Abstract
The data on gill ionocyte functions and the effects of hormones on the ion exchange in fish are summarized. The ionocytes found in freshwater zebrafish, HR (H+-ATPase-rich), NaR (Na+/K+-ATPase-rich), NCCC (Na+-Cl–-cotransporters expressing cells), SLC26C (solute transporter 26 expressing cells), and KE (K+-excreting cells), are described. Information on the functions of each type of ionocyte, as well as the characteristic ion transporters, are given. The current data on hormones that positively and negatively affect Ca2+, Na+, Cl–, H+, and \({\text{NH}}_{4}^{ + }\) transport through the gills are presented. Unresolved issues of hormonal regulation of the ion exchange in fish are listed, and further research directions are outlined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the course of evolution, vertebrates have developed complex mechanisms of ion and osmotic regulation that are necessary for maintenance of the water and salt exchange. In fish, ion- and osmoregulation is carried out by gills, kidneys, and intestines. The gills are considered the main osmoregulatory organs of adult fish: they make direct contact with the external environment and provide most of the transepithelial ion exchange (Evans et al., 2005; Takei et al., 2014; Guh et al., 2015; Yan and Hwang, 2019). In embryos and larvae of teleost fishes, which have undeveloped gills, the ion exchange function is implemented by the skin epithelium and yolk sac epithelium (Hiroi and McCormick, 2012).
Ion exchange is carried out by specialized cells, ionocytes. Ionocytes were first found in the gills of the European eel (Anguilla anguilla) and were called “chloride-secreting cells,” which reflects their function (Keys and Willmer, 1932). Later, these cells in many other teleost fishes were first called chloride cells and, even later, mitochondria-rich cells (MRCs). In modern literature, the term “ionocytes” is used. It is preferable, since these cells are involved not only in the secretion of Cl– but also in other processes. They are necessary for the ion absorption by freshwater fish, for regulation of the acid-base balance, and for the excretion of ammonium (Hiroi and McCormick, 2012).
Most fish species are stenohaline, i.e., they live either in fresh or sea water and cannot tolerate significant changes in environmental salinity. The remaining fish (it is believed that about 5% of the species) are euryhaline; they possess physiological mechanisms that allow them to adapt to a wide salinity range and changes in the aquatic environment (McCormick, 2001; Evans et al., 2005).
In marine, freshwater, and euryhaline fish, the regulation of the ion exchange is significantly different, since these fish are faced with different ion compositions and concentrations in the aquatic environment.
In particular, fish in fresh water passively absorb water and lose salt ions. In order to counteract this, they must actively absorb ions (mainly Na+ and Cl–) by the epithelial ionocytes in the gills, which are equipped to do so with a set of transporter proteins (ion channels). Ion transport is facilitated by an electrical gradient created by Na+/K+-ATPase (NKA) located on the basolateral membrane of ionocytes (Marshall and Grosell, 2006; Shaughnessy and McCormick, 2018).
In sea water, fish lose water and are “loaded” with salt ions that enter the body along the concentration gradient. In order to counteract this process, ionocytes of the gill epithelium of marine fish and euryhaline fish trapped in a marine environment (e.g., during catadromous migration) must actively excrete ions. The excretion of Cl– occurs via transcellular transport, i.e., chloride ions pass through the gill ionocytes. In this process, they first enter the ionocytes from the blood through the basolateral Na+-K+-2Cl–-cotransporter (NKCC) and are then taken out through the apical canal of cystic fibrosis transmembrane conductance regulator (CFTR). Na+ is excreted along the electrical gradient set by chloride ions via paracellular transport, i.e., through intercellular “leaky” tight junctions of the branchial epithelium (McCormick, 2001; Evans et al., 2005; Takei et al., 2014; Shaughnessy and McCormick, 2018). The excretion of these ions into the sea water is also highly dependent on NKA activity.
The ion exchange in marine fish was studied in detail earlier than that in freshwater fish, which is apparently explained by the fact that the mechanisms of ion transport in fresh water are more diverse and are carried out with the participation of a larger number of different types of cells. The high diversity of ionocytes in freshwater fish may be associated with a higher variability in the composition of the freshwater environment as compared to the marine environment (Marshall, 2002; Wilson and Laurent, 2002; Takei et al., 2014). In addition, it is assumed that the ancestors of fish were marine protovertebrates, which then repeatedly colonized fresh and brackish water bodies (Smith, 1932; Marshall, 2002; Evans et al., 2005; Ditrich, 2007; Dymowska et al., 2012). When new, fresh waterbodies form as a result of a geological event, new adaptations to this habitat can arise in fish. For example, the three-spined stickleback (Gasterosteus aculeatus) has certain features that allow this species to evolve towards freshwater forms. In sticklebacks, this evolution can take several generations, and these processes have occurred repeatedly throughout the geological history of freshwater lakes (Marshall and Grosell, 2006). Low concentrations of sodium chloride and calcium chloride in some freshwater bodies may activate completely different sets of ion transporters that are not typical of marine and brackish water; this also increases the diversity of ion transport mechanisms. For these reasons, it is clear that a single model of ion transport operation cannot explain ion regulation in all freshwater fish (Marshall and Grosell, 2006).
The osmoregulatory mechanisms are most flexible in euryhaline fish, in particular, diadromous (migratory) fish species, which experience extreme changes in the salinity of the environment during their life cycle. The survival of these fish when they move between fresh and sea waters depends on the timely switching of the gill epithelium ionocytes functioning, in which the absorption of ions is replaced by the excretion of salt or vice versa (McCormick, 2012; Takei et al., 2014; Shaughnessy and McCormick, 2018).
Euryhaline fish species are often used in the study of osmoregulation. This is especially true for two groups of migratory fish, anadromous salmonids and catadromous eels. These fish are characterized by large differences in the mechanisms of ion exchange, especially in its hormonal regulation (Takei et al., 2014). Other model species in this area of research include such euryhaline fish as tilapias (Oreochromis mossambicus, Oreochromis niloticus), mummichog (Fundulus heteroclitus), olive flounder (Paralichthys olivaceus), European seabass (Dicentrarchus labrax), medaka (Oryzias latipes), and milkfish (Chanos chanos).
This review mainly summarizes the information on ionocytes and their hormonal regulation in freshwater zebrafish (Danio rerio). In addition, some data are given on osmoregulation in euryhaline fish, medaka (Oryzias latipes), rainbow trout (Oncorhynchus mykiss), and some others. The ion transporter proteins and ion exchange mechanisms in zebrafish have been studied in greater detail than those in other stenohaline freshwater species. This is due, in particular, to the fact that the zebrafish is a classic model species. Its genome has been sequenced, and the signaling pathways of ionocyte proliferation, differentiation, and maturation have been studied most fully (Esaki et al., 2007, 2009; Guh and Hwang, 2017).
TYPES AND FUNCTIONS OF IONOCYTES IN ZEBRAFISH AND MEDAKA
Several types of ionocytes have been found in freshwater zebrafish and medaka (Dymowska et al., 2012; Guh and Hwang, 2017; Yan and Hwang, 2019).
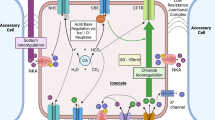
At least five types of ionocytes were identified in the gills and skin of zebrafish: (1) H+-ATPase rich cells (HR), (2) Na+/K+-ATPase rich cells (NaR), (3) Na+-Cl–-cotransporter expressing cells (NCCС), (4) solute transporter 26 expressing cells (SLC26C), and (5) K+-excreting cells (KE) (Guh and Hwang, 2017; Yan and Hwang, 2019). Table 1 presents the functions of zebrafish ionocytes and the localization of ion transporters in the cells.
The role of the NBCe1 transporter (\({\text{HCO}}_{3}^{ - }\) transporter) in the regulation of the acid-base balance is unknown. No transporters other than SLC26 and NKA have been identified in SLC26C. In addition, there is no information on any transporters other than Kir1.1 in KE cells, and the physiological significance of K+ excretion is also unknown (Guh and Hwang, 2017).
Euryhaline medaka living in fresh water has the following ionocytes: (1) Na+/H+-exchanger cells (NHEC), (2) epithelial Ca2+ channel expressing cells (ECaC-expressing cells, ECaСC), (3) NCCC, and (4) HR cells. NHEC allow the excretion of H+, \({\text{NH}}_{4}^{ + }\), and K+, as well as the uptake of Na+. The ECaСC ionocytes are responsible for Ca2+ uptake, and the NCCC are ionocytes for Na+ and Cl– uptake. The function of HR ionocytes in freshwater medaka has not been yet established (Yan and Hwang, 2019).
Ionocytes of the seawater (SW) type and accessory cells (ACs) were found in medaka acclimated to sea water. This set of ionocytes is characteristic of fish inhabiting marine environment. In medaka, they function in a manner typical of marine fish. Thus, SW ionocytes excrete H+, \({\text{NH}}_{4}^{ + }\), K+, Na+, and Cl–. Na+ is excreted via paracellular transport, through the intercellular space between ionocytes and ACs. The function of ACs has not yet been fully described (Yan and Hwang, 2019).
It is believed that gill ionocytes in fish (as well as ionocytes in the skin of fish embryos) and renal tubule cells in mammals are very similar in terms of the mechanisms of ion transport and the structure of transport proteins (Yan and Hwang, 2019). For example, HR of zebrafish and NHEC of medaka are similar to proximal renal tubule cells and intercalated α-cells of the mammalian collecting duct, since they also carry out Na+ uptake and H+ and \({\text{NH}}_{4}^{ + }\) excretion. In addition, NCCC of both species are similar to mammalian distal convoluted tubule cells, since they also uptake Na+ and Cl–; lastly, NaR cells of zebrafish and ECaC-expressing cells of medaka are similar to mammalian kidney cells in their ability to absorb (or reabsorb) Ca2+.
Therefore, the ionocytes of zebrafish and medaka have been studied in sufficient detail. Although not all of their functions have been revealed at present, we argue that these fish species are successfully used in the study of epithelial ion transport and its regulation.
CHARACTERISTICS OF IONOCYTES OF ZEBRAFISH
Ionocytes Rich in H+-ATPase
HR ionocytes possess apically localized H+-ATPase. They were first identified in the skin of embryos and gills of adult fish, as the cells most actively excreting acid (more precisely, acid equivalents, i.e., protons) (Lin et al., 2006; Horng et al., 2007; Hwang and Chou, 2013). NHE3b and Rhcg1 (\({\text{NH}}_{4}^{ + }\) transporter) are also colocalized on the apical membrane of these ionocytes. The AE1b and α1 subunits of the NKA (atp1a1a.5) are located basolaterally. Two carbonic anhydrases were also identified in these cells: membrane-bound apical carbonic anhydrase CA15a and cytosolic CA2-like carbonic anhydrase a (Esaki et al., 2007; Yan et al., 2007; Lin et al., 2008; Shih et al., 2008; Liao et al., 2009; Lee et al., 2011; Hwang and Chou, 2013).
HR ionocytes of zebrafish are similar to the cells of proximal renal tubules and intercalated kidney cells (type A) in terms of the mechanisms of the transport and expression of transporter proteins (Hwang and Chou, 2013). Thus, skin cells of zebrafish embryos can serve as a useful, alternative model for the study of ion transport in the kidneys of other vertebrates, including humans (Hwang and Chou, 2013).
The excretion of H+ through the apical membrane of HR cells in zebrafish is provided by two transporters, HA and NHE3b. It was proven experimentally that HA plays a more important role in the excretion of acid equivalents than NHE3b (Shih et al., 2012; Hwang and Chou, 2013).
Unlike mammals, which excrete urea as the main by-product of nitrogen metabolism, teleost fish are mainly ammoniotelic, i.e., they excrete nitrogen mainly as ammonium. Because teleost fish are able to excrete \({\text{NH}}_{4}^{ + }\) directly to the surrounding water, they do not need to expend energy converting \({\text{NH}}_{4}^{ + }\) to less toxic urea. It is possible that the fish gills do not excrete \({\text{NH}}_{4}^{ + }\) ions but NH3 gas, which is assumed in studies on the binding of acid equivalents in \({\text{NH}}_{4}^{ + }\). At the same time, acidification of the water layer adjacent to the gills facilitates \({\text{NH}}_{4}^{ + }\) excretion (Wright et al., 1989). It was also shown that the combined work of NHE3 and Rhcg1 not only mediates the binding of acid equivalents in the composition of excreted \({\text{NH}}_{4}^{ + }\); it is also necessary for Na+ uptake by ionocytes (Wu et al., 2010; Hwang and Chou, 2013).
The uptake of Na+ and \({\text{HCO}}_{3}^{ - }\) by HR ionocytes requires the presence of carbonic anhydrases and other basolateral transporters, which makes them similar to the cells of the proximal tubules and collecting ducts in mammalian kidneys (Hwang and Chou, 2013).
Ionocytes Rich in Na+/K+-ATPase
The difference between NaR ionocytes and HR ionocytes was first demonstrated via the labeling of the skin of zebrafish embryos with monoclonal antibodies to the NKA α subunit (Liao et al., 2007; Hwang and Chou, 2013). The ECaC channels are located apically on the membrane of these ionocytes, while PMCA, NCX, and NKA (isoform atp1a1a.1) are located basolaterally (Hwang and Chou, 2013).
Six PMCA isoforms and seven NCX isoforms have been identified in zebrafish. Of them, only PMCA2 and NCX1b are colocalized in NaR cells (Liao et al., 2007). In addition, only atp1a1a.1 is expressed in NaR cells of the six genes of α1 subunit of NKA (Liao et al., 2009). The set of Ca2+ transporters in zebrafish NaR cells is similar to that in Ca2+-absorbing cells of the kidneys and intestines of mammals (Hoenderop et al., 2005). In mammals, the calcium uptake across apical membranes is closely related to TRPV5/6 (orthologs of zebrafish ECaC), NCX1, and PMCA1b. Ca2+ transport through the TRPV6 transporter is a rate-limiting step in vitamin D-dependent Ca2+ uptake (Hoenderop et al., 2005; Hwang and Chou, 2013).
The acclimation of zebrafish to an environment with a low Ca2+ content, which is known to stimulate Ca2+ uptake (Pan et al., 2005), also increases the expression of ecac mRNA (Liao et al., 2007). In addition, the expression of ecac, but not pmca2 and ncx1b, depends on the effects of hormones, e.g., stanniocalcin (Tseng et al., 2009), cortisol (Lin et al., 2011), and vitamin D (Lin et al., 2012). In general, the literature data show that the regulation of transepithelial Ca2+ uptake due to the effect on ECaC exhibits conservative properties in the phylogenetic range from fish to mammals (Hwang and Chou, 2013).
Ionocytes Expressing Na+-Cl– Cotransporters
It was found that the cotransporter NCC2b (same NCC-like 2 or SLC12A10.2) is localized in zebrafish on the apical membrane in a separate group of ionocytes, which differ from the previously identified HR and NaR cells. These ionocytes were found to be analogous to cells of the distal convoluted tubules of mammalian kidneys, which also express Na+-Cl– cotransporters and are involved in the uptake of Na+ and Cl– (Wang et al., 2009; Hwang and Chou, 2013).
The Na+ uptake in zebrafish occurs mainly due to the work of the NHE3b transporter (in HR ionocytes), and NCC apparently plays a minimal or auxiliary role in this process (Esaki et al., 2009; Hwang and Chou, 2013). The functional redundancy associated with the participation of two transporters in Na+ metabolism, NHE3 and NCC, seems to have been conserved from zebrafish to mammals. In the proximal tubules of the mammalian kidneys, massive Na+ reabsorption is carried out by the NHE3 transporter, while fine regulation of Na+ reabsorption is achieved by other, redundant mechanisms, in particular, via NCC in the distal convoluted tubules (Hwang and Chou, 2013).
The NKA (atp1a1a.2) and NBCe1b ion transporters are located basolaterally in the NCCC ionocytes of zebrafish (Liao et al., 2009; Lee et al., 2011). The apical NCC transporter transports Na+ from the environment into cells, and the basolateral NBCe1b and NKA (atp1a1a.2) can direct intracellular Na+ ions from epithelial cells into the bloodstream; however, there is insufficient molecular physiological evidence in the literature for such work by transporters (Hwang and Chou, 2013).
Twelve genes of the chloride channels of the clc family (Cl– channel) are also expressed in zebrafish. Of these, only the clc-2c gene is coexpressed with the ncc2b gene (ncc2b expression is a marker of NCCC ionocytes) in the gills and skin of the zebrafish. Apparently, the CLC-2c channel is required for the movement of Cl– from ionocytes into the blood (Wang et al., 2015).
Ionocytes Expressing SLC26 Transporters
In mammalian collecting ducts, intercalated B-type cells coexpress apical pendrin (SLC26A4) and basolateral HA. They play a major role in the excretion of \({\text{HCO}}_{3}^{ - }\), which is necessary to maintain acid-base homeostasis (Wagner et al., 2011). In zebrafish, three members of the SLC26 family have been identified: SLC26A3, SLC26A4, and SLC26A6C (Bayaa et al., 2009; Perry et al., 2009). The mRNA encoding these proteins, as well as the SLC26A3 protein itself, is expressed in certain types of cells in the gills of embryos and adults, while a small fraction (less than 10%) of cells expressing SLC26A3 also coexpresses basolateral NKA (atp1a1a.1) (Bayaa et al., 2009; Perry et al., 2009; Hwang and Chou, 2013).
Ionocytes Excreting K+
The expression of α1 subunits of the NKA subtype atp1a1a.4 was observed in a group of zebrafish ionocytes, which differ from HR, NaR, and NCCC ionocytes (Liao et al., 2009). It turned out that mRNA of the transporter, which is orthologous to the human Kir1.1 channel, is colocalized in the skin ionocytes of zebrafish embryos, together with atp1a1a.4 (Abbas et al., 2011). Kir1.1 is the K+ channel, an ortholog of the ROMK channel (renal outer medullary K+ channel) of mammals (Abbas et al., 2011). Loss of Kir1.1 function has been shown to lead to transient tachycardia, followed by bradycardia (Abbas et al., 2011) which are the effects seen in human hyperkalemia (Kahloon et al., 2005).
Although the presence of potassium currents in these cells has not been proven, ionocytes expressing Kir1.1 are called K+-secreting cells (Abbas et al., 2011; Hwang and Chou, 2013). In our opinion, it would be more appropriate to call these cells K+-excreting, since the concept of “secretion” is associated mainly with the glands of internal and external secretion. In non-Russian scientific publications, the term “secretion” is often used to denote the transport of ions from the body, and they speak of excretion only in the case of the release of any metabolic products, e.g., in the case of \({\text{NH}}_{4}^{ + }\) ions release (Hwang and Chou, 2013).
ROLE OF HORMONES IN THE REGULATION OF ION EXCHANGE IN FISH
Environmental changes rapidly affect the ionic composition and osmotic pressure of body fluids in fish. Thus, they must quickly and efficiently regulate transepithelial ion transport in order to compensate for these effects. Since the neuroendocrine system is the main mediator between environmental signals and physiological responses, it is the most important part of the osmoregulatory mechanisms (Guh et al., 2015).
Two groups of processes can be distinguished in the hormonal regulation of ion exchange: (1) fast adjustment, which occurs within minutes or hours due to already existing ionocytes and transporter proteins, and (2) long-term regulation, which is carried out within hours or days due to changes in the ionocyte number and the expression of transporters (Hwang and Chou, 2013). The processes of rapid regulation mainly involve hormones that act through receptors of plasma membranes (e.g., adrenaline), while the role of hormones exerting effects on transcription in the cell nucleus (e.g., steroid hormones) is more pronounced in long-term regulation.
The classical mechanism of action of some hormones is to influence the genetic apparatus of the cell, but nonclassical, nongenomic mechanisms of regulation have also been shown for these hormones. In the middle of the 20th century, it was found that steroids can have rapid, nongenomic effects (Duval et al., 1983); however, their detailed study began only in recent decades, including in fish (Das et al., 2018).
Table 2 lists the hormones and other signaling molecules that regulate ion exchange in zebrafish.
Hormonal Regulation of Ca2+ Uptake
The following hormones are known to be involved in regulation of the calcium metabolism in zebrafish: prolactin, somatotropin (growth hormone), parathyroid hormone, parathyroid hormone-related protein, cortisol, vitamin D, calcitonin, and stanniocalcin (Hoshijima and Hirose, 2007; Tseng et al., 2009; Lafont et al., 2011; Lin et al., 2011, 2012).
Some of these hormones can have a positive effect on the level of Ca2+ (i.e., they are hypercalcemic, or calciotropic hormones). Others have a negative effect (hypocalcemic hormones).
Hypercalcemic hormones include prolactin, growth hormone, PTH1, PTHrP, and cortisol. Depending on its molecular form, vitamin D can exhibit both hypercalcemic and opposite effects in fish (Fraser, 2017).
It is well known that prolactin is required for acclimation to fresh water in euryhaline fish, while growth hormone is required for acclimation to sea water (Manzon, 2002; Evans et al., 2005; Hwang and Chou, 2013). In a classical study, the hypophysectomized mummihog (Fundulus heteroclitus) could survive in fresh water only if it received exogenous prolactin (Pickford and Phillips, 1959). Subsequent studies revealed that the survival of fish in this case was associated with a decrease in the loss of ions, not with stimulation of their absorption (Potts and Evans, 1966). It was shown later that prolactin injections restored blood plasma osmolality in hypophysectomized channel catfish (Ictalurus punctatus) in fresh water (Evans et al., 2005).
The hypercalcemic effect of prolactin is closely related to its osmoregulatory effect. In euryhaline teleosts, the transfer from sea water to fresh water leads to a significant increase in the level of prolactin in the blood plasma, which is necessary to limit the loss of Na+ (Arakawa et al., 1993). At the same time, prolactin enhances the absorption of Ca2+ ions from the surrounding fresh water, in which the content of these ions is lower than that in sea water. However, if there is no salt load present, then a change in Ca2+ concentration in the environment is apparently a less effective stimulator of prolactin secretion in fish that have adapted either only to fresh water or only to sea water (Arakawa et al., 1993; Wongdee and Charoenphandhu, 2013).
In the freshwater-acclimated American eel (Anguilla rostrata), the injection of ovine prolactin significantly increased the plasma Ca2+ concentration by increasing PMCA activity in gill epithelial cells, as does hyperprolactinemia caused by pituitary transplantation (Flik et al., 1989a). In Mozambique tilapia males (Oreochromis mossambicus), 8 days of prolactin injections increased the Ca2+ influx into the gills and decreased the outflow of these ions, which led to hypercalcemia (Wongdee and Charoenphandhu, 2013).
The acclimation of zebrafish embryos to fresh water diluted 20 times (0.8 μM Ca2+) stimulated the transcription of genes encoding prolactin, somatotropin, and PTH1. When 16 μM CaCl2 was added to water, the transcription of these genes returned to the initial level (Hoshijima and Hirose, 2007). However, more detailed information on the role of prolactin and somatotropin in ion homeostasis in stenohaline zebrafish is absent (Hwang and Chou, 2013).
There are few studies on the effect of somatotropic hormone on calcium metabolism in fish. It was shown that the calcium level in scales and bones was slightly, but significantly, lower in control fish than in the individuals of Mozambique tilapia (Oreochromis mossambicus), which were injected with somatotropin (Flik et al., 1993). In rainbow trout (Oncorhynchus mykiss) treated with growth hormone, the Ca2+ concentration in the plasma was higher than that in the control group (Zhu et al., 2013). Therefore, somatotropin presumably has a hypercalcemic effect, although this effect has been studied in a very limited number of fish species (Hwang and Chou, 2013).
Parathyroid hormone (PTH) is expressed in the parathyroid glands of higher vertebrates. In mammals, parathyroid hormone functions as a hypercalcemic hormone that can stimulate Ca2+ reabsorption by the kidneys, affecting the expression of the Ca2+ transporter (Hoenderop et al., 2005; van Abel et al., 2005; Guh et al., 2015).
It is assumed that the parathyroid glands of mammals are evolutionarily derived from the gill tissue of fish (Okabe and Graham, 2004). The evolution of the parathyroid glands eliminated the need for terrestrial vertebrates to absorb Ca2+ from water; this allowed them to regulate Ca2+ levels in serum through the CaSR–PTH (CaSR is calcium-sensing receptor) signaling pathway. Therefore, the parathyroid glands may be a mechanism that makes it possible for vertebrates to exist in the terrestrial environment (Okabe and Graham, 2004; Lin et al., 2014).
Fish, apparently, do not have parathyroid glands; however, two Pth paralogs, Pth1 and Pth2, have been identified (Gensure et al., 2004). The expression of Pth genes has been recorded in several tissues of the zebrafish, including gills, muscles, and brain (Okabe and Graham, 2004; Lin et al., 2014). The Ca2+ level in the blood serum of goldfish (Carassius auratus) increased after the injection of heterologous PTH1 (Suzuki et al., 2011). The same effect was observed in zebrafish (Lin et al., 2014; Guh et al., 2015; Guh and Hwang, 2017).
In addition to PTH, a number of fish have been found to have another member of the PTH family: the parathyroid hormone-related protein (PTHrP). It is believed that PTHrP plays a key role in the evolution of animals due to the role of this protein in the formation of pulmonary alveoli, which are necessary for terrestrial vertebrates to breathe in the air (Abbink and Flik, 2007; Torday, 2013). PTHrP is produced in many tissues and exerts a wide range of intracellular, paracrine, and endocrine physiological actions. The key role of PTHrP for normal life was demonstrated in mice with knockout of the Pthrp gene (or its receptor), which led to the death of animals at birth due to the absence of alveoli (Guerreiro et al., 2007; Torday, 2013).
PTHrP is a pleiotropic hormone that has also hypercalcemic effects. The action of the N-terminal peptide (1–38)PTHrP led to a dose-dependent increase in Ca2+ accumulation in the larvae of gilt-head bream (Sparus aurata) (Guerreiro et al., 2007; Guh and Hwang, 2017). PTHrP was discovered in humans in 1987 in connection with several forms of cancer that caused an increase in the Ca2+ level in the blood. This condition is called humoral hypercalcemia of malignancy (HHM) (Abbink and Flik, 2007). Hypercalcemia resulting from uncontrolled secretion of PHTrP is a consequence of bone resorption and the suppression of urinary Ca2+ excretion, which is also observed in hyperparathyroidism (Guerreiro et al., 2007).
Cortisol is another hormone that influences Ca2+ metabolism. In contrast to mammals, in which cortisol has a hypocalcemic effect (Patschan et al., 2001), this hormone acts as a stimulator of Ca2+ uptake in fish. Water with a low Ca2+ content can cause an increase in cortisol levels in freshwater rainbow trout (Oncorhynchus mykiss gairdneri) (Flik and Perry, 1989). In the same fish species, exposure to exogenous cortisol leads to activation of the expression of mRNA and ECaC protein in the gills (Shahsavarani and Perry, 2006).
In zebrafish embryos, exogenous cortisol increased Ca2+ uptake and ecac expression but did not affect the mRNA expression of Ca2+ transporters, e.g., pmca2 and ncx1b (Lin et al., 2011; Hwang and Chou, 2013). At the same time, an increase in ecac expression and Ca2+ uptake can be blocked by morpholine knockout of GR (glucocorticoid) receptors but not MR (mineralocorticoid) receptors, which is convincing evidence that the effect of cortisol on the work of ionocytes is carried out mainly through the signaling pathway of GR receptors (Lin et al., 2011; Hwang and Chou, 2013).
Since teleost fish lack aldosterone synthase, which is necessary for the synthesis of aldosterone, cortisol is the main corticosteroid in this group (Nelson, 2003). Cortisol can bind to both GR and MR, albeit with different affinities, with each of the signaling pathways having different effects on the transcription of genes encoding ion transporters in the gills (Kiilerich et al., 2007). The difference in the effect of cortisol on Ca2+ metabolism in terrestrial mammals and in fish may be related to the difference in the pathways of Ca2+ uptake in these groups of animals (Hwang and Chou, 2013).
In addition, the cortisol-GR signaling pathway is found to regulate the expression of the receptor and enzyme for vitamin D synthesis (Lin et al., 2011), which suggests that the effect of cortisol on Ca2+ uptake may be mediated by other factors (Guh and Hwang, 2017).
Vitamin D is a vital hormone that regulates Ca2+ uptake in mammals. Complexes of vitamin D3 and vitamin D receptor (VDR) can enhance the expression of proteins TRPV5 and TRPV6 (ECaC orthologs) in mammals by binding to vitamin D response elements (VDRE) in the promoter regions of calcium transporter genes. This is how the “vitamin D3–VDR” complex can activate transcription of the ecac gene (aka trpv5) and, thus, accelerate Ca2+ uptake (Hoenderop et al., 2005; Hwang and Chou, 2013). Thus, this signaling pathway is an important regulator of calcium homeostasis in mammals. The hypercalcemic effect of vitamin D is conservative and persists from fish to terrestrial vertebrates (Hwang and Chou, 2013). However, unlike terrestrial vertebrates, the form of vitamin D found in the maximum concentration in the blood of rainbow trout is not 25(OH)D3 but 1.25(OH)2D3 (Fraser, 2017).
Apparently, there is no such strict specificity of the distribution of enzymes of vitamin D3 hydroxylation in fish organs, which is the opposite of the situation in humans (Graff et al., 1999). In rainbow trout (Oncorhynchus mykiss gairdneri) (Hayes et al., 1986), Atlantic cod (Gadus morhua) (Sundell et al., 1992), common carp (Cyprinus carpio), and olive flounder (Paralichthys olivaceus) (Takeuchi, 1994), 1.25(OH)2D3 is synthesized in liver, not in kidneys, as it is in mammals. Moreover, the activity of 1α-hydroxylase, which is necessary for the synthesis of this form of the hormone, is also found in kidneys in a number of fish species (Graff et al., 1999; Fraser, 2018).
There is evidence that the biological effect of the hormone 1,25(OH)2D3 is manifested only in fresh water, and another form of the hormone, 24,25(OH)2D3, begins to act with the opposite activity after the migration of euryhaline fish into the marine environment, where it is not Ca2+ absorption but Ca2+ excretion that is required (Fraser, 2017). This change in the functioning of the two forms of the hormone is mediated by the expression of their receptors, which presumably depends on the Ca2+ concentration in the environment (Fraser, 2017).
The injection of vitamin D causes an increase in plasma Ca2+ levels in Atlantic cod (Gadus morhua) (Sundell et al., 1993), as well as dose-dependent hypercalcemia in the common carp (Cyprinus carpio) (Swarup et al., 1991). Exogenous vitamin D (1,25(OH)2D3), as well as exogenous cortisol, increased Ca2+ uptake and ecac expression in zebrafish embryos without affecting pmca and ncx expression (Lin et al., 2012; Hwang and Chou, 2013).
The hypocalcemic hormones include STC-1 and CT.
Stanniocalcin is secreted by corpuscles of Stannius, small endocrine glands attached to the kidneys of teleost fish (Wagner et al., 1986; Guh and Hwang, 2017). It was shown that STC-1 exhibits a hypocalcemic effect, i.e., it suppresses Ca2+ uptake (Wagner et al., 1986; Yan and Hwang, 2019). The hypocalcemic effect of STC-1 is well known in many fish species (Yeung et al., 2012). In zebrafish embryos, knockdown of the stc1 gene by morpholine oligonucleotides led to an increase in Ca2+ content, Ca2+ uptake, and ecac expression. These data suggest that STC-1 in zebrafish suppresses the expression of the ecac gene, which, in turn, leads to suppression of Ca2+ uptake in fish (Tseng et al., 2009; Yan and Hwang, 2019).
Chronic stress induced by dexamethasone caused a decrease in the levels of Na+, Cl–, and Ca2+ and also decreased the secretion of STC from Stannius corpuscles in rainbow trout (Oncorhynchus mykiss) (Pierson et al., 2004). This may indicate that STC-1 is involved in the homeostasis of ions other than Ca2+ (Yan and Hwang, 2019).
Calcitonin (CT) is a small peptide (32 amino acid residues) produced in mammals by parafollicular C‑cells of the thyroid gland (Baldisserotto, 2019). In teleost fishes, CT is synthesized mainly in the ultimobranchial gland (Guh et al., 2015). The ultimobranchial gland originates from the last pharyngeal sac. It is homologous to the C-cells of the mammalian thyroid gland. In mammalian embryogenesis, C-cells are part of the thyroid gland as parafollicular cells. In zebrafish, C-cells do not fuse with the thyroid gland, and these cells remain a separate ultimobranchial gland (Holden et al., 2013).
Calcitonin is considered a hypocalcemic hormone in fish and mammals (Evans et al., 2005; Guh et al., 2015). The injection of a homologous or heterologous CT was shown to induce hypocalcemic effects in various fish species, including the red stingray (Dasyatis akajei) (Sasayama et al., 1992), common carp (Cyprinus carpio) (Chakrabarti and Mukherjee, 1993), and goldfish (Carassius auratus) (Sasayama et al., 1993). In zebrafish embryos exposed to water with a high Ca2+ content, the expression of the calcitonin gene and its receptor was increased, while the expression of ecac decreased (Lafont et al., 2011). Some results suggest a biphasic CT effect. In zebrafish embryos, the CT-mediated decrease in Ca2+ uptake results in short-term hypocalcemia. In turn, this may activate hypercalcemic signaling pathways, which leads to compensation for imbalances in the calcium metabolism by hypercalcemic hormones and various Ca2+ transporters (Hwang and Chou, 2013). Therefore, “plus-minus interactions” can appear during the activity of calciotropic and hypocalcemic hormones.
Hormonal Regulation of Na+ and Cl– Uptake
Teleosts living in sea water balance the osmotic water loss by the ingestion of water, followed by the absorption of NaCl in the intestine (Hickman Jr., 1968). The resulting salt load is added to that occurring due to the diffusional permeation of salt through the gills. The total salt balance is restored due to the active excretion of NaCl by the gill epithelium, since the absence of the loop of Henle in the nephrons makes fish unable to produce urine that is more hyperosmotic than blood plasma (Evans et al., 2005).
Conversely, fish living in fresh water should actively absorb Na+ and Cl–, since the osmolality of blood plasma under these conditions is higher than that of the environment (Edwards and Marshall, 2012). The exchange of sodium and chloride ions in freshwater fish is regulated by signaling molecules, e.g., cortisol, prolactin, catecholamines, hydrogen sulfide, STC-1, CGRP peptides, and the renin-angiotensin system. Hydrogen sulfide is hyponatremic, while STC-1 and CGRP are hypochloremic.
Cortisol is considered a switch for water-salt metabolism when fish move between marine and freshwater environments (McCormick, 2001; Evans et al., 2005; Takei et al., 2014; Guh et al., 2015; Guh and Hwang, 2017). At the same time, the role of cortisol in osmoregulation is twofold, since it is involved in adaptation to both salt and fresh water in euryhaline fish species (McCormick, 2001). The combined action of cortisol and somatotropin is necessary for the differentiation of gill ionocytes according to the marine environment, while the action of cortisol and prolactin is necessary for living in fresh water (McCormick, 2001; Sakamoto and McCormick, 2006).
In zebrafish, cortisol is involved in the regulation of Na+ uptake (Kumai et al., 2012a). This cortisol function is mediated by GR, but not MR, receptors; this was shown in both pharmacological studies and experiments with gene knockdown (Guh and Hwang, 2017).
Conversely, there is evidence that cortisol can suppress Na+ loss by altering epithelial permeability. Exposure to exogenous cortisol in zebrafish larvae leads to a significant increase in the expression of tight junction proteins, occludin-a and claudin-b, which is accompanied by a decrease in paracellular permeability upon exposure to an acidic environment (Kwong and Perry, 2013). These cortisol effects are also mediated by GR receptors, since GR receptor knockdown abolishes the effect of cortisol on paracellular permeability. In addition, morphants with disabled GRs that are exposed to an acidic environment have a more pronounced increase in paracellular permeability (and a more significant loss of Na+ by diffusion) than the fish of the control group (Kwong and Perry, 2013).
The pituitary hormone prolactin is necessary for adaptation to fresh water; however, the mechanisms of the effect of prolactin on the transport of certain types of ions have long remained undetermined (Manzon, 2002; Sakamoto and McCormick, 2006; Breves et al., 2014; Guh et al., 2015).
Prolactin stimulates the differentiation of NCC-expressing ionocytes and, accordingly, the expression of NCC transporters in euryhaline Mozambique tilapia (Oreochromis mossambicus) and zebrafish exposed to fresh water (Breves et al., 2014; Guh and Hwang, 2017). Since NCC is a key factor necessary for the uptake of both Na+ and Cl– in zebrafish, these data suggest that prolactin also affects the Cl– exchange in fish.
The role of prolactin in ion uptake was further confirmed in studies on prolactin-knockout zebrafish larvae. In these studies, during adaptation to fresh water prolactin definitely plays a key role in the regulation of ion absorption, not only of osmolarity. In the branchial body region of larvae with prolactin gene knockout, the expression of NCC2b and the number of NCCC ionocytes decreased by the fifth day after fertilization; this led to a disruption in the uptake of Na+ and Cl– in these morphants on the sixth day after fertilization (Guh and Hwang, 2017).
The role of the renin-angiotensin II system (RAS) in enhancing salt reabsorption in mammalian kidneys is well known (Guh et al., 2015). There is evidence that this hormonal system affects the ion exchange in fish as well. In particular, a compensatory increase in Na+ uptake in zebrafish larvae under acute exposure to acidic or ion-poor water can be partially blocked by an angiotensin-II receptor antagonist. In addition, knockdown of the renin gene prevents stimulation of Na+ uptake after acute exposure to acidic or ion-poor water (Kumai et al., 2014). Apparently, the action of the RAS under these conditions does not depend on cortisol, since neither RU486 (GR antagonist) nor GR knockdown influence the stimulation of Na+ uptake (Kumai et al., 2014; Guh and Hwang, 2017).
Catecholamines, which are released either from nerve endings or chromaffin cells (Reid et al., 1998), play an important role in ion regulation in freshwater fish by acting on α- and β-adrenergic receptors (Evans et al., 2005; Guh et al., 2015). Catecholamines contribute to Na+ uptake by zebrafish ionocytes (Evans et al., 2005; Hwang and Chou, 2013). In particular, knockout of β-adrenergic receptors by specific morpholine oligonucleotides leads to impaired Na+ uptake in acidic or ion-poor water (Kumai et al., 2012b).
Although most research focuses on the hormonal mechanisms involved in the stimulation of Na+ and Cl– uptake, studies on zebrafish found negative regulation of the salt uptake in fish. Hydrogen sulfide (H2S), a gas that plays an important role in the regulation of cardiorespiratory function and oxygen sensing, can stimulate Ca2+ uptake (Kwong and Perry, 2015). In addition, this signaling molecule is a negative regulator of Na+ uptake. Thus, the addition of sodium sulfide (hydrolyzed to form H2S) to acidified water with zebrafish embryos led to a significant decrease in Na+ uptake. This effect is not observed in zebrafish morphants, which lack HR ionocytes due to gcm2 knockout, and Na+ is absorbed mainly by NCCC ionocytes. This suggests that H2S affects Na+ uptake via NHE3b transporters rather than via NCC2b (Guh and Hwang, 2017).
Relatively little is known about the hypochloremic effects of hormones in fish. Such effects can be exerted by STC-1 and the CGRP peptide.
It has already been mentioned above that stanniocalcin inhibits Ca2+ absorption. Further studies have shown that STC-1 has a broader effect on ion exchange and is not limited to an effect only on Ca2+ exchange (Chou et al., 2015). In particular, overexpression of STC-1 leads to a decrease in ECaC, NCC, and HA expression with a concomitant decrease in the contents of Ca2+, Na+, and Cl– in the body and in H+ secretion. Knockdown of the stc-1 gene has opposite effects. In addition, an increase in STC-1 expression causes a decrease in the number of ionocyte progenitor cells and mature ionocytes in the skin of zebrafish embryos. Therefore, STC-1 is a negative regulator of the number of ionocytes and, accordingly, reduces their functional activity (Chou et al., 2015).
Calcitonin gene-related peptide (CGRP) is another regulator of Cl– uptake. CGRP is a splice variant of calcitonin, but, unlike the latter, CGRP has a hypochloremic effect, not a hypocalcemic one. An increase in CGRP expression in zebrafish larvae leads to a decrease in NCC2b expression and a slowdown in Cl– uptake, and vice versa. This effect occurs via a change in the number of NCCC, since knockdown of crlr1 (CGRP receptor) leads to an increase in the density of cells expressing NCC2b (Guh and Hwang, 2017).
Hormonal Regulation of H+ Excretion
The information on the hormonal regulation of the acid-base balance in fish is fragmentary. Most of the research on this topic focuses on the regulation of ion transport at low pH values (“acid stress” as opposed to “alkaline stress”). The level of certain hormones, e.g., cortisol (Kumai et al., 2012a), prolactin (Flik et al., 1989b), somatolactin (Kakizawa et al., 1996), endothelin 1 (Guh et al., 2014) and angiotensin II (Kumai et al., 2014), increases after fish exposure to acidified water.
Cortisol is known to be involved in the regulation of H+ excretion in fish. The activity of HA, which excretes H+ in the gills, increases upon the chronic infusion of cortisol into the abdominal cavity of freshwater rainbow trout (Oncorhynchus mykiss) (Lin and Randall, 1993). Exposure to exogenous cortisol increases both H+ excretion and the expression of transporters associated with H+ excretion in the yolk sac membrane of zebrafish embryos (Lin et al., 2015). In addition, this enhancement is mainly mediated by GR receptors, not MR receptors; it is achieved not only via regulation of the total number of HR ionocytes but also via enhancement of the work of individual HR ionocytes (Lin et al., 2015; Guh and Hwang, 2017).
Exposure to cortisol increases the ionocyte number (including HR cells) via a GR-dependent increase in the expression of Foxi3a factor (Cruz et al., 2013). This suggests that cortisol influences H+ secretion, at least in part, by regulating the proliferation and differentiation of HR ionocytes (Cruz et al., 2013; Guh et al., 2015).
Endothelins form a family of three peptides consisting of 21 amino acid residues. The family includes endothelin 1, 2, and 3 (EDN1, EDN2, and EDN3), which are involved in many physiological processes, in particular, regulation of the vascular tone and the transport of water and ions in the kidneys (Kohan et al., 2011). EDN1 is a regulator of H+ secretion in the mammalian kidney (Wesson, 2011).
Overexpression of EDN1 increases H+ excretion from the skin of embryos, while knockdown of the endothelin receptor ednraa causes a significant decrease in proton excretion induced by EDN1 or medium acidification (Guh et al., 2014, 2015).
The estrogen-related receptor α is an orphan nuclear receptor that plays an important role in adaptive metabolic responses under conditions of increased energy expenditure, such as exposure to cold, exercising, and fasting (Villena and Kralli, 2008). It is important to note that metabolism under these conditions is usually accompanied by an increase in the production of organic acids, which can threaten the acid-base balance of the body (Guh et al., 2016). Knockdown of the esrra gene, which produces ERRα, disrupts H+ excretion both in the entire body and in individual HR ionocytes. This is accompanied by a decrease in the number of HR cells, as well as a decrease in the expression of genes required for H+ excretion and energy metabolism (Guh et al., 2016). Thus, ERRα modulates H+ excretion by affecting the expression of transporters, ionocyte differentiation, and energy metabolism (Guh and Hwang, 2017).
Stannocalcin is another regulator of H+ excretion. Overexpression of STC-1 decreases both HA expression and H+ secretion. Knockdown of the stc-1 gene causes the opposite effects (Chou et al., 2015).
Hormonal Regulation of the Excretion of \(NH_{4}^{ + }\) and Urea
Unlike mammals, which excrete urea as the end product of nitrogen metabolism, teleost fish are mainly ammoniotelic, i.e., they excrete nitrogen mainly in the form of ammonium. Since teleost fish can excrete \({\text{NH}}_{4}^{ + }\) directly into the surrounding water, they do not need to expend energy converting \({\text{NH}}_{4}^{ + }\) to less toxic urea. It is also possible that fish gills do not excrete \({\text{NH}}_{4}^{ + }\) ions but NH3 gas, which is assumed in studies on the binding of acid equivalents in \({\text{NH}}_{4}^{ + }\). At the same time, acidification of the water layer adjacent to the gills facilitates \({\text{NH}}_{4}^{ + }\) excretion (Wright et al., 1989).
Very little is known about the hormonal regulation of \({\text{NH}}_{4}^{ + }\) excretion (Wilkie, 2002). Since ammonia and ammonium are natural end products of protein catabolism in fish (Wright and Wood, 2012), hormones stimulating such catabolism (for example, cortisol) should apparently promote the formation of ammonium and, possibly, its excretion.
Not all fish species are ammoniotelic. For example, the Gulf toadfish (Opsanus beta) has a fully functional ornithine-urea cycle; it is one of the few teleost fish excreting nitrogenous waste mainly in the form of urea (i.e., it is ureotelic) at the adult stage (Fulton et al., 2017). In nature, the Gulf toadfish excretes nitrogen in the form of 50% urea and 50% ammonia. The excretion of urea serves as a “chemical cloak,” that partially masks the smell of ammonia and protects against predators (Barimo, 2004). Under laboratory conditions, when a stressful environment is created (overcrowding or limited mobility) and is accompanied by an increase in the cortisol level, almost 100% ureotely can be induced in the Gulf toadfish (Hopkins et al., 1995; Wood et al., 1997, 2001). This process is associated with the activation of enzymes for urea production (Hopkins, et al., 1995) and an increase in the expression of tUT transporter protein in the gills, which is necessary to facilitate the diffusion of urea (Walsh et al., 2000). In this case, urea is excreted through tUT in separate impulses lasting from 1 to 3 h once or twice a day (Wood et al., 1995, 1997, 1998). In addition, cortisol is involved in the regulation of the pulsatile excretion of urea, since plasma cortisol levels fall 2 to 4 h before the urea-excretion pulse; excretion then occurs, and the cortisol level recovers quickly (Wood et al., 1997, 2001). A decreased cortisol level does not directly induce pulsatile urea secretion (Wood et al., 1997, 2001, 2003). This effect is believed to be mediated by serotonin and its receptor (Wood et al., 2003; Fulton et al., 2017).
Ureotelic fish are adapted to life in an alkaline environment with a very high pH (Wilkie and Wood, 1996). It is quite possible that production and secretion of urea are regulated by cortisol in these fish, too.
CONCLUSIONS
The successful existence of any fish species in a certain range of environmental salinity depends on the effectiveness of osmoregulatory mechanisms, in particular, the capacity for hormonal regulation. It is believed that modern teleost fish evolved from marine protovertebrates (Evans et al., 2005), which then repeatedly colonized fresh and brackish water bodies (Smith, 1932; Marshall, 2002; Ditrich, 2007; Dymowska et al., 2012). Colonization of the fresh water bodies would be impossible without adaptive rearrangements of the endocrine system and effector organs that performed osmoregulation, i.e., gills, intestines, and kidneys. The variety of gill ionocytes and regulating hormones in different fish species may be a consequence of the repeated colonization of fresh water bodies. This diversity was undoubtedly facilitated by the duplication of the entire genome that occurred during the formation of teleost fish and thus provided the genetic material necessary for evolution.
At the organ level, an essential feature of the fish endocrine system is the absence of parathyroid glands, and the presence of two additional glands that are absent in mammals, the corpuscles of Stannius and the urophysis (Bentley, 2002). The urophysis is located in the caudal part of the spinal cord and produces two hormones necessary for the existence of fish in a marine environment: urotensin I and urotensin II. On the example of several species of marine fish, it was reported that urotensin I stimulates the Cl– excretion, while urotensin II inhibits this process (Marshall, 2019). A detailed consideration of the function of urotensins is beyond the scope of this review, since it mainly examines the regulation of ion exchange in a freshwater environment.
Despite the observed diversity, many systems of ion regulation are conservative. First, this conservatism is manifested in the similarity of the transporter proteins that allow the ion transport in both fish gills and the kidneys of mammals, including humans. Second, some systems of hormonal regulation are rather conservative, e.g., the structure and signaling pathways of parathyroid hormone, calcitonin, vitamin D, and their receptors.
The mechanisms of hormonal regulation of ion exchange in fish have been discussed in many review articles and monographs (Bentley, 2002; Norris and Lopez, 2011; Dymowska et al., 2012; McCormick, 2012; Norris and Carr, 2013; Takei et al., 2014; Guh and Hwang, 2017; Baldisserotto, 2019; Yan and Hwang, 2019). Despite the active study of these mechanisms, there are many scientific issues that remain unresolved. In particular, the dual role of cortisol, which is manifested in the adaptation of fish to both sea and fresh water, is still unexplained. Cortisol is a stress hormone. Does this mean that stress can contribute to adaptation to changes in environmental salinity? Does this manifest itself in all fish, including those undergoing metamorphosis associated with spawning migration (e.g., salmonids and eels)? How does this relate to stress reactivity in different fish species?
It is also known that cortisol exerts both glucocorticoid and mineralocorticoid effects, both of which are mediated through GRs. The functional ligand(s) of the MRs remain unclear. What is the role of these receptors? Is it the same in fish of different groups?
The mechanism of the action of vitamin D is also a hot topic in fish biochemistry research. There have been many questions regarding this hormone-vitamin, some of which have been answered only in recent years. Where do fish get their vitamin D? If exposure to sunlight is necessary for this, then how can fish get this exposure, living at great depths underwater? Why do some fish have high levels of vitamin D? How is vitamin D deficiency manifested in fish?
In recent years, much attention has been paid to the paracrine and autocrine regulators, in particular, the so-called gasotransmitters, which include nitrogen monoxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S). There are a number of questions still unanswered. Is there the effect of such signaling molecules transmitted from individual to individual, especially in schooling fish? How do these molecules interact with industrial pollutants? How conservative are the effects of these mediators?
The presence of a large number of regulators suggests the possibility of cross-talk of their signaling pathways. The synergism of the effects of cortisol and growth hormone is an example of the intersection of signaling pathways in the mechanisms of osmoregulation. It is known that somatotropin increases both the expression of cortisol receptors in zebrafish gills and the sensitivity of the interrenal tissue of the kidneys to ACTH. However, interactions are also possible between different body systems. For example, glucocorticoids have an immunosuppressive effect in mammals. To what extent are these effects manifested in fish of different ecological and systematic groups? How does this affect the processes of osmoregulation?
Thus, the mechanisms of the regulation of ion exchange in fish are implemented by a complex system of ionocytes, ion channels, and hormones necessary for their coordinated work. Despite the significant advances in biology in recent decades, many questions remain to be answered in further research.
REFERENCES
Abbas, L., Hajihashemi, S., Stead, L.F., et al., Functional and developmental expression of a zebrafish Kir1.1 (ROMK) potassium channel homologue Kcnj1, J. Physiol., 2011, vol. 589, no. 6, pp. 1489–1503.
Abbink, W. and Flik, G., Parathyroid hormone-related protein in teleost fish, Gen. Comp. Endocrinol., 2007, vol. 152, nos. 2–3, pp. 243–251.
Arakawa, E., Hasegawa, S., Kaneko, T., and Hirano, T., Effects of changes in environmental calcium on prolactin secretion in Japanese eel, Anguilla japonica, J. Comp. Physiol. B., 1993, vol. 163, no. 2, pp. 99–106.
Barimo, J.F., Dogmas and controversies in the handling of nitrogenous wastes: ureotely and ammonia tolerance in early life stages of the gulf toadfish, Opsanus beta, J. Exp. Biol., 2004, vol. 207, no. 12, pp. 2011–2020.
Bayaa, M., Vulesevic, B., Esbaugh, A., et al., The involvement of SLC26 anion transporters in chloride uptake in zebrafish (Danio rerio) larvae, J. Exp. Biol., 2009, vol. 212, no. 20, pp. 3283–3295.
Bentley, P.J., The fishes, in Endocrines and Osmoregulation: A Comparative Account in Vertebrates, Berlin: Springer-Verlag, 2002, pp. 187–231.
Breves, J.P., McCormick, S.D., and Karlstrom, R.O., Prolactin and teleost ionocytes: new insights into cellular and molecular targets of prolactin in vertebrate epithelia, Gen. Comp. Endocrinol., 2014, vol. 203, pp. 21–28.
Chakrabarti, P. and Mukherjee, D., Studies on the hypocalcemic actions of salmon calcitonin and ultimobranchial gland extracts in the freshwater teleost Cyprinus carpio, Gen. Comp. Endocrinol., 1993, vol. 90, no. 3, pp. 267–273.
Chou M.-Y., Lin, C.-H., Chao P.-L., et al., Stanniocalcin-1 controls ion regulation functions of ion-transporting epithelium other than calcium balance, Int. J. Biol. Sci., 2015, vol. 11, no. 2, pp. 122–132.
Cruz, S.A., Chao, P.-L., and Hwang, P.-P., Cortisol promotes differentiation of epidermal ionocytes through Foxi3 transcription factors in zebrafish (Danio rerio), Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2013, vol. 164, no. 1, pp. 249–257.
Das, C., Thraya, M., and Vijayan, M.M., Nongenomic cortisol signaling in fish, Gen. Comp. Endocrinol., 2018, vol. 265, pp. 121–127.
Ditrich, H., The origin of vertebrates: a hypothesis based on kidney development, Zool. J. Linn. Soc., 2007, vol. 150, no. 2, pp. 435–441.
Duval, D., Durant, S., and Homo-Delarche, F., Non-genomic effects of steroids interactions of steroid molecules with membrane structures and functions, Biochim. Biophys. Acta, Rev. Biomembr., 1983, vol. 737, nos. 3–4, pp. 409–442.
Dymowska, A.K., Hwang, P.-P., and Goss, G.G., Structure and function of ionocytes in the freshwater fish gill, Respir. Physiol. Neurobiol., 2012, vol. 184, no. 3, pp. 282–292.
Edwards, S.L. and Marshall, W.S., Principles and patterns of osmoregulation and euryhalinity in fishes, in Fish Physiology, McCormick, S.D., Farrell, A.P., and Brauner, C.J., Eds., Amsterdam: Elsevier, 2012, vol. 32, pp. 1–44.
Esaki, M., Hoshijima, K., Kobayashi, S., et al., Visualization in zebrafish larvae of Na+ uptake in mitochondria-rich cells whose differentiation is dependent on foxi3a, Am. J. Physiol.: Integr. Comp. Physiol., 2007, vol. 292, no. 1, pp. R470–R480.
Esaki, M., Hoshijima, K., Nakamura, N., et al., Mechanism of development of ionocytes rich in vacuolar-type H+-ATPase in the skin of zebrafish larvae, Dev. Biol., 2009, vol. 329, no. 1, pp. 116–129.
Evans, D.H., Piermarini, P.M., and Choe, K.P., The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste, Physiol. Rev., 2005, vol. 85, no. 1, pp. 97–177.
Fish Osmoregulation, Baldisserotto, B., Mancera Romero, J.M., and Kapoor, B.G., Eds., Boca Raton, FL: CRC Press, 2019.
Flik, G. and Perry, S.F., Cortisol stimulates whole body calcium uptake and the branchial calcium pump in freshwater rainbow trout, J. Endocrinol., 1989, vol. 120, no. 1, pp. 75–82.
Flik, G., Fenwick, J.C., and Wendelaar Bonga, S.E., Calcitropic actions of prolactin in freshwater North American eel (Anguilla rostrata LeSueur), Am. J. Physiol.: Integr. Comp. Physiol., 1989a, vol. 257, no. 1, pp. R74–R79.
Flik, G., van der Velden, J.A., Seegers, H.C.M., et al., Prolactin cell activity and sodium fluxes in tilapia (Oreochromis mossambicus) after long-term acclimation to acid water, Gen. Comp. Endocrinol., 1989b, vol. 75, no. 1, pp. 39–45.
Flik, G., Atsma, W., Fenwick, J.C., et al., Homologous recombinant growth hormone and calcium metabolism in the tilapia, Oreochromis mossambicus, adapted to fresh water, J. Exp. Biol., 1993, vol. 185, no. 1, pp. 107–119.
Fraser, D.R., Evolutionary biology: mysteries of vitamin D in fish, in Vitamin D, Vol. 1: Biochemistry, Physiology and Diagnostics, Elsevier, 2018, pp. 13–27.
Fulton, J., LeMoine C.M.R., Bucking C., et al., A waterborne chemical cue from Gulf toadfish, Opsanus beta, prompts pulsatile urea excretion in conspecifics, Physiol. Behav., 2017, vol. 171, pp. 92–99.
Gensure, R.C., Ponugoti, B., Gunes, Y., et al., Identification and characterization of two parathyroid hormone-like molecules in zebrafish, Endocrinology., 2004, vol. 145, no. 4, pp. 1634–1639.
Graff, L.E., Lie, O., and Aksnes, L., In vitro hydroxylation of vitamin D3 and 25-hydroxy vitamin D3 in tissues of Atlantic salmon Salmo salar, Atlantic mackerel Scomber scombrus, Atlantic halibut Hippoglossus hippoglossus and Atlantic cod Gadus morhua, Aquat. Nutr., 1999, vol. 5, no. 1, pp. 23–32.
Guerreiro, P.M., Renfro, J.L., Power, D.M., et al., The parathyroid hormone family of peptides: structure, tissue distribution, regulation, and potential functional roles in calcium and phosphate balance in fish, Am. J. Physiol.: Integr. Comp. Physiol., 2007, vol. 292, no. 2, pp. R679–R696.
Guh, Y.-J. and Hwang, P.-P., Insights into molecular and cellular mechanisms of hormonal actions on fish ion regulation derived from the zebrafish model, Gen. Comp. Endocrinol., 2017, vol. 251, pp. 12–20.
Guh, Y.-J., Lin, C.-H., and Hwang, P.-P., Osmoregulation in zebrafish: ion transport mechanisms and functional regulation, EXCLI J., 2015, vol. 14, pp. 627–659.
Guh, Y.-J., Tseng, Y.-C., Yang, C.-Y., and Hwang, P.-P., Endothelin-1 regulates H+-ATPase-dependent transepithelial H+ secretion in zebrafish, Endocrinology, 2014, vol. 155, no. 5, pp. 1728–1737.
Guh, Y.-J., Yang, C.-Y., Liu, S.-T., et al., Oestrogen-related receptor α is required for transepithelial H+ secretion in zebrafish, Proc. R. Soc. B, 2016, vol. 283, no. 1825, p. 20152582.
Hayes, M.E., Guilland-Cumming, D.F., Russell, R.G.G., and Henderson, I.W., Metabolism of 25-hydroxycholecalciferol in a teleost fish, the rainbow trout (Salmo gairdneri), Gen. Comp. Endocrinol., 1986, vol. 64, no. 1, pp. 143–150.
Hickman, C.P., Jr., Ingestion, intestinal absorption, and elimination of seawater and salts in the southern flounder, Paralichthys lethostigma, Can. J. Zool., 1968, vol. 46, no. 3, pp. 457–466.
Hiroi, J. and McCormick, S.D., New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish, Respir. Physiol. Neurobiol., 2012, vol. 184, no. 3, pp. 257–268.
Hoenderop, J.G.J., Nilius, B., and Bindels, R.J.M., Calcium absorption across epithelia, Physiol. Rev., 2005, vol. 85, no. 1, pp. 373–422.
Holden, J.A., Layfield, L.L., and Matthews, J.L., The Zebrafish: Atlas of Macroscopic and Microscopic Anatomy, Cambridge: Cambridge Univ. Press, 2013.
Hopkins, T.E., Wood, C.M., and Walsh, P.J., Interactions of cortisol and nitrogen metabolism in the ureogenic gulf toadfish Opsanus beta, J. Exp. Biol., 1995, vol. 198, no. 10, pp. 2229–2235.
Horng, J.-L., Lin, L.-Y., Huang, C.-J., et al., Knockdown of V-ATPase subunit A (atp6v1a) impairs acid secretion and ion balance in zebrafish (Danio rerio), Am. J. Physiol.: Integr. Comp. Physiol., 2007, vol. 292, no. 5, pp. R2068–R2076.
Hoshijima, K. and Hirose, S., Expression of endocrine genes in zebrafish larvae in response to environmental salinity, J. Endocrinol., 2007, vol. 193, no. 3, pp. 481–491.
Hwang, P.-P. and Chou, M.-Y., Zebrafish as an animal model to study ion homeostasis, Pflügers Arch. Eur. J. Physiol., 2013, vol. 465, no. 9, pp. 1233–1247.
Kahloon, M.U., Aslam, A.K., Aslam, A.F., et al., Hyperkalemia induced failure of atrial and ventricular pacemaker capture, Int. J. Cardiol., 2005, vol. 105, no. 2, pp. 224–226.
Kakizawa, S., Kaneko, T., and Hirano, T., Elevation of plasma somatolactin concentrations during acidosis in rainbow trout (Oncorhynchus mykiss), J. Exp. Biol., 1996, vol. 199, no. 5, pp. 1043–1051.
Keys, A. and Willmer, E.N., ‘Chloride secreting cells’ in the gills of fishes, with special reference to the common eel, J. Physiol., 1932, vol. 76, no. 3, pp. 368–378.
Kiilerich, P., Kristiansen, K., and Madsen, S.S., Cortisol regulation of ion transporter mRNA in Atlantic salmon gill and the effect of salinity on the signaling pathway, J. Endocrinol., 2007, vol. 194, no. 2, pp. 417–427.
Kohan, D.E., Inscho, E.W., Wesson, D., and Pollock, D.M., Physiology of endothelin and the kidney, in Comprehensive Physiology, Hoboken, NJ: Wiley, 2011, pp. 883–919.
Kumai, Y., Bernier, N.J., and Perry, S.F., Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water, J. Endocrinol., 2014, vol. 220, no. 3, pp. 195–205.
Kumai, Y., Nesan, D., Vijayan, M.M., and Perry, S.F., Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor, Mol. Cell. Endocrinol., 2012a, vol. 364, nos. 1–2, pp. 113–125.
Kumai, Y., Ward, M.A.R., and Perry, S.F., β-Adrenergic regulation of Na+ uptake by larval zebrafish Danio rerio in acidic and ion-poor environments, Am. J. Physiol.: Integr. Comp. Physiol., 2012b, vol. 303, no. 10, pp. R1031–R1041.
Kwong, R.W.M. and Perry, S.F., Cortisol regulates epithelial permeability and sodium losses in zebrafish exposed to acidic water, J. Endocrinol., 2013, vol. 217, no. 3, pp. 253–264.
Kwong, R.W.M. and Perry, S.F., Hydrogen sulfide promotes calcium uptake in larval zebrafish, Am. J. Physiol.: Physiol., 2015, vol. 309, no. 1, pp. C60–C69.
Lafont, A.-G., Wang, Y.-F., Chen, G.-D., et al., Involvement of calcitonin and its receptor in the control of calcium-regulating genes and calcium homeostasis in zebrafish (Danio rerio), J. Bone Miner. Res., 2011, vol. 26, no. 5, pp. 1072–1083.
Lee, Y.-C., Yan, J.-J., Cruz, S.A., et al., Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+-ATPase-rich cells, Am. J. Physiol.: Physiol., 2011, vol. 300, no. 2, pp. C295–C307.
Liao, B.-K., Chen, R.-D., and Hwang, P.-P., Expression regulation of Na+-K+-ATPase α1-subunit subtypes in zebrafish gill ionocytes, Am. J. Physiol.: Integr. Comp. Physiol., 2009, vol. 296, no. 6, pp. R1897–R1906.
Liao, B.-K., Deng, A.-N., Chen, S.-C., et al., Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells, BMC Genomics, 2007, vol. 8, no. 1, p. 354.
Lin, H. and Randall, D.J., H+-ATPase activity in crude homogenates of fish gill tissue: inhibitor sensitivity and environmental and hormonal regulation, J. Exp. Biol., 1993, vol. 180, no. 1, pp. 163–174.
Lin, L.-Y., Horng, J.-L., Kunkel, J.G., and Hwang, P.-P., Proton pump-rich cell secretes acid in skin of zebrafish larvae, Am. J. Physiol.: Physiol., 2006, vol. 290, no. 2, pp. C371–C378.
Lin, T.-Y., Liao, B.-K., Horng, J.-L., et al., Carbonic anhydrase 2-like a and 15α are involved in acid-base regulation and Na+ uptake in zebrafish H+-ATPase-rich cells, Am. J. Physiol.: Physiol., 2008, vol. 294, no. 5, pp. C1250–C1260.
Lin, C.-H., Shih, T.-H., Liu, S.-T., et al., Cortisol regulates acid secretion of H+-ATPase-rich ionocytes in zebrafish (Danio rerio) embryos, Front. Physiol., 2015, vol. 6, p. 328.
Lin, C.-H., Su, C.-H., and Hwang, P.-P., Calcium-sensing receptor mediates Ca2+ homeostasis by modulating expression of PTH and stanniocalcin, Endocrinology, 2014, vol. 155, no. 1, pp. 56–67.
Lin, C.-H., Su, C.-H., Tseng, D.-Y., et al., Action of vitamin D and the receptor, VDRa, in calcium handling in zebrafish (Danio rerio), PLoS One, 2012, vol. 7, no. 9, p. e45650.
Lin, C.-H., Tsai, I.-L., Su, C.-H., et al., Reverse effect of mammalian hypocalcemic cortisol in fish: cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism, PLoS One, 2011, vol. 6, no. 8, p. e23689.
Manzon, L.A. The role of prolactin in fish osmoregulation: a review, Gen. Comp. Endocrinol., 2002, vol. 125, no. 2, pp. 291–310.
Marshall, W.S., Na+, Cl–, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis, J. Exp. Zool., 2002, vol. 293, no. 3, pp. 264–283.
Marshall, W.S., Rapid regulation of ion transport in mitochondrion-rich cells, in Fish Osmoregulation, Boca Raton, FL: CRC Press, 2019, pp. 395–426.
Marshall, W.S. and Grosell, M., Ion transport, osmoregulation, and acid-base balance, in The Physiology of Fishes, Boca Raton, FL: CRC Press, 2006, pp. 177–230.
McCormick, S.D., Endocrine control of osmoregulation in teleost fish, Am. Zool., 2001, vol. 41, no. 4, pp. 781–794.
McCormick, S.D., Smolt physiology and endocrinology, in Euryhaline Fishes, Amsterdam: Elsevier, 2012, pp. 199–251.
Nelson, D.R., Comparison of P450s from human and fugu: 420 million years of vertebrate P450 evolution, Arch. Biochem. Biophys., 2003, vol. 409, no. 1, pp. 18–24.
Norris, D.O. and Carr, J.A., Vertebrate Endocrinology, Amsterdam: Elsevier, 2013.
Norris, D.O. and Lopez, K.H., Hormones and Reproduction of Vertebrates, Vol. 1: Fishes, Amsterdam: Elsevier, 2011.
Okabe, M. and Graham, A., The origin of the parathyroid gland, Proc. Natl. Acad. Sci. U.S.A., 2004, vol. 101, no. 51, pp. 17716–17719.
Pan, T.-C., Liao, B.-K., Huang, C.-J., et al., Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish, Am. J. Physiol.: Integr. Comp. Physiol., 2005, vol. 289, no. 4, pp. R1202–R1211.
Patschan, D., Loddenkemper, K., and Buttgereit, F., Molecular mechanisms of glucocorticoid-induced osteoporosis, Bone, 2001, vol. 29, no. 6, pp. 498–505.
Perry, S.F., Vulesevic, B., Grosell, M., and Bayaa, M., Evidence that SLC26 anion transporters mediate branchial chloride uptake in adult zebrafish (Danio rerio), Am. J. Physiol.: Integr. Comp. Physiol., 2009, vol. 297, no. 4, pp. R988–R997.
Pickford, G.E. and Phillips, J.G., Prolactin, a factor in promoting survival of hypophysectomized killifish in fresh water, Science, 1959, vol. 130, no. 3373, pp. 454–455.
Pierson, P.M., Lamers, A., Flik, G., and Mayer-Gostan, N., The stress axis, stanniocalcin, and ion balance in rainbow trout, Gen. Comp. Endocrinol., 2004, vol. 137, no. 3, pp. 263–271.
Potts, W.T.W. and Evans, D.H., The effects of hypophysectomy and bovine prolactin on salt fluxes in freshwater-adapted Fundulus heteroclitus, Biol. Bull., 1966, vol. 131, no. 2, pp. 362–368.
Reid, S.G., Bernier, N.J., and Perry, S.F., The adrenergic stress response in fish: control of catecholamine storage and release, Comp. Biochem. Physiol. Part C: Pharmacol. Toxicol. Endocrinol., 1998, vol. 120, no. 1, pp. 1–27.
Sakamoto, T. and McCormick, S.D., Prolactin and growth hormone in fish osmoregulation, Gen. Comp. Endocrinol., 2006, vol. 147, no. 1, pp. 24–30.
Sasayama, Y., Suzuki, N., Oguro, C., et al., Calcitonin of the stingray: comparison of the hypocalcemic activity with other calcitonins, Gen. Comp. Endocrinol., 1992, vol. 86, no. 2, pp. 269–274.
Sasayama, Y., Ukawa, K.-I., Kai-Ya, H., et al., Goldfish calcitonin: purification, characterization, and hypocalcemic potency, Gen. Comp. Endocrinol., 1993, vol. 89, no. 2, pp. 189–194.
Shahsavarani, A. and Perry, S.F., Hormonal and environmental regulation of epithelial calcium channel in gill of rainbow trout (Oncorhynchus mykiss), Am. J. Physiol.: Integr. Comp. Physiol., 2006, vol. 291, no. 5, pp. R1490–R1498.
Shaughnessy, C.A. and McCormick, S.D., Reduced thermal tolerance during salinity acclimation in brook trout (Salvelinus fontinalis) can be rescued by prior treatment with cortisol, J. Exp. Biol., 2018, vol. 221, no. 6, p. jeb169557.
Shih, T.-H., Horng, J.-L., Hwang, P.-P., and Lin, L.-Y., Ammonia excretion by the skin of zebrafish (Danio rerio) larvae, Am. J. Physiol.: Physiol., 2008, vol. 295, no. 6, pp. C1625–C1632.
Shih, T.-H., Horng, J.-L., Lai, Y.-T., and Lin, L.-Y., Rhcg1 and Rhbg mediate ammonia excretion by ionocytes and keratinocytes in the skin of zebrafish larvae: H+-ATPase‑linked active ammonia excretion by ionocytes, Am. J. Physiol.: Integr. Comp. Physiol., 2013, vol. 304, no. 12, pp. R1130–R1138.
Shih, T.-H., Horng, J.-L., Liu, S.-T., et al., Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water, Am. J. Physiol.: Integr. Comp. Physiol., 2012, vol. 302, no. 1, pp. R84–R93.
Smith, H.W., Water regulation and its evolution in the fishes, Q. Rev. Biol., 1932, vol. 7, no. 1, pp. 1–26.
Sundell, K., Bishop, J.E., Björnsson, B.T., and Norman, A.W., 1,25-Dihydroxyvitamin D3 in the Atlantic cod: plasma levels, a plasma binding component, and organ distribution of a high affinity receptor, Endocrinology, 1992, vol. 131, no. 5, pp. 2279–2286.
Sundell, K., Norman, A.W., and Björnsson, B.T., 1,25(OH)2 Vitamin D3 increases ionized plasma calcium concentrations in the immature Atlantic cod Gadus morhua, Gen. Comp. Endocrinol., 1993, vol. 91, no. 3, pp. 344–351.
Suzuki, N., Danks, J.A., Maruyama, Y., et al., Parathyroid hormone 1 (1–34) acts on the scales and involves calcium metabolism in goldfish, Bone, 2011, vol. 48, no. 5, pp. 1186–1193.
Swarup, K., Das, V.K., and Norman, A.W., Dose-dependent vitamin D3 and 1,25-dihydroxyvitamin D3-induced hypercalcemia and hyperphosphatemia in male cyprinoid Cyprinus carpio, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1991, vol. 100, no. 2, pp. 445–447.
Takei, Y., Hiroi, J., Takahashi, H., and Sakamoto, T., Diverse mechanisms for body fluid regulation in teleost fishes, Am. J. Physiol.: Integr. Comp. Physiol., 2014, vol. 307, no. 7, pp. R778–R792.
Takeuchi, A., Comparative studies on vitamin D in vertebrates, especially in fish, Vitamins (Japan), 1994, vol. 68, pp. 55–64.
Torday, J.S., Evolution and cell physiology. 1. Cell signaling is all of biology, Am. J. Physiol.: Physiol., 2013, vol. 305, no. 7, pp. C682–C689.
Tseng, D.-Y., Chou, M.-Y., Tseng, Y.-C., et al., Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo, Am. J. Physiol.: Integr. Comp. Physiol., 2009, vol. 296, no. 3, pp. R549–R557.
van Abel, M., Hoenderop, J.G.J., van der Kemp, A.W.C.M., et al., Coordinated control of renal Ca2+ transport proteins by parathyroid hormone, Kidney Int., 2005, vol. 68, no. 4, pp. 1708–1721.
Villena, J.A. and Kralli, A., ERRα: a metabolic function for the oldest orphan, Trends Endocrinol. Metab., 2008, vol. 19, no. 8, pp. 269–276.
Wagner, G.F., Hampong, M., Park, C.M., and Copp, D.H., Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius, Gen. Comp. Endocrinol., 1986, vol. 63, no. 3, pp. 481–491.
Wagner, C.A., Mohebbi, N., Capasso, G., and Geibel, J.P., The anion exchanger pendrin (SLC26A4) and renal acid-base homeostasis, Cell. Physiol. Biochem., 2011, vol. 28, no. 3, pp. 497–504.
Walsh, P.J., Heitz, M.J., Campbell, C.E., et al., Molecular characterization of a urea transporter in the gill of the gulf toadfish (Opsanus beta), J. Exp. Biol., 2000, vol. 203, no. 15, pp. 2357–2364.
Wang, Y.-F., Tseng, Y.-C., Yan, J.-J., et al., Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl uptake mechanism in zebrafish (Danio rerio), Am. J. Physiol.: Integr. Comp. Physiol., 2009, vol. 296, no. 5, pp. R1650–R1660.
Wang, Y.-F., Yan, J.-J., Tseng, Y.-C., et al., Molecular physiology of an extra-renal Cl– uptake mechanism for body fluid Cl– homeostasis, Int. J. Biol. Sci., 2015, vol. 11, no. 10, pp. 1190–1203.
Wesson, D.E., Endothelins and kidney acidification, in Endothelin in Renal Physiology and Disease, Basel: Karger, 2011, pp. 84–93.
Wilkie, M.P., Ammonia excretion and urea handling by fish gills: present understanding and future research challenges, J. Exp. Zool., 2002, vol. 293, no. 3, pp. 284–301.
Wilkie, M.P. and Wood, C.M., The adaptations of fish to extremely alkaline environments, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1996, vol. 113, no. 4, pp. 665–673.
Wilson, J.M. and Laurent, P., Fish gill morphology: inside out, J. Exp. Zool., 2002, vol. 293, no. 3, pp. 192–213.
Wongdee, K. and Charoenphandhu, N., Regulation of epithelial calcium transport by prolactin: from fish to mammals, Gen. Comp. Endocrinol., 2013, vol. 181, pp. 235–240.
Wood, C., Hopkins, T., Hogstrand, C., and Walsh, P., Pulsatile urea excretion in the ureagenic toadfish Opsanus beta: an analysis of rates and routes, J. Exp. Biol., 1995, vol. 198, no. 8, pp. 1729–1741.
Wood, C., Hopkins, T., and Walsh, P., Pulsatile urea excretion in the toadfish (Opsanus beta) is due to a pulsatile excretion mechanism, not a pulsatile production mechanism, J. Exp. Biol., 1997, vol. 200, no. 6, pp. 1039–1046.
Wood, C.M., Gilmour, K.M., Perry, S.F., et al., Pulsatile urea excretion in gulf toadfish (Opsanus beta): evidence for activation of a specific facilitated diffusion transport system, J. Exp. Biol., 1998, vol. 201, no. 6, pp. 805–817.
Wood, C.M., McDonald, M.D., Sundin, L., et al., Pulsatile urea excretion in the gulf toadfish: mechanisms and controls, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2003, vol. 136, no. 4, pp. 667–684.
Wood, C.M., Warne, J.M., Wang, Y., et al., Do circulating plasma AVT and/or cortisol levels control pulsatile urea excretion in the gulf toadfish (Opsanus beta)? Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2001, vol. 129, no. 4, pp. 859–872.
Wright, P.A. and Wood, C.M., Seven things fish know about ammonia and we don’t, Respir. Physiol. Neurobiol., 2012, vol. 184, no. 3, pp. 231–240.
Wright, P.A., Randall, D.J., and Perry, S.F., Fish gill water boundary layer: a site of linkage between carbon dioxide and ammonia excretion, J. Comp. Physiol. B, 1989, vol. 158, no. 6, pp. 627–635.
Wu, S.-C., Horng, J.-L., Liu, S.-T., et al., Ammonium-dependent sodium uptake in mitochondrion-rich cells of medaka (Oryzias latipes) larvae, Am. J. Physiol.: Physiol., 2010, vol. 298, no. 2, pp. C237–C250.
Yan, J.-J., Chou, M.-Y., Kaneko, T., and Hwang, P.-P., Gene expression of Na+/H+ exchanger in zebrafish H+-ATPase-rich cells during acclimation to low-Na+ and acidic environments, Am. J. Physiol.: Physiol., 2007, vol. 293, no. 6, pp. C1814–C1823.
Yan, J.-J. and Hwang, P.-P., Novel discoveries in acid-base regulation and osmoregulation: a review of selected hormonal actions in zebrafish and medaka, Gen. Comp. Endocrinol., 2019, vol. 277, pp. 20–29.
Yeung, B.H.Y., Law, A.Y.S., and Wong, C.K.C., Evolution and roles of stanniocalcin, Mol. Cell. Endocrinol., 2012, vol. 349, no. 2, pp. 272–280.
Zhu, T., Zhang, T., Wang, Y., et al., Effects of growth hormone (GH) transgene and nutrition on growth and bone development in common carp, J. Exp. Zool. A, 2013, vol. 319, no. 8, pp. 451–460.
Funding
The work was carried out within the framework of the State Theme no. 0218-2019-0076 (agreement no. AAAA-A17-117031710039-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
Translated by D. Martynova
Rights and permissions
About this article
Cite this article
Rendakov, N.L. Ionocyte Functions and Hormonal Regulation of Ion Exchange in Fish. Biol Bull Rev 11, 616–631 (2021). https://doi.org/10.1134/S2079086421060074
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086421060074