework of a capillary model together with the results of determination of their specific conductivity in solutions of electrolytes of different natures. The free solvent transport number in an MF-4SK membrane in solutions of LiCl, NaCl, KCl, CsCl, and HCl has been calculated for the first time. A parameter characterizing the fraction of through mesopores and their tortuosity in the structure of the membrane has been introduced for the correct calculation. It has been shown that the specified parameter is substantially higher in an MF-4SK noncrosslinked membrane than in an MK-40 heterogeneous membrane and increases with the increase in the specific water content of the membrane and radius of the counterion in the hydrated state, which is in agreement with the peculiarity of their structural organization.
REFERENCES
M. Yaqub and W. Lee, Sci. Total Environ. 681, 551 (2019).
J. Havelka, H. Fárová, T. Jiříček, T. Kotala, and J. Kroupa, Water Sci. Technol. 79, 1580 (2019).
G. J. Doornbusch, M. Tedesco, J. W. Post, Z. Borneman, and K. Nijmeijer, Desalination 464, 105 (2019).
K. V. Protasov, S. A. Shkirskaya, N. P. Berezina, and V. I. Zabolotskii, Russ. J. Electrochem. 46, 1131 (2010).
L. Han, S. Galier, and H. Roux-de Balmann, Desalination 373, 38 (2015).
A. H. Galama, M. Saakes, H. Bruning, H. H. M. Rijnaarts, and J. W. Post, Desalination 342, 61 (2013).
B. Sun, M. Zhang, S. Huang, Z. Cao, L. Lu, and X. Zhang, Sep. Purif. Technol. 281, 119907 (2022).
B. Sun, M. Zhang, S. Huang, J. Wang, X. Zhang, Desalination 498, 114793 (2021).
S. Porada, W. J. van Egmond, J. W. Post, M. Saakes, and H. V. M. Hamelers, J. Membr. Sci. 552, 22 (2018).
J. O. Bockris and K. N. Reddy, Modern Electrochemistry. Ionics (Kluwer Academic Publishers, London, 2002).
G. Xie and T. Okada, Electrochim. Acta 41, 1569 (1996).
R. Sprocati and M. Rolle, Water Res. 213, 118161 (2022).
T. Yamanaka, T. Takeguchi, H. Takahashi, and W. Ueda, J. Electrochem. Soc. 156, B831 (2009).
J. Garrido, V. Compan, M. L. Lopez, and D. G. Miller, J. Phys. Chem. 101, 5740 (1997).
C. Larchet, B. Auclair, and V. Nikonenko, Electrochim. Acta 49, 1711 (2004).
H. M. Park and Y. J. Choi, Int. J. Heat Mass Transfer 52, 4279 (2009).
P. Schaetzel, Q. T. Nguyen, and B. Riffault, J. Membr. Sci. 240, 25 (2004).
A. N. Filippov, Colloid J. 80, 716 (2018).
Y. Xin, Y.-X. Zheng, and Y.-X. Yu, Mol. Phys. 114, 2328 (2016).
P. Meares, J. Polymer Science 20, 507 (1956).
I. V. Falina, V. I. Zabolotsky, O. A. Demina, and N. V. Sheldeshov, J. Membr. Sci. 573, 520 (2019).
Yu. M. Vol’fkovich, Elektrokhimiya 20, 669 (1984).
N. P. Berezina, S. A. Shkirskaya, A. A.-R. Sycheva, and M. V. Krishtopa, Colloid J. 71, 397 (2008).
N. Kononenko, V. Nikonenko, D. Grande, C. Larchet, L. Dammak, M. Fomenko, and Yu. Volfkovich, Adv. Colloid Interface Sci. 246, 196 (2017).
M. I. Bakeev, Hydration and Physicochemical Properties of Electrolyte Solutions (Nauka, Alma-Ata, 1978) [in Russian].
I. T. Goronovskii, Yu. P. Nazarenko, and E. F. Nekryach, Quick Reference Guide to Chemistry (Naukova Dumka, Kiev, 1987) [in Russian].
R. A Robinson and R. H. Stoks, Electrolyte Solutions, 2nd Ed. (Dover Publications, 2002).
Handbook of Electrochemistry, Ed. by A. M. Sukhotin (Khimiya, Leningrad, 1981) [in Russian].
N. P. Berezina, N. A. Kononenko, O. A. Dyomina, and N. P. Gnusin, Adv. Colloid Interface Sci. 139, 3 (2008).
E. Yu. Safronova, V. I. Volkov, A. A. Pavlov, A. V. Chernyak, E. V. Volkov, and A. B. Yaroslavtsev, Russ. J. Inorg. Chem. 56, 156 (2011).
N. H. Jalani and R. Datta, J. Membr. Sci. 264, 167 (2005).
I. A. Stenina, P. Sistat, A. I. Rebrov, G. Pourcelly, and A. B. Yaroslavtsev, Desalination 170, 49 (2004).
G. Pourcelly, A. Oikonomou, and C. Gavach, J. Electroanal. Chem. 287, 43 (1990).
V. I. Volkov, E. V. Volkov, S. V. Timofeev, E. A. Sanginov, A. A. Pavlov, E. Yu. Safronova, I. A. Stenina, and A. B. Yaroslavtsev, Russ. J. Inorg. Chem. 55, 315 (2010).
I. V. Falina, O. A. Demina, and V. I. Zabolotskii, Membr. Membr. Tekhnol. 9, 81 (2019).
A. N. Filippov, E. Yu. Safronova, and A. B. Yaroslavtsev, J. Membr. Sci. 471, 110 (2014).
K. D. Kreuer, S. J. Paddison, E. Spohr, and M. Schuster, Chem. Rev. 104, 4637 (2004).
Funding
This research was financially supported by the Kuban Science Foundation within scientific project no. H-21.1/23/21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Falina, I.V., Kononenko, N.A., Shkirskaya, S.A. et al. Experimental and Theoretical Study of Influence of Nature of Counterion on Electroosmotic Water Transport in Sulfonated Cation-Exchange Membranes. Membr. Membr. Technol. 4, 281–289 (2022). https://doi.org/10.1134/S2517751622050043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751622050043