Abstract
The endocrine system is an essential regulator of the osmoregulatory organs that enable euryhaline fishes to maintain hydromineral balance in a broad range of environmental salinities. Because branchial ionocytes are the primary site for the active exchange of Na+, Cl−, and Ca2+ with the external environment, their functional regulation is inextricably linked with adaptive responses to changes in salinity. Here, we review the molecular-level processes that connect osmoregulatory hormones with branchial ion transport. We focus on how factors such as prolactin, growth hormone, cortisol, and insulin-like growth-factors operate through their cognate receptors to direct the expression of specific ion transporters/channels, Na+/K+-ATPases, tight-junction proteins, and aquaporins in ion-absorptive (freshwater-type) and ion-secretory (seawater-type) ionocytes. While these connections have historically been deduced in teleost models, more recently, increased attention has been given to understanding the nature of these connections in basal lineages. We conclude our review by proposing areas for future investigation that aim to fill gaps in the collective understanding of how hormonal signaling underlies ionocyte-based processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishes, the most numerous and diverse vertebrates, consist of three major classes: Agnatha (jawless fishes), Chondrichthyes (cartilaginous fishes), and Osteichthyes (bony fishes) (Moyle and Cech 2004). Teleosts (class Osteichthyes; subclass Actinopterygii; infraclass Neopterygii; division Teleostei) and lampreys (members of class Agnatha) typically maintain extracellular fluids between 270 and 400 mOsm/kg, with Na+ and Cl− constituting the major dissolved ions (Hwang and Lin 2014; Ferreira-Martins et al. 2016). Therefore, when residing in dilute freshwater (FW) environments, they are at risk for both excessive hydration and salt loss across body surfaces. To counterbalance this situation, the gill actively absorbs ions (Na+, Cl−, and Ca2+) from the external environment, while the kidney and urinary bladder produce large volumes of dilute urine (Marshall and Grosell 2006; Kaneko et al. 2008). Lampreys and teleosts residing in seawater (SW), on the other hand, must excrete ions gained by passive diffusion from the surrounding environment and replace water that is lost via osmosis. While multiple segments of the gastrointestinal tract work in concert to promote solute-linked water absorption (Barany et al. 2020; Takei 2021), the gill and kidney secrete monovalent (Na+, Cl−) and divalent (Mg2+, Ca2+, and SO42−) ions into the external environment, respectively (Kaneko et al. 2008). Cartilaginous fishes are typically marine in their distribution and operate as osmoconformers by retaining urea and trimethylamine oxide while maintaining internal Na+ and Cl− concentrations below those of SW (Hwang and Lin 2014). Hagfishes (members of class Agnatha) are marine osmoconformers with limited capacities to regulate internal ion concentrations.
While most fishes inhabit a single aquatic environment characterized as either FW (≤ 0.5‰) or SW (30–40‰), a relatively small percentage of species (~ 5%) are considered “euryhaline” and can withstand both conditions (Schultz and McCormick 2013). Euryhaline species possess the capacity to rapidly modulate ion- and water-transporting activities within the gill, gastrointestinal tract, kidney, and urinary bladder following changes in salinity (Takei et al. 2014). In turn, they offer valuable opportunities to resolve how cellular and molecular processes within osmoregulatory organs enable fish to transition between environmental salinities. Since the branchial exchange of ions with the external environment is critical for maintaining osmoregulatory balance, decades of focused investigation have pursued how “ionocytes”, cells specialized for Na+, Cl−, and Ca2+ transport, operate relative to environmental salinity (Evans et al. 2005; Dymowska et al. 2012).
Molecular aspects of ionocyte function
Freshwater-type ionocytes in teleosts
Historically, various models have been put forth to explain how the branchial ionocytes of FW-acclimated fishes actively absorb ions against strong electrochemical gradients (Hwang and Lin 2014). The contrasting models of FW-type ionocytes reflect, in part, the evolution of different strategies for Na+ and Cl− uptake across the teleost lineage (Dymowska et al. 2012; Takei et al. 2014; Yan and Hwang 2019). For euryhaline teleosts, the most comprehensive models of FW-type ionocytes are derived from rainbow trout (Oncorhyncus mykiss), Mozambique tilapia (Oreochromis mossambicus), and Japanese medaka (Oryzias latipes) (Dymowska et al. 2012; Hsu et al. 2014; Inokuchi et al. 2022). For basal fishes, recent progress has been made in the development of FW-type ionocyte models for sea lamprey (Petromyzon marinus) (Ferreira-Martins et al. 2021). Without question, insights into how ionocytes operate in stenohaline zebrafish (Danio rerio) have supported progress in the euryhaline species listed above (Guh et al. 2015).
In FW-type ionocyte models for salmonids, largely conceived from findings in rainbow trout, two distinct subtypes absorb environmental Na+, Cl−, and Ca2+. In one subtype, termed peanut lectin agglutinin positive (PNA+) cells, Na+/H+ exchangers 2 and 3 (Nhe2 and -3; Slc9a2 and -3), epithelial Ca2+ channel (ECaC), and an Slc26-family anion exchanger are expressed in the apical membrane. Na+/K+-ATPase (Nka) mediates the basolateral movement of Na+, while an uncharacterized pathway allows for the exit of Cl− (Ivanis et al. 2008; Dymowska et al. 2012). The other ionocyte subtype, termed PNA− cells, expresses an apical Na+ channel, purported to be acid-sensing ion channel 4 (Asic4), along with apical H+-ATPase. Na+/HCO3− cotransporter 1 (Nbce1; Slc4a4) and Nka are expressed in PNA− cells to mediate the basolateral exit of Na+ (Parks et al. 2007; Dymowska et al. 2014).
Like in trout, there are multiple FW-type ionocytes operating within the branchial epithelium of euryhaline Mozambique tilapia. “Type II” ionocytes express a Na+/Cl− cotransporter (Ncc) in the apical membrane to transport Na+ and Cl− into the cell interior (Hiroi et al. 2008). This Ncc is denoted Ncc2 (Slc12a10) and is not a member of the “conventional” Ncc1 (Slc12a3) clade (Motoshima et al. 2023). Nka and Clc family Cl− channel 2c (Clc2c) support the basolateral transport of Na+ and Cl− from the ionocyte interior into the blood plasma, respectively (Pérez-Rius et al. 2015; Wang et al. 2015; Breves et al. 2017b). While Ncc2-expressing ionocytes operate in euryhaline and stenohaline species spanning teleost clades (Wang et al. 2009; Hsu et al. 2014; Inokuchi et al. 2017; Lema et al. 2018), they are conspicuously absent in salmonids (Hiroi and McCormick 2012). In tilapia, a second type of Na+-absorptive ionocyte which expresses Nka, coined “Type III” ionocytes, is characterized by the apical localization of Nhe3 (Hiroi et al. 2008). The density of Type III ionocytes (along with nhe3 expression) increases in the gill filaments of tilapia exposed to low-Na+ conditions (Inokuchi et al. 2008, 2009).
Freshwater-type ionocytes in basal fishes
In lampreys, two FW-adaptive ionocytes have been proposed to support ion uptake (Bartels and Potter 2004; Reis-Santos et al. 2008; Ferreira-Martins et al. 2021). These two ionocytes differ most notably in their expression of Nka and H+-ATPase. A “larval FW ionocyte” highly expresses H+-ATPase but shows low expression of Nka, whereas a “FW ionocyte” (observed in larvae as well as post-metamorphic and adult stages) strongly expresses both H+-ATPase and Nka. Branchial H+-ATPase E subunit (atp6v1e) expression markedly decreases when lamprey acclimate to elevated salinities (Reis-Santos et al. 2008; Ferreira-Martins et al. 2016). The ionoregulatory role of H+-ATPase in FW gills entails coordination with a pathway for the electrochemically neutral uptake of environmental Na+. The absorption of environmental Na+ by lampreys appears to involve the epithelial Na+ channel (ENaC) (Ferreira-Martins et al. 2016), while Ncc supports both Na+ and Cl− uptake (Barany et al. 2021b). Accordingly, both ENaC and Ncc are highly expressed in the gills of FW-acclimated lamprey and exhibit decreased expression during SW acclimation, although which particular cell-types express these transporters has not been fully elucidated. The co-involvement of an apical carbonic anhydrase-powered Cl−/HCO3− exchanger and a basolateral Cl−-channel in Cl− uptake has also been proposed, but the molecular identities of these transporters are unresolved (Bartels and Potter 2004; Ferreira-Martins et al. 2021).
Seawater-type ionocytes in teleosts
Within the branchial epithelium of marine/SW-acclimated teleosts, SW-type ionocytes actively secrete excess Na+ and Cl− into the environment. SW-type ionocytes express Nka and Na+/K+/2Cl− cotransporter 1 (Nkcc1; Slc12a2) in the basolateral membrane to energize and facilitate the Na+- and K+-coupled passage of Cl− from blood plasma into the cell interior (Marshall and Grosell 2006; Kaneko et al. 2008). The catalytic α-subunit of the Nka enzyme contains binding sites for ATP, Na+, and K+ (Geering 2008). Two distinct isoforms of the α-subunit (α1a and α1b) were identified in salmonids, first by Richards et al. (2003). In salmonids and cichlids, these isoforms have functional capacities exclusive to either FW (α1a) or SW (α1b), with branchial expression “switching” from one to the other during salinity transitions (Bystriansky et al. 2006; Nilsen et al. 2007; McCormick et al. 2009; Tipsmark et al. 2011; Dalziel et al. 2014). Apically located cystic fibrosis transmembrane conductance regulator 1 (Cftr1) enables Cl− to exit SW-type ionocytes and to enter the external environment (Marshall and Grosell 2006). With Nkcc1 and Cftr1 forming the pathway for transcellular Cl− excretion, tight-junction complexes composed of claudins (Cldns) between ionocytes and adjacent accessory cells provide the paracellular route for Na+ to exit the gill (Marshall and Grosell 2006; Tipsmark et al. 2008b; Bui and Kelly 2014). Attendant increases in branchial Nka, Nkcc1, and Cftr1 expression coincide with SW-acclimation. For this reason, all three ion transporters are widely employed as markers of branchial ion-secretory capacity.
Seawater-type ionocytes in basal fishes
The pathways for branchial Cl− secretion are far less resolved in basal fishes than in teleosts. Cftr orthologs are present in the genomes of sturgeon, bichir, and coelacanth (Shaughnessy and Breves 2021), yet none of these orthologs have been functionally characterized. A single Cftr ortholog was identified in sea lamprey; however, cftr expression is low in all larval, juvenile, and adult tissues aside from intestine (Ren et al. 2015). Moreover, compared with human Cftr, lamprey Cftr exhibits limited Cl− conductance and reduced activation by cAMP (Cui et al. 2019). Given the limited Cl− conductance of lamprey Cftr and the lack of a cftr transcriptional response to SW exposure (Shaughnessy et al. unpublished), it is questionable whether Cftr mediates the secretion of Cl− by lamprey ionocytes known to express Nka and Nkcc1 (Shaughnessy and McCormick 2020). A recent analysis of the updated inshore hagfish (Eptatretus burgeri) genome assembly (Yu et al. 2023; Marlétaz et al. 2023) indicates that a cftr ortholog may be absent in hagfishes altogether (Yamaguchi et al. 2023).
Hormones and ionocytes
The endocrine system has long been appreciated as a central player in the homeostatic regulation of salt and water balance in fishes. Perturbations in internal osmotic and ionic conditions caused by changes in environmental salinity elicit the secretion of hormones that modulate ion- and water-transport by key osmoregulatory organs. Because these regulatory connections are indispensable to maintaining hydromineral balance, there is no shortage of literature that discusses how hormones impact the osmoregulatory physiology of fishes at the organismal, organ, and cellular levels (Hirano 1986; McCormick 2001; Manzon 2002; Evans et al. 2005; Sakamoto and McCormick 2006; Takei and McCormick 2013; Takei et al. 2014). Therefore, in this review, we do not address all established hormonal actions within the gills of fishes; rather, we focus on how hormones control the molecular components of ionocytes. We focus on the regulatory connections identified in euryhaline species but, in several instances, reference stenohaline zebrafish for added context. An expansive collection of endocrine factors undeniably contributes to regulating branchial ionocytes (Evans et al. 2005; Takei et al. 2014); however, the identification of molecular endocrine targets is largely based on studies that focused upon the “classical” FW- and SW-adapting hormones in fishes, namely prolactin (Prl), growth hormone (Gh), and cortisol. While this review is heavily weighted toward describing the actions of these three hormones, we also highlight promising areas for future investigations into how additional endocrine factors regulate ionocytes.
Freshwater-adaptive endocrine control
Prolactin
Euryhaline models, and most famously, mummichogs (Fundulus heteroclitus), supported the discovery that pituitary hormones are key regulators of osmoregulatory organs (Pickford and Atz 1957). Pickford (1953) and Burden (1956) reported that hypophysectomized mummichogs could not survive in FW, and that pituitary brei injections rescued them from death. Prl was subsequently identified as the pituitary factor that enables individuals to reside in dilute environments (Pickford and Phillips 1959). Over the succeeding decades, it was firmly established that through its highly conserved actions on teleost osmoregulatory organs, Prl stimulates a spectrum of activities befitting FW-acclimation (Loretz and Bern 1982; Hirano 1986; Manzon 2002; Sakamoto and McCormick 2006; Breves et al. 2014a, 2020). Accordingly, pituitary prl expression and plasma Prl levels rise when fish acclimate to low-salinity conditions (Lee et al. 2006; Hoshijima and Hirose 2007; Fuentes et al. 2010; Seale et al. 2012). The notion that ionocytes are targets of Prl signaling was supported decades ago by the observation that Prl influences ionocyte populations in Mozambique and Nile (O. niloticus) tilapia (Herndon et al. 1991; Pisam et al. 1993; Flik et al. 1994). With respect to directing ionoregulatory function, Zhou et al. (2003) showed that exogenous Prl stimulated ion uptake in rainbow trout branchial epithelium. Patterns of Prl binding and prl receptor (prlr) gene expression reported in both euryhaline and stenohaline FW species further associated Prl signaling with ionocytes (Dauder et al. 1990; Prunet and Auperin 1994; Weng et al. 1997; Rouzic et al. 2002; Santos et al. 2001; Lee et al. 2006; Huang et al. 2007; Fiol et al. 2009; Breves et al. 2013). Additionally, the Prlr was localized to branchial ionocytes of tilapia and sea bream (Sparus aurata) (Weng et al. 1997; Santos et al. 2001).
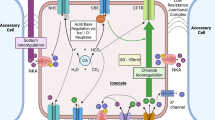
Only recently have investigations into the actions of Prl become unencumbered by a paucity of molecular tools to study FW-type ionocytes. For example, the characterization of tilapia Type II ionocytes by Hiroi et al. (2008) provided an opportunity to link Prl with a specific molecular pathway for ion uptake, particularly Ncc2. Prl enables hypophysectomized tilapia to recruit Ncc2-expressing ionocytes during FW acclimation, an activity that does not require systemic intermediaries (Breves et al. 2010c; Inokuchi et al. 2015; Watanabe et al. 2016) (Fig. 1). Prl similarly regulates branchial ncc2 expression in euryhaline mummichogs (Breves et al. 2022) and Japanese medaka (Bossus et al. 2017), as well as in stenohaline zebrafish (Breves et al. 2013). Activated Prlrs can modulate the transcription of target genes through JAK/STAT and ERK/MAPK signaling (Huang et al. 2007; Fiol et al. 2009; Chen et al. 2011). In medaka, Prl stimulates ncc2 via STAT5 activation rather than through ERK- or AKT-dependent pathways (Bollinger et al. 2018). Since Clc2c is expressed within Ncc2-expressing ionocytes to facilitate basolateral Cl− movement (Pérez-Rius et al. 2015; Wang et al. 2015), it is fitting that Prl coordinately promotes clc2c and ncc2 expression (Breves et al. 2017b; Breves 2019) (Fig. 1). In contrast, branchial clc3 expression in tilapia is not controlled by Prl (Tang and Lee 2011; Breves et al. 2017b).
Schematic diagrams of “Type II” and “Type III” ionocytes in Mozambique tilapia showing the stimulatory (arrows with a “ + ”) effects of prolactin (Prl) (see text for citations). Nka-α1a and Clc2c are included in these models based upon the expression of their associated gene transcripts; however, they have yet to be definitively assigned to tilapia ionocytes. Apical and basolateral sides are presented at the top and bottom of cells, respectively. Aqp3 aquaporin 3, Clc2c Clc family Cl− channel 2c, Ncc2 Na+/Cl− cotransporter 2, Nka Na+/K+-ATPase, Prl prolactin
The potential for Ncc-dependent pathways to operate in the osmoregulatory organs of cartilaginous and jawless fishes has recently received increased attention. In Japanese-banded houndshark (Triakis scyllium), a “conventional” ncc1 (slc12a3) is expressed within a subpopulation of gill ionocytes, termed type-B cells, where its expression increases upon transfer from full-strength SW to 30% SW (Takabe et al. 2016). Given that elasmobranch genomes are devoid of Ncc2-encoding genes (Motoshima et al. 2023), Ncc1 may assume a role in branchial Na+ and Cl− absorption in elasmobranchs. Similarly, the branchial expression of ncca (ncc1) in sea lamprey is attenuated during SW acclimation (Ferreira-Martins et al. 2016; Barany et al. 2021b). Given the Prlr expression in lamprey gills, the next step is to assess whether the recently found Prl participates in modulating ncca when lamprey transition between FW and marine environments (Gong et al. 2020).
In two lampreys (P. marinus and Lethenteron reissneri), the expression of gene transcripts encoding ENaC subunits increases under low-Na+ conditions (Ferreira-Martins et al. 2016; Tseng et al. 2022). Thus, ENaC may provide a means for lampreys to absorb Na+ from FW; this strategy for Na+ absorption is absent in cartilaginous and ray-finned fishes (Ferreira-Martins et al. 2021). Curiously, branchial gene expression of an ENaC subunit, scnn1a, decreases when inshore hagfish experience high-salinity conditions (Yamaguchi et al. 2023). Despite hagfishes exhibiting plasma Na+ concentrations close to SW, this response suggests that Na+ movement in the gill may be more complex than previously thought. To our knowledge, endocrine control of ENaC subunit expression has not been addressed in any cyclostome and, in an analogous fashion as ncca, should be probed for links to the Prlrs identified in hagfish and lamprey (Gong et al. 2020).
While branchial ionocytes leveraging Ncc operate in species across the three major fish lineages, they are not found within salmonids (Hiroi and McCormick 2012). In turn, an apically located Cl−/HCO3− exchanger (Slc26a6) may provide a pathway for Cl− absorption by PNA+ ionocytes in rainbow trout and other salmonids (Boyle et al. 2014; Leguen et al. 2015). Branchial slc26a6a2 is elevated in FW- versus SW-acclimated Atlantic salmon (Takvam et al. 2021) and is a transcriptional target of Prl signaling (Breves et al. unpublished). Therefore, Slc26a6a2 may constitute a pathway for Prl-stimulated Cl− uptake in species lacking Ncc-expressing ionocytes (Zhou et al. 2003). Because Leguen et al. (2015) reported clc2 expression in trout ionocytes (putative PNA+ cells), Prl-based control of salmonid clc2 isoforms also warrants investigation. Studies of this nature will enable comparisons of Prl-Clc2 connectivity between species that do, and do not, leverage Ncc2-expressing ionocytes.
Within the PNA− ionocytes of trout, Nbce1 supports the absorption of environmental Na+ by cotransporting Na+ and HCO3− across the basolateral membrane (Parks et al. 2007; Leguen et al. 2015). The apical entry of Na+ into PNA− cells was proposed to occur via Asic4 through its electrochemical linkage to H+-ATPase (Dymowska et al. 2014). Under this scenario, intracellular HCO3− is supplied by carbonic anhydrase (Parks et al. 2007). In tilapia, Nbce1 operates in the basolateral membrane of Ncc2-expressing ionocytes (Furukawa et al. 2011). To our knowledge, Nbce1, Asic4, H+-ATPase, and carbonic anhydrase have not been associated with Prl signaling in trout or tilapia.
In addition to Type II ionocytes, a second type of Na+-absorptive ionocyte in tilapia (Type III ionocytes) is characterized by the apical expression of Nhe3 (Hiroi et al. 2008). Prl promotes nhe3 gene expression in tilapia gill filaments (Inokuchi et al. 2015; Watanabe et al. 2016) whereas it has no such effect in mummichogs or zebrafish (Breves et al. 2013, 2022) (Fig. 1). Because salmonids express Nhe2 and -3 within PNA+ ionocytes, they will prove key in resolving the extent to which Prl regulates Nhes among teleosts (Ivanis et al. 2008; Hiroi and McCormick 2012). Unfortunately, the lack of information on Nhes in lamprey ionocytes precludes consideration of a Prl-Nhe connection (Ferreira-Martins et al. 2021). Recent pharmacological experiments performed in zebrafish implicated K+-dependent Na+/Ca2+ exchangers (Nckxs) in mediating Na+ absorption (Clifford et al. 2022). Should roles emerge for Nckxs in supporting Na+ uptake by euryhaline species, Nckx isoforms would be additional candidates for regulation by Prl.
Nka plays a critical role in energizing ion transport by FW- and SW-type ionocytes, with the reciprocal expression of nka-α1a and -α1b first described in salmonids transitioning between FW and SW environments (Richards et al. 2003; Mackie et al. 2005; Bystriansky et al. 2006; Madsen et al. 2009; McCormick et al. 2009; Dalziel et al. 2014). Tilapia also undergo nka-α1a and -α1b “switching” upon salinity changes, and Prl stimulates the “FW-inducible” nka-α1a isoform (Tipsmark et al. 2011; Breves et al. 2014b; Inokuchi et al. 2015; Watanabe et al. 2016) (Fig. 1). Thus far, the capacity for Prl to promote nka-α1a expression seems specific to tilapia, as Prl fails to stimulate nka-α1a in Atlantic salmon (Tipsmark and Madsen 2009; Breves et al. unpublished). In zebrafish, nka-α1a1a.2 is expressed in Ncc2-expressing ionocytes responsible for Cl− uptake (Liao et al. 2009); however, Prl has no effect on branchial nka-α1a1a.2 expression (Breves 2019). The auxiliary γ-subunit of Nka (also called Fxyd) participates in the regulation of enzymatic activity by associating with the Na+/K+ pump complex (Geering 2008; Pavlovic et al. 2013). Among the Fxyd isoforms identified in teleosts, Fxyd11 is predominately expressed in the gills where it interacts with Nka (Tipsmark 2018; Wang et al. 2008; Saito et al. 2010). In tilapia, Prl and cortisol synergistically promote fxyd11 expression in FW (Tipsmark et al. 2011).
For teleosts residing in FW, greater than 90% of whole-body Ca2+ uptake is mediated by branchial/epidermal ionocytes (Flik et al. 1996; Lin and Hwang 2016). Transcellular Ca2+ uptake entails the apical entry of Ca2+ through ECaC (Trpv5/6) followed by basolateral exit via Ca2+-ATPase 2 (Pmca2) and Na+/Ca2+ exchanger 1 (Ncx1) (Flik et al. 1996; Liao et al. 2007). Prl is hypercalcemic in multiple teleosts (Pang et al. 1978; Fargher and McKeown 1989; Flik et al. 1989, 1994; Kaneko and Hirano 1983; Chakraborti and Mukherjee 1995; Wongdee and Charoenphandhu 2013), at least in part by stimulating branchial Pmca activity (Flik et al. 1996). Future investigations employing both euryhaline and stenohaline FW models are needed to determine whether Prl promotes ECaC and Ncx1 expression in parallel with promoting Pmca activity to sustain Ca2+ uptake.
Aquaporins (Aqps) constitute a superfamily of integral membrane proteins that facilitate passive movements of water and small non-ionic compounds across cell membranes (Cerdà and Finn 2010). Multiple branchial cell types, including ionocytes, express a subset of Aqps (Lignot et al. 2002; Hirata et al. 2003; Watanabe et al. 2005; Tse et al. 2006; Brunelli et al. 2010; Tingaud-Sequeira et al. 2010; Tipsmark et al. 2010; Jung et al. 2012; Breves et al. 2016; Ruhr et al. 2020). Prl stimulates the expression of the aquaglyceroporin, Aqp3, in Mozambique tilapia (Breves et al. 2016) (Fig. 1), Japanese medaka (Ellis et al. 2019), and mummichogs (Breves et al. 2022). On the other hand, Prl does not promote branchial aqp1 expression (Ellis et al. 2019). Although the Aqp-specific effects of Prl suggest that Aqp3 plays an important role in FW-acclimated fish, there is still no clear picture of how it underlies adaptive processes. A role for Aqp3 in enhancing transepithelial water movement appears unlikely because branchial water exchange is disadvantageous to systemic hydromineral balance. Alternatively, Aqp3 may render ionocytes osmosensitive to extracellular conditions and/or capable of efficiently regulating their volume (Cutler and Cramb 2002; Watanabe et al. 2005; Tipsmark et al. 2010).
Prl has long been recognized for its effects on membrane permeability which result in a general “tightening” to minimize diffusive ion loss (Potts and Evans 1966; Hirano 1986). Paracellular solute movements across epithelia are governed in large part by the barrier properties of tight-junction complexes composed of Cldn and occludin proteins (Chasiotis et al. 2012). In tilapia and medaka, FW acclimation entails the increased expression of branchial cldn28a and -28b, respectively (Tipsmark et al. 2008a; Bossus et al. 2015). In Atlantic salmon and medaka, Prl stimulates cldn28a and -28b gene expression (Tipsmark et al. 2009; Bossus et al. 2017). Prl-Cldn28 connectivity thus provides a means to regulate tight-junction properties for minimizing ion loss in FW. Occludin expression is also correlated with environmental salinity (Chasiotis et al. 2009; Kumai et al. 2011; Whitehead et al. 2011), making it a good candidate for regulation by Prl; however, to our knowledge, this link has yet to be examined.
Teleosts express two separate Prlrs, denoted Prlr1 (Prlra) and -2 (Prlrb), that differ in their responses to salinity changes (Huang et al. 2007; Pierce et al. 2007; Fiol et al. 2009; Tomy et al. 2009; Rhee et al. 2010; Breves et al. 2011, 2013; Chen et al. 2011; Flores and Shrimpton 2012). Branchial prlr1 has emerged as a transcriptional target of Prl in tilapia, mummichogs, and zebrafish (Inokuchi et al. 2015; Breves et al. 2013, 2022). In turn, Prl seemingly upregulates the expression of Prlr1 to enhance the sensitivity of ionocytes to circulating hormone during FW acclimation (Weng et al. 1997). Alternatively, prlr2/b is typically insensitive to Prl (Breves et al. 2013, 2022; Inokuchi et al. 2015), which is not surprising given that its expression is upregulated by the hyperosmotic extracellular conditions associated with SW acclimation (Fiol et al. 2009; Inokuchi et al. 2015; Seale et al. 2019).
In tandem with initiating active ion uptake, euryhaline species must attenuate branchial ion secretion when transitioning from SW to FW. While promoting the recruitment of FW-type ionocytes and the expression of their associated ion transporters, Prl simultaneously dampens cellular and molecular phenotypes appropriate for SW conditions. For instance, Herndon et al. (1991) observed that Prl reduced the size and number of SW-type ionocytes in tilapia. At the molecular level, Prl inhibits the transcription of nkcc1 and cftr1 within the SW-type ionocytes of medaka and mummichogs (Bossus et al. 2017; Breves et al. 2022) (Fig. 2). Prl also inhibits branchial Nka activity and nka-α1b expression (Pickford et al. 1970a; Sakamoto et al. 1997; Shrimpton and McCormick 1998; Kelly et al. 1999; Mancera et al. 2002; Tipsmark and Madsen 2009), which, like nkcc1 and cftr1, are elevated in SW to support ion secretion. Recall that while Cftr1 is the conduit for Cl− to exit SW-type ionocytes, tight junction complexes between ionocytes and accessory cells provide the paracellular path for Na+ to exit the gill. The cation-selective tight-junctions adjacent to ionocytes are composed of multiple Cldn10 isoforms (Tipsmark et al. 2008b; Bui and Kelly 2014). Among the four mummichog cldn10 genes (cldn10c, -10d, -10e, and -10f) upregulated in response to SW (Marshall et al. 2018), cldn10f is the only transcript downregulated by Prl (Breves et al. 2022) (Fig. 2). Collectively, these nkcc1, cftr1, and cldn10f responses illustrate the various means by which Prl inhibits branchial salt secretion.
Schematic diagrams of FW (freshwater)- and SW (seawater)-type ionocytes in mummichogs showing the stimulatory (arrows with a “ + ”) and inhibitory (blocked lines with a “−”) effects of prolactin (Prl) (see text for citations). Where Cl− transport is indicated with a question mark, a pathway is presumed to exist but remains uncharacterized. Apical and basolateral sides are presented at the top and bottom of cells, respectively. Aqp3 aquaporin 3, Cftr1 cystic fibrosis transmembrane conductance regulator 1, Cldn10f claudin 10f, Ncc2 Na+/Cl− cotransporter 2, Nka Na+/K+-ATPase, Nkcc1 Na+/K+/2Cl− cotransporter 1, Prl prolactin, TJ tight-junction. Figure adapted from Breves et al. (2022)
Growth hormone and somatolactin
As discussed in "Growth hormone and insulin-like growth-factors", Gh is conventionally regarded as a “SW-adapting hormone” because it promotes the survival of euryhaline fishes (and especially salmonids) in hyperosmotic environments (Björnsson 1997; Takei et al. 2014). To our knowledge, there is no direct evidence that Gh plays a role in regulating FW-type ionocytes. Nonetheless, Gh receptors (Ghrs) are expressed in the gills of euryhaline species regardless of whether they are acclimated to FW or SW (Pierce et al. 2007; Poppinga et al. 2007; Breves et al. 2011; Link et al. 2010); therefore, Ghrs are at least present to mediate any direct regulatory connections between circulating Gh and FW-type ionocytes. It is certainly plausible that Gh may indirectly regulate FW-type ionocytes through the synthesis of insulin-like growth-factors (Igfs) (Reinecke et al. 1997; Berishvili et al. 2006; Reindl and Sheridan 2012). In fact, black-chinned tilapia (Sarotherodon melanotheron) exhibit enhanced ghr and igf1 expression in the gill during FW acclimation (Link et al. 2010). Similarly, zebrafish exhibit elevated pituitary gh and branchial ghr (ghra and -b), igf1a, and -2a expression when challenged with ion-poor conditions (Hoshijima and Hirose 2007; Breves et al. unpublished). However, whether the Gh/Igf system supports the molecular responses of tilapia and zebrafish ionocytes to FW/ion-poor conditions has yet to be determined.
Somatolactin (Sl), a member of the Gh/Prl-family of pituitary hormones, is a putative regulator of various physiological processes in fishes, particularly Ca2+ homeostasis (Kaneko and Hirano 1983). Rainbow trout transferred to Ca2+-rich FW exhibit reduced sl gene expression in the pituitary, a response that is consistent with Sl having hypercalcemic activity (Kakizawa et al. 1993). Given the substantial progress made toward understanding how ionocytes absorb environmental Ca2+ (Lin and Hwang 2016), a reassessment of whether Sl is indeed hypercalcemic is warranted by probing targets such as ECaC, Pmca2, and Ncx1.
Cortisol
Cortisol is typically deemed a “SW-adapting hormone” because it directly stimulates the activities and/or expression of transporters tied to branchial ion-secretion ("Corticosteroids"). The recognition that cortisol also promotes ion uptake in some teleosts arrived after its SW-adaptive role was firmly established (McCormick 2001; Takei and McCormick 2013). Morphological responses to cortisol in the gills of rainbow trout and American eel (Anguilla rostrata) suggested that FW-type ionocytes are targets of cortisol signaling (Perry et al. 1992), a notion that would be later supported with the development of molecular tools to more precisely study FW-type ionocytes. In tilapia, medaka, and zebrafish, Nhe3 and Ncc2 are expressed in distinct ionocyte subtypes (Hiroi and McCormick 2012; Hsu et al. 2014; Guh et al. 2015). In zebrafish, cortisol stimulates Na+ uptake in a fashion dependent upon the presence of Nhe3b-expressing ionocytes and promotes the differentiation of Ncc2-expressing ionocytes from a progenitor population (Kumai et al. 2012; Cruz et al. 2013a). While cortisol similarly promotes ncc2 expression in medaka (Bossus et al. 2017; Ellis et al. 2019), this is not the case in tilapia (Breves et al. 2014b; Watanabe et al. 2016).
The FW-adaptive role of cortisol in zebrafish appears to be mediated solely by the glucocorticoid receptor (Gr) rather than the mineralocorticoid receptor (Mr) (Cruz et al. 2013b). The zebrafish Gr is expressed by Nka-rich branchial and epidermal ionocytes, with knockdown of gr, but not mr, disrupting the development of FW-type ionocytes through the action of forkheadbox I3 transcription factors (Foxi3a and -b) (Cruz et al. 2013b). Exogenous cortisol increases nhe3b, H+-ATPase α-subunit (atp6v1a), and ecac expression in zebrafish embryos. In medaka embryos, knockdown of gr2, but not gr1 or mr, decreases the total number of epidermal ionocytes (Trayer et al. 2013). Conversely, in FW-acclimated tilapia, it was suggested that the Mr, rather than the Gr, controls cortisol-mediated development of Nka-rich branchial ionocytes (Wu et al. 2023). Accordingly, mr expression occurs in ionocyte precursors/epidermal stem cells (Wu et al. 2023).
In Atlantic salmon, cortisol upregulates gene transcription and protein abundance of the “FW-inducible” Nka-α1a isoform (Kiilerich et al. 2007b; McCormick et al. 2008; Tipsmark and Madsen 2009). Cortisol also upregulates the “SW-inducible” Nka-α1b isoform (Kiilerich et al. 2007b; Tipsmark and Madsen 2009; Breves et al. 2024), and thus, the capacity of cortisol to increase the expression of both Nka-α1a and -α1b is indicative of its dual role in promoting FW- and SW-adaptive processes. While cortisol was shown to stimulate branchial carbonic anhydrase activity in trout (Gilmour et al. 2011), to our knowledge, no ion transporters expressed in salmonid FW-type ionocytes outside of Nka (e.g., Nhe2, -3, Asic4, ECaC, and Nbce1) have been linked with cortisol. This is a significant knowledge gap, especially given that cortisol is known to stimulate Ca2+ uptake by ECaC-expressing ionocytes in zebrafish (Lin and Hwang 2016). Reminiscent of the scenario for Prl ("Prolactin"), future work is warranted to resolve whether cortisol affects Ca2+ uptake pathways in euryhaline species.
In addition to promoting key mediators of ion uptake (e.g., Ncc2, Nhe3, and Nka-α1a), cortisol promotes FW acclimation by decreasing the paracellular permeability of the branchial epithelium (Kelly and Wood 2002; Zhou et al. 2003; Kolosov and Kelly 2017). This important contribution to FW acclimation is achieved through the regulation of specific tight-junction proteins. For instance, cortisol increases the expression of cldn8d, -10c, -10d, -10e, -10f, -11a, -27a, -30c, and -33b in various euryhaline species (Tipsmark et al. 2009; Bui et al. 2010; Bossus et al. 2017; Kolosov and Kelly 2017). Finally, it certainly must be recognized that cortisol can promote FW acclimation by acting in concert with Prl (Eckert et al. 2001; McCormick 2001). For instance, from a molecular perspective, Prl and cortisol act synergistically to promote branchial nka-α1a and cldn28b expression in tilapia and medaka, respectively (Watanabe et al. 2016; Bossus et al. 2017).
Thyroid hormones
Although limited, there is evidence that thyroid hormones are involved in the control of FW-adaptive branchial processes. Unfortunately, information is particularly scant regarding plasma thyroxine (T4) and 3-3′-5-triiodothyronine (T3) levels in euryhaline species undergoing FW acclimation. In sea bream, plasma T4 levels increase following transfer from SW to FW (Klaren et al. 2007). Alternatively, Mozambique tilapia acclimating to FW exhibit rapid declines in both plasma T4 and T3 (Seale et al. 2021). While the dynamics of T4 and T3 in tilapia suggest a hyposmotically-induced suppression of thyroid hormone production at the systemic level, at the level of the gill, these changes coincide with an increase in the outer-ring deiodination activity of deiodinase 2 (Dio2). As shown in mummichogs, Dio2 expression/activity is activated by hyposmotic stress (López-Bojórquez et al. 2007). Thus, increased branchial Dio2 activity supports the local production of T3 at a time when the recruitment of ionocytes is activated following entry into FW (Hiroi et al. 2008; Breves et al. 2021). Accordingly, tilapia treated with T4 exhibit an increase in the density and size of presumed FW-type ionocytes (Peter et al. 2000). It remains to be seen whether these cellular responses to T4 manifest changes in branchial ncc2, nhe3, and clc2c expression.
Seawater-adaptive endocrine control
Growth hormone and insulin-like growth-factors
Although much of the early attention given to the Gh/Igf system in fishes was driven by its potential application to understanding growth in aquaculture settings, the osmoregulatory actions of both Gh and Igf1 have emerged as important aspects of the hormonal control of osmoregulation. In salmonids, Gh is integral to the timing of parr-smolt transformation and the associated development of SW tolerance (Hoar 1988; Björnsson 1997; McCormick 2013), and accordingly, plasma Gh levels increase during smolting (Boeuf et al. 1989; Prunet et al. 1989; Young et al. 1989; McCormick et al. 2007, 2013; Nilsen et al. 2008). The SW-adaptive role for Gh is not restricted to salmonids, as in both salmonid and non-salmonid teleost species, exposure to SW corresponds with elevated plasma Gh levels alongside with increased gh gene expression, Gh protein content, and somatotroph numbers in the pituitary (Deane and Woo 2009). As shown in Mozambique tilapia, somatotrophs release Gh in direct response to hyperosmotic extracellular conditions (Seale et al. 2002). Importantly, treatment with Gh upregulates branchial Nka activity and improves the SW tolerance of several euryhaline teleosts (Madsen 1990a, b; McCormick 1996; Xu et al. 1997; Mancera and McCormick 1998; Pelis and McCormick 2001). Intraperitoneal injection of Gh also increases Nkcc1 protein abundance within SW-type ionocytes (Pelis and McCormick 2001) and stimulates nka-α1b and nkcc1 expression (Tipsmark and Madsen 2009), although these effects were most pronounced when Gh was co-administered with cortisol.
Ghrs are expressed in teleost gills (Gray et al. 1990; Yao et al. 1991; Sakamoto and Hirano 1991); however, they have yet to be localized to any discrete branchial cell-types. It was initially reported that rainbow trout acclimating to SW do not exhibit changes in branchial Gh binding (Sakamoto and Hirano 1991). More recent molecular analyses describe variable branchial ghr expression patterns with respect to SW acclimation. In Atlantic salmon, ghr expression has been seen to increase (Kiilerich et al. 2007a; Nilsen et al. 2008) or not change at all (Breves et al. 2017a) during smolting. Likewise, there is little consistency in branchial ghr patterns following SW exposure, with increases, decreases, and no changes in expression all having been observed across several species (Kiilerich et al. 2007a; Nilsen et al. 2008; Breves et al. 2010a, b; Flores and Shrimpton 2012; Einarsdóttir et al. 2014; Breves et al. 2017a; Link et al. 2022). Additionally, Gh-treated gill explants from coho salmon (Oncorhynchus kisutch) and Nile tilapia did not exhibit changes in Nka activity, or nka-α1b and nkcc1 gene expression (McCormick et al. 1991; Breves et al. 2014b). Rather than directly regulating the expression of specific ion-transporters, Gh may exert cytogenic effects that promote the recruitment of branchial ionocytes (Madsen 1990a, b; Flik et al. 1993; Prunet et al. 1994). For instance, Gh-elicited increases in Nka activity and Nkcc1 in Atlantic salmon were coincident with the increased abundance of ionocytes (Pelis and McCormick 2001).
Gh is the primary regulator of the production and release of Igf1 and -2 from the liver (Pierce et al. 2011; Reindl and Sheridan 2012). Branchial igf1 receptor (igf1r) expression increases during smolting and upon exposure to SW (Nilsen et al. 2008; Shimomura et al. 2012), and increased circulating Igf1 levels correlate with elevated branchial Nka activity (Agustsson et al. 2001; McCormick et al. 2007; Shimomura et al. 2012). However, not all studies have observed rises in plasma Igf1 during smolting (Nilsen et al. 2008; Breves et al. 2017a). Intraperitoneal injection of Atlantic salmon with Igf1 increases SW tolerance but only marginally impacts gill Nka activity (McCormick 1996) whereas Nkcc1 in isolated Japanese eel (Anguilla japonica) gill cells is stimulated by Igf1 (Tse et al. 2007). In addition to exerting osmoregulatory actions as endocrine signals (i.e., secreted from the liver and acting upon ionocytes) (Madsen and Bern 1993), Igf1 and -2 may also operate as autocrine/paracrine signals (i.e., produced by and acting upon ionocytes) (Berishvili et al. 2006; Tipsmark and Madsen 2009). In Atlantic salmon, Nilsen et al. (2008) reported increases in gill igf1 and igf1r during smolting and SW acclimation, even when no increase in circulating Igf1 was detected. Similarly, Breves et al. (2017a) observed increases in branchial igf2 and igf1ra expression in smolts following SW exposure.
The promotion of SW-adaptive ionoregulatory capacities by Gh may be best explained by its interaction with cortisol to promote both the proliferation of ionocytes and their responsiveness to cortisol (McCormick 2013). Studies using salmonids demonstrated that cortisol interacts with the Gh/Igf system to affect SW-type ionocytes. The co-administration of cortisol with either Gh or Igf1 increases gill Nka activity to levels beyond those induced by treatment with each hormone individually (Madsen 1990a, b; Madsen and Korsgaard 1991; McCormick 1996). Scenarios proposed to underlie the apparent synergistic actions of cortisol and Gh include, (1) Gh promotes Gr abundance in ionocytes, thereby increasing the capacity for cortisol to affect ion transporter expression, and (2) Gh promotes ionocyte proliferation while cortisol promotes the differentiation of ionocytes (McCormick 2013). Thus, future work should leverage recent insights into the regulators of ionocyte differentiation, such as forkhead box transcription factors (Hsiao et al. 2007), to elucidate how Gh and cortisol shape SW-type ionocyte populations.
Recent studies also describe the potential for Gh and Igf1 to regulate SW-adaptive branchial processes in lampreys. Kawauchi et al. (2002) were the first to identify a lamprey Gh capable of stimulating hepatic igf1 expression. Later, Gh-like cells in the lamprey pituitary were shown to increase in abundance during metamorphosis (Nozaki et al. 2008). Discovery of the Ghr, Prlr, and Prl itself in sea lamprey spurred recent investigations into their regulatory roles (Gong et al. 2022). Although pituitary gh and prl expression are upregulated during sea lamprey metamorphosis (Gong et al. 2022), it was later shown that gh also increases in the pituitary of non-metamorphosing larvae over the same period (Ferreira-Martins et al. 2023). Thus, such increases in gh expression may be seasonal, and it remains unclear whether the same is true for pituitary prl expression. In any case, branchial ghr and prlr gene expression also increases during metamorphosis (Gong et al. 2020; Ferreira-Martins et al. 2023). Because similar increases do not occur in non-metamorphosing larval lamprey (Ferreira-Martins et al. 2023), heightened ghr and prlr expression likely underlies developmental (as opposed to seasonal) processes. Substantial increases in hepatic and branchial igf1 expression also occur throughout metamorphosis, and therefore, endocrine as well as autocrine/paracrine actions of Igf1 may operate in lamprey (Ferreira-Martins et al. 2023). Surprisingly, SW exposure does not affect pituitary gh expression, hepatic igf expression, or branchial ghr and igf1 expression (Gong et al. 2020, 2022; Ferreira-Martins et al. 2023) and treatment with recombinant Gh does not affect branchial ion transporters (Gong et al. 2022). Future studies in lamprey are warranted to assess whether Gh and Igf1 promote the recruitment of SW-type ionocytes through cytogenic actions.
Corticosteroids
In lobe-finned fishes (Sarcopterygii) and tetrapods, cortisol (or, in some cases, corticosterone) and aldosterone are the products of the corticosteroid biosynthesis pathway and the predominant circulating hormones. Cortisol and aldosterone separately regulate carbohydrate metabolism and osmoregulation by interacting with the Gr and Mr, respectively. In all other fishes, corticosteroids and their receptors mediate both carbohydrate metabolism and osmoregulation. However, important differences exist between fish groups, particularly with respect to the milieu of corticosteroids in circulation and the identity and expression of receptors that mediate their actions. Here, we focus on corticosteroids that are known to directly regulate branchial processes in fishes.
Non-sarcopterygian fishes lack aldosterone synthase (Cyp11b2) and consequently the ability to synthesize aldosterone (Baker 2003; Takahashi and Sakamoto 2013). In actinopterygian fishes, cortisol is the predominant corticosteroid present in circulation, with 11-deoxycorticosterone and corticosterone present at far lower concentrations (Prunet et al. 2006). Among the circulating corticosteroids in actinopterygians, cortisol has both glucocorticoid and mineralocorticoid activity. To a far lesser extent, 11-deoxycorticosterone also exhibits mineralocorticoid-like actions (Takahashi and Sakamoto 2013). Chondrichthyan fishes produce a novel steroid biosynthetic product, 1α-hydroxycorticosterone, which exhibits some mineralocorticoid-like action (Anderson 2012). However, chondrichthyans do not utilize branchial processes for bulk ion secretion but rather use the salt-secretory rectal gland (Wright and Wood 2015); therefore, the potential ionoregulatory actions of 1α-hydroxycorticosterone will not be discussed here. Lampreys apparently lack 11β-hydroxylase (Cyp11b1) and cannot produce cortisol or corticosterone (Bridgham et al. 2006; Close et al. 2010; Rai et al. 2015). Thus, 11-deoxycortisol and 11-deoxycorticosterone are the most abundant circulating corticosteroids in lampreys and exhibit capacities to regulate branchial ionoregulatory activities (Close et al. 2010; Shaughnessy et al. 2020).
Chondrichthyan and actinopterygian fishes express both classes of corticosteroid receptors (Gr and Mr). In actinopterygians, it has long been held that the ionoregulatory actions of corticosteroids result from cortisol acting through the Gr. While this remains true, recent discoveries have added some nuance to this perspective. For instance, particular teleosts express two distinct Gr orthologs (Bury et al. 2003) as well as an Mr (Colombe et al. 2000). Knowledge of these three corticosteroid receptor subtypes has motivated investigations into how the actions of cortisol and 11-deoxycorticosterone are differentially mediated by these receptors (see below). Interestingly, lamprey do not express Gr or Mr but rather an ancestral “corticoid receptor” (Cr) that facilitates the osmoregulatory actions of 11-deoxycortisol (Bridgham et al. 2006; Close et al. 2010; Shaughnessy et al. 2020).
Using adult sea lamprey, Close et al. (2010) demonstrated that 11-deoxycortisol elicits an increase in branchial Nka activity. Later, Shaughnessy et al. (2020) described how 11-deoxycortisol supports the acquisition of SW tolerance during metamorphosis. Plasma 11-deoxycortisol levels and gill Cr abundance both increase during metamorphosis and are positively correlated with gill Nka activity. Accordingly, the treatment of mid-metamorphic lamprey with 11-deoxycortisol improves SW tolerance and increases gill Nka and Nkcc1 protein expression (Shaughnessy et al. 2020; Barany et al. 2021a). Likewise, 11-deoxycortisol increases the expression of nka and nkcc1 transcripts in gill explants (Shaughnessy et al. 2020). Interestingly, 11-deoxycorticosterone can elicit modest increases in branchial nka and nkcc1 expression but is far less potent than 11-deoxycortisol (Shaughnessy et al. 2020). Future studies are warranted to further elucidate the ionoregulatory roles of 11-deoxycortisol and 11-deoxycorticosterone, and particularly whether they interact with Gh and Prl.
Cortisol has long been known to support the acclimation of teleosts to SW. Multiple lines of evidence have described this role, including early studies demonstrating that plasma cortisol increases during salmonid parr-smolt transformation and upon exposure to SW (Fontaine and Hatey 1954; Specker and Schreck 1982; Langhorn and Simpson 1986; Shrimpton et al. 1994), and that SW tolerance is increased following cortisol treatment (Bisbal and Specker 1991). Elevations in plasma cortisol following exposure to SW also occur in numerous non-salmonid species (McCormick 2001). Early work described the direct action of cortisol to increase gill Nka activity, which correlated with the development of SW tolerance during smolting (Langhorn and Simpson 1986; McCormick and Saunders 1987). Additional studies showed that gill Nka activity can be impacted in vivo by cortisol injections (Pickford et al. 1970b; Bisbal and Specker 1991; McCormick et al. 1991) and in vitro by exposing gill explants to cortisol-containing media (McCormick and Bern 1989).
More recently, cortisol was shown to regulate proteins and gene transcripts expressed by SW-type ionocytes, such as Nka, Nkcc1, and Cftr (Fig. 3). Atlantic salmon interperitoneally injected with cortisol increase the expression of nka-α1b (McCormick et al. 2008; Tipsmark and Madsen 2009; Breves et al. 2020, 2024) and the protein abundance of Nka and Nkcc1 (Pelis and McCormick 2001). In gill explants from FW- and SW-acclimated Atlantic salmon, cortisol increases nka-α1b and nkcc1 expression (Tipsmark et al. 2002; Kiilerich et al. 2007b, 2011a, b, c). In vivo treatment with cortisol increases cftr1 expression in Atlantic salmon parr and smolts (Singer et al. 2003; Breves et al. 2020, 2024), and in vitro exposure of gill explants to cortisol increases cftr1 and nkcc1 (Kiilerich et al. 2007b). Likewise, cortisol promotes cftr1 and nkcc1 expression in the gills of FW-acclimated trout and medaka (Tipsmark et al. 2002; Kiilerich et al. 2011a; Bossus et al. 2017). In tilapia and striped bass (Morone saxatilis), cortisol similarly promotes branchial nkcc1 expression (Kiilerich et al. 2011c). Cortisol also promotes components of SW-type ionocytes in non-teleost models, such as Nka and Nkcc1 in Atlantic and Persian sturgeon (Acipenser oxyrhynchus and A. persicus) (Khodabandeh et al. 2009; McCormick et al. 2020).
Schematic diagram of SW (seawater)-type ionocytes showing the stimulatory (arrows with a “+”) and inhibitory (blocked lines with a “−”) effects of cortisol (Cort) (see text for citations). Apical and basolateral sides are presented at the top and bottom of cells, respectively. Aqp3 aquaporin 3, Cftr1 cystic fibrosis transmembrane conductance regulator 1, Cldn10s claudin 10 isoforms, Cort cortisol, Nka Na+/K+-ATPase, Nkcc1 Na+/K+/2Cl− cotransporter 1, TJ tight-junction
Fewer studies have examined the molecular actions of 11-deoxycorticosterone, as it circulates at far lower concentrations than cortisol. Intraperitoneal injection of 11-deoxycorticosterone has no effect on SW tolerance or branchial nka-α1a and -α1b expression in Atlantic salmon (McCormick et al. 2008). The in vitro effects of 11-deoxycorticosterone vary depending on whether treated filaments are collected from salmon acclimated to either FW or SW. 11-deoxycorticosterone is more effective in stimulating nka-α1a versus -α1b expression (Kiilerich et al. 2007b, 2011a, b), although this effect is generally far less consistent than that of cortisol.
The role of the Gr in mediating the ionoregulatory actions of cortisol in teleosts has also received considerable attention. Early studies demonstrated that a corticosteroid receptor expressed in the gills with high binding affinity for cortisol increases during parr-smolt transformation and SW acclimation (Weisbart et al. 1987; Maule and Schreck 1990; Shrimpton and Randall 1994; Shrimpton et al. 1994; Marsigliante et al. 2000). Moreover, Gr expression is strongly correlated with the capacity for cortisol to stimulate branchial Nka activity (Shrimpton and McCormick 1999). Following the discovery of two distinct Grs (Bury et al. 2003) and an Mr (Colombe et al. 2000; Sturm et al. 2005) in teleost fishes, studies using selective receptor antagonists investigated their individual roles in mediating the actions of cortisol and 11-deoxycorticosterone. It was proposed that the Gr and Mr underlie the duality of cortisol operating as a FW- and SW-adapting hormone (Prunet et al. 2006). In support of this, the upregulation of gr expression occurs in the gills of several species during smolting or following SW exposure (Mazurais et al. 1998; Mizuno et al. 2001; Kiilerich et al. 2007a; Nilsen et al. 2008; Yada et al. 2014; Bernard et al. 2020), and a potential role for the Mr in FW ionoregulation has been suggested (Sloman et al. 2001; Scott et al. 2005; Kiilerich et al. 2011a). The ionoregulatory role of the Mr in FW may entail activation by both cortisol and 11-deoxycorticosterone, as the Mr is potently activated by both hormones (Sturm et al. 2005; Katsu et al. 2018). Investigations into the regulation of gr and mr during smolting or SW acclimation have generally presented mixed results. In some studies, only the gr is upregulated during smolting (Kiilerich et al. 2007a, 2011b; Nilsen et al. 2008), and in others, the transcriptional upregulation of both receptors occurred (Yada et al. 2014; Bernard et al. 2020). Similarly, there seems to be little consistency in how gr and mr are transcriptionally regulated during SW acclimation in salmonids (Kiilerich et al. 2007b, 2011a; Nilsen et al. 2008; Flores and Shrimpton 2012) as well as in non-salmonids (Aruna et al. 2012a, b).
Several in vivo and in vitro studies have employed receptor blockade approaches, including the cotreatment of corticosteroids with mammalian Gr and Mr antagonists (e.g., RU486 and spironolactone, respectively). Cotreatment with RU486 blocks the upregulation of branchial nka-α1a and -α1b by cortisol, whereas cotreatment with spironolactone has no effect on SW tolerance or nka-α1a and -α1b expression (McCormick et al. 2008). Kiilerich et al. (2007b) demonstrated using Atlantic salmon gill explants that both RU486 and spironolactone can block the ability of cortisol to upregulate nka-α1a, -α1b, and cftr1. However, these results were not consistent across species or salinities (Kiilerich et al. 2007b, 2011b, c). In teleosts, RU486 antagonizes both Gr1 and -2, with more potent effects on Gr1 (Bury et al. 2003). On the other hand, spironolactone is now known to agonize the fish Mr, activating it with similar potency as cortisol, 11-deoxycorticosterone, and aldosterone (Sugimoto et al. 2016; Fuller et al. 2019). Thus, studies which use RU486 and spironolactone to differentially block the Gr and Mr should be interpreted with caution. Considering the challenges associated with pharmacologically targeting the fish Gr and Mr, advanced molecular approaches using transcriptional knockdown or transgenic knockout have emerged to investigate the Gr and Mr (Faught and Vijayan 2018; Yan and Hwang 2019). To date, these approaches have mostly been leveraged to investigate the metabolic, developmental, and ionoregulatory actions of corticosteroids in zebrafish (Faught and Vijayan 2018; Yan and Hwang 2019), which cannot tolerate SW. However, Japanese medaka offer a promising euryhaline model for knockdown or knockout approaches (Yan and Hwang 2019) and is therefore poised to delineate the Gr- and Mr-mediated actions of corticosteroids on SW-type ionocytes.
In tetrapods, the interaction of aldosterone with the Mr is facilitated by coexpression of the Mr with the cortisol-inactivating enzyme, 11β-hydroxylase 2 (Cyp11b2). Interestingly, a strong transcriptional upregulation of cyp11b2 occurs in the gills of smolting Atlantic salmon (Kiilerich et al. 2007a; Nilsen et al. 2008). It was also shown in trout branchial epithelial cells that cortisol increases cyp11b2 expression (Kolosov and Kelly 2019). These findings suggest the operation of a tissue-level mechanism to regulate cortisol signaling. A better understanding of which branchial cell-types specifically express cyp11b2 is needed to assess its role in tuning the actions of cortisol on ionocytes.
The role of corticosteroids in regulating permeability of the branchial epithelium has also received considerable attention. This work has largely focused on the FW-adaptive, rather than the SW-adaptive, roles of corticosteroids, as the increased expression of tight-junction proteins generally promotes epithelial tightening. However, “leaky” tight-junction complexes composed of Cldn10s contribute to SW-adaptation by facilitating the paracellular excretion of Na+ (Tipsmark et al. 2008b; Bui and Kelly 2014). Acclimation to SW increases the expression of cldn10 isoforms in puffer fish (Tetraodon nigroviridis) (Bui et al. 2010) and exposing gill explants to cortisol stimulates multiple cldn10s in medaka (Bossus et al. 2017). Cortisol and 11-deoxycorticosterone generally upregulate the expression of Cldns through processes mediated by both the Gr and Mr (Tipsmark et al. 2009; Bui et al. 2010; Chasiotis and Kelly 2011, 2012; Kelly and Chasiotis 2011; Bossus et al. 2017; Kolosov et al. 2017b; Kolosov and Kelly 2019). In sea lamprey, multiple claudins have been identified that are expressed in the gill, and among those investigated, cldn3 and -10 orthologs increase their expression after exposure to ion-poor water and exhibit decreases during SW acclimation (Kolosov et al. 2017a, 2020). Future studies in lamprey should seek to address whether 11-deoxycortisol and 11-deoxycorticosterone control branchial barrier functions via Cldns.
Cortisol was the first hormone linked with the expression of branchial Aqps. FW-acclimated eels infused with cortisol show a marked decrease in the expression of aqp3 in the gill (Cutler et al. 2007) (Fig. 3). Choi et al. (2013) subsequently reported that cortisol diminishes branchial aqp3 and -8 expression in sockeye salmon (Oncorhynchus nerka). These patterns suggest that SW-induced increases in plasma cortisol are responsible for rapidly attenuating aqp3 expression upon entry into hyperosmotic environments (Cutler and Cramb 2002; Cutler et al. 2007). Furthermore, cortisol blocks the stimulatory action of Prl on aqp3 (Breves et al. 2016). The regulation of branchial Aqp3 is a clear example of antagonistic, rather than synergistic, roles for cortisol and Prl in promoting salinity acclimation.
Thyroid hormones
In addition to supporting FW acclimation ("Thyroid hormones"), there is evidence that thyroid hormones promote SW-adaptive processes by acting directly on ionocytes and through interactions with the Gh/Igf system (McCormick 2001). For example, coho salmon and mummichogs increase plasma T4 levels in response to SW (Knoeppel et al. 1982; Specker and Kobuke 1987), and Atlantic salmon and summer flounder (Paralichthys dentatus) treated with T4 or T3 exhibit increased SW tolerance (Refstie 1982; Saunders et al. 1985; Schreiber and Specker 1999). Accordingly, when summer flounder and mummichogs are treated with thiourea (an inhibitor of T4 synthesis), they exhibit diminished hyposmoregulatory capacities (Knoeppel et al. 1982; Schreiber and Specker 1999). Thiourea diminishes the SW tolerance of flounder by disrupting the thyroid-mediated development of SW-type ionocytes during metamorphosis (Schreiber and Specker 2000). To our knowledge, there has been no direct assessment of whether the rapid recruitment of SW-type ionocytes that occurs in euryhaline species when they encounter SW is linked with thyroid hormone signaling.
Future perspectives
The availability of genomic resources and molecular tools over the last two decades has given rise to an increasingly mechanistic understanding of how hormones regulate ionocytes. This trend will undoubtedly continue with manipulative molecular tools such as gene editing ushering in new opportunities to link hormones and their cognate receptors with specific ion transporters. Zebrafish have already proven to be a valuable model for this purpose, supporting progress toward understanding the ontogeny and function of ion-absorptive ionocytes (Chen et al. 2019). Nonetheless, the poor salinity tolerance of zebrafish precipitates the need for a similarly amenable euryhaline model, a need that Japanese medaka seem poised to fill (Yan and Hwang 2019). In a similar vein, refined methods for primary cell culture of the branchial epithelium would accelerate the use of advanced molecular manipulations; however, progress in this endeavor has been limited.
The various modes by which endocrine factors can affect branchial processes deserve continued attention. For example, it is necessary to better resolve the cytogenic (controlling ionocyte abundance), molecular (controlling the expression of ion transporters), and physiological (controlling the function of ion transporters) actions of hormones (Breves et al 2014a; Shir-Mohammadi and Perry 2020). Important in this endeavor will be the characterization of, (1) the factors influencing the differentiation of SW-type ionocytes from precursor cells (analogous to how Foxi3a and -b regulate FW-type ionocyte differentiation in zebrafish), (2) the regulatory elements in the promoters and distal regulatory regions of genes encoding ion transporters, and (3) the functional elements of the ion transporters themselves (such as the motifs facilitating ATP binding and phosphorylation).
Despite the recent progress, there are still many gaps to fill in the collective understanding of how ionocytes operate—this is especially true for non-teleost fishes. For example, it stands unresolved whether Slc26-family anion exchangers, Clc family Cl− channels, and Cftr sustain Cl− transport in the ionocytes of lampreys and sturgeons (Ferreira-Martins et al. 2021; Shaughnessy and Breves 2021). We foresee that some of these transporters/channels will emerge as hormone targets. The recent expansion of genomic resources in non-teleosts will certainly support work of this nature (Amemiya et al. 2013; Smith et al. 2013, 2018; Braasch et al. 2016; Vialle et al. 2018; Cheng et al. 2019; Du et al. 2020; Yamaguchi et al. 2020; Marlétaz et al. 2023).
Finally, future work should seek to better understand how systemic hormones interact with the osmotic stress signaling cascades that permit ionocytes to directly perceive salinity changes (Fiol and Kültz 2007). For instance, cortisol promotes the expression of osmotic stress transcription factor 1 (Ostf1) during the acute phase of SW acclimation (McGuire et al. 2010). While Prl inhibits the activity of SW-type ionocytes (Fig. 2), it remains to be seen whether Prl dampens the expression of intracellular and paracrine factors that respond to hyperosmotic conditions (e.g., Ostf1, serum- and glucocorticoid-inducible kinase 1, 14-3-3 proteins, MAPKs, endothelin 1, interleukins, and tumor necrosis factor α) (Fiol and Kültz 2007; Notch et al. 2012; Kültz 2015; Lai et al. 2015). Given the multifactorial nature of osmotic stress signaling (Fiol and Kültz 2007), and the myriad hormones that impact branchial processes (Evans et al. 2005; Takei et al. 2014), it will be interesting to learn the extent to which ionocytes are a hub for interactions between intracellular, paracrine, and systemic signals.
References
Agustsson T, Sundell K, Sakamoto T et al (2001) Growth hormone endocrinology of Atlantic salmon (Salmo salar): pituitary gene expression, hormone storage, secretion and plasma levels during parr-smolt transformation. J Endocrinol 170:227–234. https://doi.org/10.1677/joe.0.1700227
Amemiya CT, Alföldi J, Lee AP et al (2013) The African coelacanth genome provides insights into tetrapod evolution. Nature 496(7445):311–316. https://doi.org/10.1038/nature12027
Anderson WG (2012) The endocrinology of 1α-hydroxycorticosterone in elasmobranch fish: a review. Comp Biochem Physiol A 162:73–80. https://doi.org/10.1016/j.cbpa.2011.08.015
Aruna A, Nagarajan G, Chang CF (2012a) Involvement of corticotrophin-releasing hormone and corticosteroid receptors in the brain–pituitary–gill of tilapia during the course of seawater acclimation. J Neuroendocrinol 24:818–830. https://doi.org/10.1111/j.1365-2826.2012.02282.x
Aruna A, Nagarajan G, Chang CF (2012b) Differential expression patterns and localization of glucocorticoid and mineralocorticoid receptor transcripts in the osmoregulatory organs of tilapia during salinity stress. Gen Comp Endocrinol 179:465–476. https://doi.org/10.1016/j.ygcen.2012.08.028
Baker ME (2003) Evolution of glucocorticoid and mineralocorticoid responses: go fish. Endocrinology 144:4223–4225. https://doi.org/10.1210/en.2003-0843
Barany A, Shaughnessy CA, Fuentes J, Mancera JM, McCormick SD (2020) Osmoregulatory role of the intestine in the sea lamprey (Petromyzon marinus). Am J Physiol Regul Integr Comp Physiol 318(2):R410–R417. https://doi.org/10.1152/ajpregu.00033.2019
Barany A, Shaughnessy CA, McCormick SD (2021a) Corticosteroid control of Na+/K+-ATPase in the intestine of the sea lamprey (Petromyzon marinus). Gen Comp Endocrinol 307:113756. https://doi.org/10.1016/j.ygcen.2021.113756
Barany A, Shaughnessy CA, Pelis RM et al (2021b) Tissue and salinity specific Na+/Cl- cotransporter (NCC) orthologues involved in the adaptive osmoregulation of sea lamprey (Petromyzon marinus). Sci Rep 11:22698. https://doi.org/10.1038/s41598-021-02125-1
Bartels H, Potter IC (2004) Cellular composition and ultrastructure of the gill epithelium of larval and adult lampreys: implications for osmoregulation in fresh and seawater. J Exp Biol 207:3447–3462. https://doi.org/10.1242/jeb.01157
Berishvili G, D’Cotta H, Baroiller JF, Segner H, Reinecke M (2006) Differential expression of IGF-I mRNA and peptide in the male and female gonad during early development of a bony fish, the tilapia Oreochromis niloticus. Gen Comp Endocrinol 146(3):204–210. https://doi.org/10.1016/j.ygcen.2005.11.008
Bernard B, Leguen I, Mandiki SNM et al (2020) Impact of temperature shift on gill physiology during smoltification of Atlantic salmon smolts (Salmo salar L.). Comp Biochem Physiol A Mol Integr Physiol 244:110685. https://doi.org/10.1016/j.cbpa.2020.110685
Bisbal GA, Specker JL (1991) Cortisol stimulates hypo-osmoregulatory ability in Atlantic salmon, Salmo salar L. J Fish Biol 39:421–432. https://doi.org/10.1111/j.1095-8649.1991.tb04373.x
Björnsson BT (1997) The biology of salmon growth hormone: from daylight to dominance. Fish Physiol Biochem 17:9–24. https://doi.org/10.1023/A:1007712413908
Boeuf G, Le Bail PY, Prunet P (1989) Growth hormone and thyroid hormones during Atlantic salmon, Salmo salar L., smolting, and after transfer to seawater. Aquaculture 82:257–268. https://doi.org/10.1016/0044-8486(89)90413-4
Bollinger RJ, Ellis LV, Bossus MC, Tipsmark CK (2018) Prolactin controls Na+, Cl– cotransporter via Stat5 pathway in the teleost gill. Mol Cell Endocrinol 477:163–171. https://doi.org/10.1016/j.mce.2018.06.014
Bossus MC, Madsen SS, Tipsmark CK (2015) Functional dynamics of claudin expression in Japanese medaka (Oryzias latipes): response to environmental salinity. Comp Biochem Physiol A Mol Integr Physiol 187:74–85. https://doi.org/10.1016/j.cbpa.2015.04.017
Bossus MC, Bollinger RJ, Reed PJ, Tipsmark CK (2017) Prolactin and cortisol regulate branchial claudin expression in Japanese medaka. Gen Comp Endocrinol 240:77–83. https://doi.org/10.1016/j.ygcen.2016.09.010
Boyle D, Clifford AM, Orr E, Chamot D, Goss GG (2014) Mechanisms of Cl- uptake in rainbow trout: cloning and expression of slc26a6, a prospective Cl-/HCO3- exchanger. Comp Biochem Physiol A Mol Integr Physiol 180:43–50. https://doi.org/10.1016/j.cbpa.2014.11.001
Braasch I, Gehrke AR, Smith JJ et al (2016) The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet 48(4):427–437. https://doi.org/10.1038/ng.3526
Breves JP (2019) Prolactin controls branchial clcn2c but not atp1a1a.2 in zebrafish Danio rerio. J Fish Biol 94(1):168–172. https://doi.org/10.1111/jfb.13854
Breves JP, Fox BK, Pierce AL et al (2010a) Gene expression of growth hormone family and glucocorticoid receptors, osmosensors, and ion transporters in the gill during seawater acclimation of Mozambique tilapia, Oreochromis mossambicus. J Exp Zool 313A:432–441. https://doi.org/10.1002/jez.613
Breves JP, Hasegawa S, Yoshioka M et al (2010b) Acute salinity challenges in Mozambique and Nile tilapia: differential responses of plasma prolactin, growth hormone and branchial expression of ion transporters. Gen Comp Endocrinol 167:135–142. https://doi.org/10.1016/j.ygcen.2010.01.022
Breves JP, Watanabe S, Kaneko T et al (2010c) Prolactin restores branchial mitochondrion-rich cells expressing Na+/Cl− cotransporter in hypophysectomized Mozambique tilapia. Am J Physiol Regul Integr Comp Physiol 299(2):R702–R710. https://doi.org/10.1152/ajpregu.00213.2010
Breves JP, Seale AP, Helms RE et al (2011) Dynamic gene expression of GH/PRL-family hormone receptors in gill and kidney during freshwater-acclimation of Mozambique tilapia. Comp Biochem Physiol A 158(2):194–200. https://doi.org/10.1016/j.cbpa.2010.10.030
Breves JP, Serizier SB, Goffin V et al (2013) Prolactin regulates transcription of the ion uptake Na+/Cl- cotransporter (ncc) gene in zebrafish gill. Mol Cell Endocrinol 369(1–2):98–106. https://doi.org/10.1016/j.mce.2013.01.021
Breves JP, McCormick SD, Karlstrom RO (2014a) Prolactin and teleost ionocytes: new insights into cellular and molecular targets of prolactin in vertebrate epithelia. Gen Comp Endocrinol 203:21–28. https://doi.org/10.1016/j.ygcen.2013.12.014
Breves JP, Seale AP, Moorman BP et al (2014b) Pituitary control of branchial NCC, NKCC and Na+, K+-ATPase α-subunit gene expression in Nile tilapia, Oreochromis niloticus. J Comp Physiol B 184:513–523. https://doi.org/10.1007/s00360-014-0817-0
Breves JP, Inokuchi M, Yamaguchi Y et al (2016) Hormonal regulation of aquaporin 3: opposing actions of prolactin and cortisol in tilapia gill. J Endocrinol 230(3):325–337. https://doi.org/10.1530/JOE-16-0162
Breves JP, Fujimoto CK, Phipps-Costin SK et al (2017a) Variation in branchial expression among insulin-like growth-factor binding proteins (igfbps) during Atlantic salmon smoltification and seawater exposure. BMC Physiol 17:1–11. https://doi.org/10.1186/s12899-017-0028-5
Breves JP, Keith PLK, Hunt BL et al (2017b) clc-2c is regulated by salinity, prolactin and extracellular osmolality in tilapia gill. J Mol Endocrinol 59(4):391–402. https://doi.org/10.1530/JME-17-0144
Breves JP, Springer-Miller RH, Chenoweth DA et al (2020) Cortisol regulates insulin-like growth-factor binding protein (igfbp) gene expression in Atlantic salmon parr. Mol Cell Endocrinol 518:110989. https://doi.org/10.1016/j.mce.2020.110989
Breves JP, Nelson NN, Koltenyuk V et al (2021) Enhanced expression of ncc1 and clc2c in the kidney and urinary bladder accompanies freshwater acclimation in Mozambique tilapia. Comp Biochem Physiol A 260:111021. https://doi.org/10.1016/j.cbpa.2021.111021
Breves JP, Puterbaugh KM, Bradley SE et al (2022) Molecular targets of prolactin in mummichogs (Fundulus heteroclitus): Ion transporters/channels, aquaporins, and claudins. Gen Comp Endocrinol 325:114051. https://doi.org/10.1016/j.ygcen.2022.114051
Breves JP, Runiewicz ER, Richardson SG et al (2024) Transcriptional regulation of esophageal, intestinal, and branchial solute transporters by salinity, growth hormone, and cortisol in Atlantic salmon. J Exp Zool Part Ecol Integr Physiol 341:107–117. https://doi.org/10.1002/jez.2766
Bridgham JT, Carroll SM, Thornton JW (2006) Evolution of hormone-receptor complexity by molecular exploitation. Science 312:97–101. https://doi.org/10.1126/science.1123348
Brunelli E, Mauceri A, Salvatore F et al (2010) Localization of aquaporin 1 and 3 in the gills of the rainbow wrasse Coris julis. Acta Histochem 112(3):251–258. https://doi.org/10.1016/j.acthis.2008.11.030
Bui P, Kelly SP (2014) Claudin-6, -10d and -10e contribute to seawater acclimation in the euryhaline puffer fish Tetraodon nigroviridis. J Exp Biol 217(Pt 10):1758–1767. https://doi.org/10.1242/jeb.099200
Bui P, Bagherie-Lachidan M, Kelly SP (2010) Cortisol differentially alters claudin isoforms in cultured puffer fish gill epithelia. Mol Cell Endocrinol 317:120–126. https://doi.org/10.1016/j.mce.2009.12.002
Burden CE (1956) The failure of hypophysectomized Fundulus heteroclitus to survive in fresh water. Biol Bull 110:8–28
Bury NR, Sturm A, Le Rouzic P et al (2003) Evidence for two distinct functional glucocorticoid receptors in teleost fish. J Mol Endocrinol 31:141–156. https://doi.org/10.1677/jme.0.0310141
Bystriansky JS, Richards JG, Schulte PM, Ballantyne JS (2006) Reciprocal expression of gill Na+/K+-ATPase alpha-subunit isoforms alpha1a and alpha1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J Exp Biol 209(Pt 10):1848–1858. https://doi.org/10.1242/jeb.02188
Cerdà J, Finn RN (2010) Piscine aquaporins: an overview of recent advances. J Exp Zool A 313(10):623–650. https://doi.org/10.1002/jez.634
Chakraborti P, Mukherjee D (1995) Effects of prolactin and fish pituitary extract on plasma calcium levels in common carp, Cyprinus carpio. Gen Comp Endocrinol 97(3):320–326. https://doi.org/10.1006/gcen.1995.1032
Chasiotis H, Kelly SP (2011) Effect of cortisol on permeability and tight junction protein transcript abundance in primary cultured gill epithelia from stenohaline goldfish and euryhaline trout. Gen Comp Endocrinol 172:494–504. https://doi.org/10.1016/j.ygcen.2011.04.023
Chasiotis H, Kelly SP (2012) Effects of elevated circulating cortisol levels on hydromineral status and gill tight junction protein abundance in the stenohaline goldfish. Gen Comp Endocrinol 175:277–283. https://doi.org/10.1016/j.ygcen.2011.11.024
Chasiotis H, Effendi JC, Kelly SP (2009) Occludin expression in goldfish held in ion-poor water. J Comp Physiol B 179(2):145–154. https://doi.org/10.1007/s00360-008-0297-1
Chasiotis H, Kolosov D, Bui P, Kelly SP (2012) Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: a review. Respir Physiol Neurobiol 184:269–281. https://doi.org/10.1016/j.resp.2012.05.020
Chen M, Huang X, Yuen DS, Cheng CH (2011) A study on the functional interaction between the GH/PRL family of polypeptides with their receptors in zebrafish: evidence against GHR1 being the receptor for somatolactin. Mol Cell Endocrinol 337(1–2):114–121. https://doi.org/10.1016/j.mce.2011.02.006
Chen YC, Liao BK, Lu YF et al (2019) Zebrafish Klf4 maintains the ionocyte progenitor population by regulating epidermal stem cell proliferation and lateral inhibition. PLoS Genet 15(4):e1008058. https://doi.org/10.1371/journal.pgen.1008058
Cheng P, Huang Y, Du H et al (2019) Draft genome and complete Hox-cluster characterization of the sterlet (Acipenser ruthenus). Front Genet 10:776. https://doi.org/10.3389/fgene.2019.00776
Choi YJ, Shin HS, Kim NN et al (2013) Expression of aquaporin-3 and -8 mRNAs in the parr and smolt stages of sockeye salmon, Oncorhynchus nerka: effects of cortisol treatment and seawater acclimation. Comp Biochem Physiol A 165(2):228–236. https://doi.org/10.1016/j.cbpa.2013.03.013
Clifford AM, Tresguerres M, Goss GG, Wood CM (2022) A novel K+-dependent Na+ uptake mechanism during low pH exposure in adult zebrafish (Danio rerio): new tricks for old dogma. Acta Physiol (oxf) 234(3):e13777. https://doi.org/10.1111/apha.13777
Close DA, Yun S-S, McCormick SD et al (2010) 11-Deoxycortisol is a corticosteroid hormone in the lamprey. Proc Natl Acad Sci USA 107:13942–13947. https://doi.org/10.1073/pnas.0914026107
Colombe L, Fostier A, Bury N et al (2000) A mineralocorticoid-like receptor in the rainbow trout, Oncorhynchus mykiss: cloning and characterization of its steroid binding domain. Steroids 65:319–328. https://doi.org/10.1016/S0039-128X(00)00090-8
Cruz SA, Chao PL, Hwang PP (2013a) Cortisol promotes differentiation of epidermal ionocytes through Foxi3 transcription factors in zebrafish (Danio rerio). Comp Biochem Physiol A 164(1):249–257. https://doi.org/10.1016/j.cbpa.2012.09.011
Cruz SA, Lin CH, Chao PL, Hwang PP (2013b) Glucocorticoid receptor, but not mineralocorticoid receptor, mediates cortisol regulation of epidermal ionocyte development and ion transport in zebrafish (Danio rerio). PLoS One 8:e77997. https://doi.org/10.1371/journal.pone.0077997
Cui G, Hong J, Chung-Davidson YW et al (2019) An ancient CFTR ortholog informs molecular evolution in ABC transporters. Dev Cell 51(4):421-430.e3. https://doi.org/10.1016/j.devcel.2019.09.017
Cutler CP, Cramb G (2002) Branchial expression of an aquaporin 3 (AQP-3) homologue is downregulated in the European eel Anguilla anguilla following seawater acclimation. J Exp Biol 205(Pt 17):2643–2651. https://doi.org/10.1242/jeb.205.17.2643
Cutler CP, Phillips C, Hazon N, Cramb G (2007) Cortisol regulates eel (Anguilla anguilla) aquaporin 3 (AQP3) mRNA expression levels in gill. Gen Comp Endocrinol 152:310–313. https://doi.org/10.1016/j.ygcen.2007.01.031
Dalziel AC, Bittman J, Mandic M et al (2014) Origins and functional diversification of salinity-responsive Na+, K+ ATPase α1 paralogs in salmonids. Mol Ecol 23(14):3483–3503. https://doi.org/10.1111/mec.12828
Dauder S, Young G, Hass L, Bern HA (1990) Prolactin receptors in liver, kidney, and gill of the tilapia (Oreochromis mossambicus): characterization and effect of salinity on specific binding of iodinated ovine prolactin. Gen Comp Endocrinol 77(3):368–377. https://doi.org/10.1016/0016-6480(90)90226-c
Deane EE, Woo NYS (2009) Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: a review. Rev Fish Biol Fish 19:97–120. https://doi.org/10.1007/s11160-008-9091-0
Du K, Stöck M, Kneitz S et al (2020) The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization. Nat Ecol Evol 4(6):841–852. https://doi.org/10.1038/s41559-020-1166-x
Dymowska AK, Hwang PP, Goss GG (2012) Structure and function of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol 184(3):282–292. https://doi.org/10.1016/j.resp.2012.08.025
Dymowska AK, Schultz AG, Blair SD et al (2014) Acid-sensing ion channels are involved in epithelial Na+ uptake in the rainbow trout Oncorhynchus mykiss. Am J Physiol Cell Physiol 307(3):C255–C265. https://doi.org/10.1152/ajpcell.00398.2013
Eckert SM, Yada T, Shepherd BS et al (2001) Hormonal control of osmoregulation in the channel catfish Ictalurus punctatus. Gen Comp Endocrinol 122(3):270–286. https://doi.org/10.1006/gcen.2001.7633
Einarsdóttir IE, Gong N, Jönsson E et al (2014) Plasma growth hormone-binding protein levels in Atlantic salmon Salmo salar during smoltification and seawater transfer. J Fish Biol 85:1279–1296. https://doi.org/10.1111/jfb.12473
Ellis LV, Bollinger RJ, Weber HM et al (2019) Differential expression and localization of branchial AQP1 and AQP3 in Japanese medaka (Oryzias latipes). Cells 8(5):422. https://doi.org/10.3390/cells8050422
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85(1):97–177. https://doi.org/10.1152/physrev.00050.2003
Fargher RC, McKeown BA (1989) The effect of prolactin on calcium homeostasis in coho salmon (Oncorhynchus kisutch). Gen Comp Endocrinol 73(3):398–403. https://doi.org/10.1016/0016-6480(89)90197-4
Faught E, Vijayan MM (2018) The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci Rep 8:18081. https://doi.org/10.1038/s41598-018-36681-w
Ferreira-Martins D, Coimbra J, Antunes C, Wilson JM (2016) Effects of salinity on upstream-migrating, spawning sea lamprey, Petromyzon marinus. Conserv Physiol 4:1–16. https://doi.org/10.1093/conphys/cov064
Ferreira-Martins D, Wilson JM, Kelly SP et al (2021) A review of osmoregulation in lamprey. J Gt Lakes Res 47:S59–S71. https://doi.org/10.1016/j.jglr.2021.05.003
Ferreira-Martins D, Walton E, Karlstrom RO et al (2023) The GH/IGF axis in the sea lamprey during metamorphosis and seawater acclimation. Mol Cell Endocrinol. https://doi.org/10.1016/j.mce.2023.111937
Fiol DF, Kültz D (2007) Osmotic stress sensing and signaling in fishes. FEBS J 274(22):5790–5798. https://doi.org/10.1111/j.1742-4658.2007.06099.x
Fiol DF, Sanmarti E, Sacchi R, Kültz D (2009) A novel tilapia prolactin receptor is functionally distinct from its paralog. J Exp Biol 212(Pt 13):2007–2015. https://doi.org/10.1242/jeb.025601
Flik G, Fenwick JC, Wendelaar Bonga SE (1989) Calcitropic actions of prolactin in freshwater North American eel (Anguilla rostrata LeSueur). Am J Physiol 257(1 Pt 2):R74-79. https://doi.org/10.1152/ajpregu.1989.257.1.R74
Flik G, Atsma W, Fenwick JC et al (1993) Homologous recombinant growth hormone and calcium metabolism in the tilapia, Oreochromis mossambicus, adapted to fresh water. J Exp Biol 185:107–119. https://doi.org/10.1242/jeb.185.1.107
Flik G, Rentier-Delrue F, Wendelaar Bonga SE (1994) Calcitropic effects of recombinant prolactins in Oreochromis mossambicus. Am J Physiol 266(4 Pt 2):R1302-1308. https://doi.org/10.1152/ajpregu.1994.266.4.R1302
Flik G, Klaren PHM, Schoenmakers TJM et al (1996) Cellular calcium transport in fish: unique and universal mechanisms. Physiol Biochem Zool 69:403–417. https://doi.org/10.1086/physzool.69.2.30164192
Flores AM, Shrimpton MJ (2012) Differential physiological and endocrine responses of rainbow trout, Oncorhynchus mykiss, transferred from fresh water to ion-poor or salt water. Gen Comp Endocrinol 175:244–250. https://doi.org/10.1016/j.ygcen.2011.11.002
Fontaine M, Hatey J (1954) Sur la teneur en 17-hydroxycorticosteroides du plasma de saumon (Salmo salar L.). CR Acad Sci Ser D 239:319–321
Fuentes J, Brinca L, Guerreiro PM, Power DM (2010) PRL and GH synthesis and release from the sea bream (Sparus auratus L.) pituitary gland in vitro in response to osmotic challenge. Gen Comp Endocrinol 168(1):95–102. https://doi.org/10.1016/j.ygcen.2010.04.005
Fuller PJ, Yao YZ, Jin R et al (2019) Molecular evolution of the switch for progesterone and spironolactone from mineralocorticoid receptor agonist to antagonist. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1903172116
Furukawa F, Watanabe S, Inokuchi M, Kaneko T (2011) Responses of gill mitochondria-rich cells in Mozambique tilapia exposed to acidic environments (pH 4.0) in combination with different salinities. Comp Biochem Physiol A 158(4):468–476. https://doi.org/10.1016/j.cbpa.2010.12.003
Geering K (2008) Functional roles of Na, K-ATPase subunits. Curr Opin Nephrol Hypertens 17(5):526–532. https://doi.org/10.1097/MNH.0b013e3283036cbf
Gilmour KM, Collier CL, Dey CJ, Perry SF (2011) Roles of cortisol and carbonic anhydrase in acid-base compensation in rainbow trout, Oncorhynchus mykiss. J Comp Physiol B 181(4):501–515. https://doi.org/10.1007/s00360-010-0540-4
Gong N, Ferreira-Martins D, McCormick SD, Sheridan MA (2020) Divergent genes encoding the putative receptors for growth hormone and prolactin in sea lamprey display distinct patterns of expression. Sci Rep 10:1674. https://doi.org/10.1038/s41598-020-58344-5
Gong N, Ferreira-Martins D, Norstog JL et al (2022) Discovery of prolactin-like in lamprey: role in osmoregulation and new insight into the evolution of the growth hormone/prolactin family. Proc Natl Acad Sci USA 119:e2212196119. https://doi.org/10.1073/pnas.2212196119
Gray ES, Young G, Bern HA (1990) Radioreceptor assay for growth hormone in coho salmon (Oncorhynchus kisutch) and its application to the study of stunting. J Exp Zool 256:290–296. https://doi.org/10.1002/jez.1402560308
Guh YJ, Lin CH, Hwang PP (2015) Osmoregulation in zebrafish: ion transport mechanisms and functional regulation. EXCLI J 14:627–659. https://doi.org/10.17179/excli2015-246
Herndon TM, McCormick SD, Bern HA (1991) Effects of prolactin on chloride cells in opercular membrane of seawater-adapted tilapia. Gen Comp Endocrinol 83(2):283–289. https://doi.org/10.1016/0016-6480(91)90032-2
Hirano T (1986) The spectrum of prolactin action in teleosts. Prog Clin Biol Res 205:53–74
Hirata T, Kaneko T, Ono T et al (2003) Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Physiol Regul Integr Comp Physiol 284(5):R1199–R1212. https://doi.org/10.1152/ajpregu.00267.2002
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184(3):257–268. https://doi.org/10.1016/j.resp.2012.07.019
Hiroi J, Yasumasu S, McCormick SD et al (2008) Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211(Pt 16):2584–2599. https://doi.org/10.1242/jeb.018663
Hoar WS (1988) The physiology of smolting salmonids. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, pp 275–343
Hoshijima K, Hirose S (2007) Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J Endocrinol 193(3):481–491. https://doi.org/10.1677/JOE-07-0003
Hsiao CD, You MS, Guh YJ et al (2007) A positive regulatory loop between foxi3a and foxi3b is essential for specification and differentiation of zebrafish epidermal ionocytes. PLoS One 2(3):e302. https://doi.org/10.1371/journal.pone.0000302
Hsu HH, Lin LY, Tseng YC et al (2014) A new model for fish ion regulation: identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes). Cell Tissue Res 357(1):225–243. https://doi.org/10.1007/s00441-014-1883-z
Huang X, Jiao B, Fung CK et al (2007) The presence of two distinct prolactin receptors in seabream with different tissue distribution patterns, signal transduction pathways and regulation of gene expression by steroid hormones. J Endocrinol 194(2):373–392. https://doi.org/10.1677/JOE-07-0076
Hwang PP, Lin LY (2014) Gill ionic transport, acid-base regulation, and nitrogen excretion. In: Evans DH, Claiborne JB, Currie S (eds) The physiology of fishes, 4th edn. CRC Press, Boca Raton, p 453
Inokuchi M, Hiroi J, Watanabe S et al (2008) Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp Biochem Physiol A 151(2):151–158. https://doi.org/10.1016/j.cbpa.2008.06.012
Inokuchi M, Hiroi J, Watanabe S et al (2009) Morphological and functional classification of ion-absorbing mitochondria-rich cells in the gills of Mozambique tilapia. J Exp Biol 2212(Pt 7):1003–1010. https://doi.org/10.1242/jeb.025957
Inokuchi M, Breves JP, Moriyama S et al (2015) Prolactin 177, prolactin 188, and extracellular osmolality independently regulate the gene expression of ion transport effectors in gill of Mozambique tilapia. Am J Physiol Regul Integr Comp Physiol 309(10):R1251-1263. https://doi.org/10.1152/ajpregu.00168.2015
Inokuchi M, Nakamura M, Miyanishi H et al (2017) Functional classification of gill ionocytes and spatiotemporal changes in their distribution after transfer from seawater to freshwater in Japanese seabass. J Exp Biol 220(Pt 24):4720–4732. https://doi.org/10.1242/jeb.167320
Inokuchi M, Hiroi J, Kaneko T (2022) Why can Mozambique tilapia acclimate to both freshwater and seawater? Insights from the plasticity of ionocyte functions in the euryhaline teleost. Front Physiol 13:914277. https://doi.org/10.3389/fphys.2022.914277
Ivanis G, Esbaugh AJ, Perry SF (2008) Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 211(Pt 15):2467–2477. https://doi.org/10.1242/jeb.017491
Jung D, MacIver B, Jackson BP et al (2012) A novel aquaporin 3 in killifish (Fundulus heteroclitus) is not an arsenic channel. Toxicol Sci 127(1):101–109. https://doi.org/10.1093/toxsci/kfs078
Kakizawa S, Kaneko T, Hasegawa S, Hirano T (1993) Activation of somatolactin cells in the pituitary of the rainbow trout Oncorhynchus mykiss by low environmental calcium. Gen Comp Endocrinol 91(3):298–306. https://doi.org/10.1006/gcen.1993.1130
Kaneko T, Hirano T (1983) Role of prolactin and somatolactin in calcium regulation in fish. J Exp Biol 184(1):31–45. https://doi.org/10.1242/jeb.184.1.31
Kaneko T, Watanabe S, Lee KM (2008) Functional morphology of mitochondrion-rich cells in euryhaline and stenohaline teleosts. Aqua BioSci Monogr 1:1–62. https://doi.org/10.5047/absm.2008.00101.0001
Katsu Y, Oka K, Baker ME (2018) Evolution of human, chicken, alligator, frog, and zebrafish mineralocorticoid receptors: allosteric influence on steroid specificity. Sci Signal 11:35–37. https://doi.org/10.1126/scisignal.aao1520
Kawauchi H, Suzuki K, Yamazaki T et al (2002) Identification of growth hormone in the sea lamprey, an extant representative of a group of the most ancient vertebrates. Endocrinology 143(12):4916–4921. https://doi.org/10.1210/en.2002-220810
Kelly SP, Chasiotis H (2011) Glucocorticoid and mineralocorticoid receptors regulate paracellular permeability in a primary cultured gill epithelium. J Exp Biol 214:2308–2318. https://doi.org/10.1242/jeb.055962
Kelly SP, Wood CM (2002) Cultured gill epithelia from freshwater tilapia (Oreochromis niloticus): effect of cortisol and homologous serum supplements from stressed and unstressed fish. J Membr Biol 190(1):29–42. https://doi.org/10.1007/s00232-002-1020-x
Kelly SP, Chow IN, Woo NY (1999) Effects of prolactin and growth hormone on strategies of hypoosmotic adaptation in a marine teleost, Sparus sarba. Gen Comp Endocrinol 113(1):9–22. https://doi.org/10.1006/gcen
Khodabandeh S, Mosafer S, Khoshnood Z (1999) Effects of cortisol and salinity acclimation on Na+/K+/2Cl- cotransporter gene expression and Na+, K+-ATPase activity in the gill of Persian sturgeon, Acipenser persicus, fry. Sci Mar 73S1:111–116. https://doi.org/10.3989/scimar.2009.73s1111
Kiilerich P, Kristiansen K, Madsen SS (2007a) Hormone receptors in gills of smolting Atlantic salmon, Salmo salar: expression of growth hormone, prolactin, mineralocorticoid and glucocorticoid receptors and 11β-hydroxysteroid dehydrogenase type 2. Gen Comp Endocrinol 152:295–303. https://doi.org/10.1016/j.ygcen.2006.12.018
Kiilerich P, Kristiansen K, Madsen SS (2007b) Cortisol regulation of ion transporter mRNA in Atlantic salmon gill and the effect of salinity on the signaling pathway. J Endocrinol 194:417–427. https://doi.org/10.1677/JOE-07-0185
Kiilerich P, Milla S, Sturm A et al (2011a) Implication of the mineralocorticoid axis in rainbow trout osmoregulation during salinity acclimation. J Endocrinol 209:221–235. https://doi.org/10.1530/JOE-10-0371
Kiilerich P, Pedersen SH, Kristiansen K, Madsen SS (2011b) Corticosteroid regulation of Na+, K+-ATPase α1-isoform expression in Atlantic salmon gill during smolt development. Gen Comp Endocrinol 170:283–289. https://doi.org/10.1016/j.ygcen.2010.02.014
Kiilerich P, Tipsmark CK, Borski RJ, Madsen SS (2011c) Differential effects of cortisol and 11-deoxycorticosterone on ion transport protein mRNA levels in gills of two euryhaline teleosts, Mozambique tilapia (Oreochromis mossambicus) and striped bass (Morone saxatilis). J Endocrinol 209:115–126. https://doi.org/10.1530/JOE-10-0326
Klaren PH, Guzmán JM, Reutelingsperger SJ et al (2007) Low salinity acclimation and thyroid hormone metabolizing enzymes in gilthead seabream (Sparus auratus). Gen Comp Endocrinol 152(2–3):215–222. https://doi.org/10.1016/j.ygcen.2007.02.010
Knoeppel SJ, Atkins DL, Packer RK (1982) The role of the thyroid in osmotic and ionic regulation in Fundulus heteroclitus acclimated to freshwater and seawater. Comp Biochem Physiol A 73:25–29. https://doi.org/10.1016/0300-9629(82)90087-1
Kolosov D, Kelly SP (2017) Claudin-8d is a cortisol-responsive barrier protein in the gill epithelium of trout. J Mol Endocrinol 59(3):299–310. https://doi.org/10.1530/JME-17-0108
Kolosov D, Kelly SP (2019) The mineralocorticoid receptor contributes to barrier function of a model fish gill epithelium. J Exp Biol 222(Pt 11):jeb192096. https://doi.org/10.1242/jeb.192096
Kolosov D, Bui P, Donini A et al (2017a) A role for tight junction-associated MARVEL proteins in larval sea lamprey (Petromyzon marinus) osmoregulation. J Exp Biol 220:3657–3670. https://doi.org/10.1242/jeb.161562
Kolosov D, Donini A, Kelly SP (2017b) Claudin-31 contributes to corticosteroid-induced alterations in the barrier properties of the gill epithelium. Mol Cell Endocrinol 439:457–466. https://doi.org/10.1016/j.mce.2016.10.034
Kolosov D, Bui P, Wilkie MP, Kelly SP (2020) Claudins of sea lamprey (Petromyzon marinus)—organ-specific expression and transcriptional responses to water of varying ion content. J Fish Biol. https://doi.org/10.1111/jfb.14274
Kültz D (2015) Physiological mechanisms used by fish to cope with salinity stress. J Exp Biol 218(Pt 12):1907–1914. https://doi.org/10.1242/jeb.118695
Kumai Y, Bahubeshi A, Steele S, Perry SF (2011) Strategies for maintaining Na+ balance in zebrafish (Danio rerio) during prolonged exposure to acidic water. Comp Biochem Physiol A 160(1):52–62. https://doi.org/10.1016/j.cbpa.2011.05.001
Kumai Y, Nesan D, Vijayan MM, Perry SF (2012) Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor. Mol Cell Endocrinol 364(1–2):113–125. https://doi.org/10.1016/j.mce.2012.08.017
Lai KP, Li JW, Gu J et al (2015) Transcriptomic analysis reveals specific osmoregulatory adaptive responses in gill mitochondria-rich cells and pavement cells of the Japanese eel. BMC Genom 16:1072. https://doi.org/10.1186/s12864-015-2271-0
Langhorn P, Simpson TH (1986) The interrelationship of cortisol, gill Na+/K+ ATPase, and homeostasis during the parr-smolt transformation of Atlantic salmon (Salmo salar L.). Gen Comp Endocrinol 61:203–213. https://doi.org/10.1016/0016-6480(86)90198-X
Lee KM, Kaneko T, Aida K (2006) Prolactin and prolactin receptor expressions in a marine teleost, pufferfish Takifugu rubripes. Gen Comp Endocrinol 146(3):318–328. https://doi.org/10.1016/j.ygcen.2005.12.003
Leguen I, Le Cam A, Montfort J et al (2015) Transcriptomic analysis of trout gill ionocytes in fresh water and sea water using laser capture microdissection combined with microarray analysis. PLoS One 10(10):e0139938. https://doi.org/10.1371/journal.pone.0139938
Lema SC, Carvalho PG, Egelston JN et al (2018) Dynamics of gene expression responses for ion transport proteins and aquaporins in the gill of a euryhaline pupfish during freshwater and high-salinity acclimation. Physiol Biochem Zool 91(6):1148–1171. https://doi.org/10.1086/700432
Liao BK, Deng AN, Chen SC et al (2007) Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genom 8:354. https://doi.org/10.1186/1471-2164-8-354
Liao BK, Chen RD, Hwang PP (2009) Expression regulation of Na+-K+-ATPase alpha1-subunit subtypes in zebrafish gill ionocytes. Am J Physiol Regul Integr Comp Physiol 296(6):R1897–R1906. https://doi.org/10.1152/ajpregu.00029.2009
Lignot JH, Cutler CP, Hazon N, Cramb G (2002) Immunolocalisation of aquaporin 3 in the gill and the gastrointestinal tract of the European eel Anguilla anguilla (L.). J Exp Biol 205(Pt 17):2653–2663. https://doi.org/10.1242/jeb.205.17.2653
Lin CH, Hwang PP (2016) The control of calcium metabolism in zebrafish (Danio rerio). Int J Mol Sci 17(11):1783. https://doi.org/10.3390/ijms17111783
Link K, Berishvili G, Shved N et al (2010) Seawater and freshwater challenges affect the insulin-like growth factors IGF-I and IGF-II in liver and osmoregulatory organs of the tilapia. Mol Cell Endocrinol 327(1–2):40–46. https://doi.org/10.1016/j.mce.2010.05.011
Link K, Shved N, Serrano N et al (2022) Effects of seawater and freshwater challenges on the Gh/Igf system in the saline-tolerant blackchin tilapia (Sarotherodon melanotheron). Front Endocrinol 13:976488. https://doi.org/10.3389/fendo.2022.976488
López-Bojórquez L, Villalobos P, García-G C et al (2007) Functional identification of an osmotic response element (ORE) in the promoter region of the killifish deiodinase 2 gene (FhDio2). J Exp Biol 210(Pt 17):3126–3132. https://doi.org/10.1242/jeb.004150
Loretz CA, Bern HA (1982) Prolactin and osmoregulation in vertebrates: an update. Neuroendocrinology 35(4):292–304. https://doi.org/10.1159/000123397
Mackie P, Wright PA, Glebe BD, Ballantyne JS (2005) Osmoregulation and gene expression of Na+/K+ ATPase in families of Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 62(11):2661–2672. https://doi.org/10.1139/f05-168
Madsen SS (1990a) Enhanced hypoosmoregulatory response to growth hormone after cortisol treatment in immature rainbow trout, Salmo gairdneri. Fish Physiol Biochem 8:271–279. https://doi.org/10.1007/BF00003422
Madsen SS (1990b) The role of cortisol and growth hormone in seawater adaptation and development of hypoosmoregulatory mechanisms in sea trout parr (Salmo trutta trutta). Gen Comp Endocrinol 79:1–11. https://doi.org/10.1016/0016-6480(90)90082-W
Madsen SS, Bern HA (1993) In-vitro effects of insulin-like growth factor-I on gill Na+, K+-ATPase in coho salmon, Oncorhynchus kisutch. J Endocrinol 138(1):23–30. https://doi.org/10.1677/joe.0.1380023
Madsen SS, Korsgaard B (1991) Opposite effects of 17β-estradiol and combined growth hormone-cortisol treatment on hypo-osmoregulatory performance in sea trout presmolts, Salmo trutta. Gen Comp Endocrinol 83:276–282. https://doi.org/10.1016/0016-6480(91)90031-Z
Madsen SS, Kiilerich P, Tipsmark CK (2009) Multiplicity of expression of Na+, K+-ATPase alpha-subunit isoforms in the gill of Atlantic salmon (Salmo salar): cellular localisation and absolute quantification in response to salinity change. J Exp Biol 212(Pt 1):78–88. https://doi.org/10.1242/jeb.024612
Mancera JM, McCormick SD (1998) Evidence for growth hormone/insulin-like growth factor I axis regulation of seawater acclimation in the euryhaline teleost Fundulus heteroclitus. Gen Comp Endocrinol 111:103–112. https://doi.org/10.1006/gcen.1998.7086
Mancera JM, Carrión RL, del Río MPM (2002) Osmoregulatory action of PRL, GH, and cortisol in the gilthead seabream (Sparus aurata L.). Gen Comp Endocrinol 129(2):95–103. https://doi.org/10.1016/s0016-6480(02)00522-1
Manzon LA (2002) The role of prolactin in fish osmoregulation: a review. Gen Comp Endocrinol 125(2):291–310. https://doi.org/10.1006/gcen.2001.7746
Marlétaz F, de la Calle-Mustienes E, Acemel RD et al (2023) The little skate genome and the evolutionary emergence of wing-like fins. Nature 616(7957):495–503. https://doi.org/10.1038/s41586-023-05868-1
Marshall WS, Grosell M (2006) Ion transport, osmoregulation, and acid-base balance. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn. CRC Press, Boca Raton, pp 177–230
Marshall WS, Breves JP, Doohan EM et al (2018) claudin-10 isoform expression and cation selectivity change with salinity in salt-secreting epithelia of Fundulus heteroclitus. J Exp Biol 221(Pt 1):jeb168906. https://doi.org/10.1242/jeb.168906
Marsigliante S, Barker S, Jimenez E, Storelli C (2000) Glucocorticoid receptors in the euryhaline teleost Anguilla anguilla. Mol Cell Endocrinol 162:193–201. https://doi.org/10.1016/S0303-7207(99)00262-2
Maule AG, Schreck CB (1990) Glucocorticoid receptors in leukocytes and gill of juvenile coho salmon (Oncorhynchus kisutch). Gen Comp Endocrinol 77:448–455. https://doi.org/10.1016/0016-6480(90)90236-f
Mazurais D, Ducouret B, Tujague M et al (1998) Regulation of the glucocorticoid receptor mRNA levels in the gills of Atlantic salmon (Salmo salar) during smoltification. Bull Fr Pêche Piscic 350–351:499–510. https://doi.org/10.1051/kmae:1998019
McCormick SD (1996) Effects of growth hormone and insulin-like growth factor I on salinity tolerance and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar): interaction with cortisol. Gen Comp Endocrinol 101:3–11. https://doi.org/10.1006/gcen.1996.0002
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794. https://doi.org/10.1093/icb/41.4.781
McCormick SD (2013) Smolt physiology and endocrinology. In: McCormick SD, Farrell AP, Brauner CJ (eds) Fish Physiology: Euryhaline Fishes. Academic Press Inc, Amsterdam, pp 191–251
McCormick SD, Bern HA (1989) In vitro stimulation of Na+-K+-ATPase activity and ouabain binding by cortisol in coho salmon gill. Am J Physiol Regul Integr Comp Physiol 256:R707-715. https://doi.org/10.1152/ajpregu.1989.256.3.r707
McCormick SD, Saunders RL (1987) Preparatory physiological adaptations for marine life of salmonids: osmoregulation, growth, and metabolism. Am Fish Soc Symp 1:211–229. https://doi.org/10.1016/0022-4596(90)90074-8
McCormick SD, Dickhoff WW, Duston J et al (1991) Developmental differences in the responsiveness of gill Na+, K+-ATPase to cortisol in salmonids. Gen Comp Endocrinol 84:308–317. https://doi.org/10.1016/0016-6480(91)90054-A
McCormick SD, Shrimpton JM, Moriyama S, Björnsson BT (2007) Differential hormonal responses of Atlantic salmon parr and smolt to increased daylength: a possible developmental basis for smolting. Aquaculture 273:337–344. https://doi.org/10.1016/j.aquaculture.2007.10.015
McCormick SD, Regish A, O’Dea MF, Shrimpton JM (2008) Are we missing a mineralocorticoid in teleost fish? Effects of cortisol, deoxycorticosterone and aldosterone on osmoregulation, gill Na+, K+-ATPase activity and isoform mRNA levels in Atlantic salmon. Gen Comp Endocrinol 157:35–40. https://doi.org/10.1016/j.ygcen.2008.03.024
McCormick SD, Regish AM, Christensen AK (2009) Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J Exp Biol 212(Pt 24):3994–4001. https://doi.org/10.1242/jeb.037275
McCormick SD, Regish AM, Christensen AK, Björnsson BT (2013) Differential regulation of sodium–potassium pump isoforms during smolt development and seawater exposure of Atlantic salmon. J Exp Biol 216:1142–1151. https://doi.org/10.1242/jeb.080440
McCormick SD, Taylor ML, Regish AM (2020) Cortisol is an osmoregulatory and glucose-regulating hormone in Atlantic sturgeon, a basal ray-finned fish. J Exp Biol 223:jeb220251. https://doi.org/10.1242/jeb.220251
McGuire A, Aluru N, Takemura A et al (2010) Hyperosmotic shock adaptation by cortisol involves upregulation of branchial osmotic stress transcription factor 1 gene expression in Mozambique tilapia. Gen Comp Endocrinol 165(2):321–329. https://doi.org/10.1016/j.ygcen.2009.07.016
Mizuno S, Ura K, Onodera Y et al (2001) Changes in transcript levels of gill cortisol receptor during smoltification in wild masu salmon, Oncorhynchus masou. Zool Sci 18:853–860. https://doi.org/10.2108/zsj.18.853
Motoshima T, Nagashima A, Ota C et al (2023) Na+/Cl- cotransporter 2 is not fish-specific and is widely found in amphibians, non-avian reptiles, and select mammals. Physiol Genom 55(3):113–131. https://doi.org/10.1152/physiolgenomics.00143.2022
Moyle PB, Cech JJ (2004) Fishes: an introduction to ichthyology, 4th edn. Prentice Hall, Upper Saddle River
Nilsen TO, Ebbesson LO, Madsen SS et al (2007) Differential expression of gill Na+, K+-ATPase alpha- and beta-subunits, Na+, K+, 2Cl− cotransporter and CFTR anion channel in juvenile anadromous and landlocked Atlantic salmon Salmo salar. J Exp Biol 210(Pt 16):2885–2896. https://doi.org/10.1242/jeb.002873
Nilsen TO, Ebbesson LOE, Kiilerich P et al (2008) Endocrine systems in juvenile anadromous and landlocked Atlantic salmon (Salmo salar): seasonal development and seawater acclimation. Gen Comp Endocrinol 155:762–772. https://doi.org/10.1016/j.ygcen.2007.08.006
Notch EG, Chapline C, Flynn E et al (2012) Mitogen activated protein kinase 14-1 regulates serum glucocorticoid kinase 1 during seawater acclimation in Atlantic killifish, Fundulus heteroclitus. Comp Biochem Physiol A 162(4):443–448. https://doi.org/10.1016/j.cbpa.2012.04.025
Nozaki M, Ominato K, Shimotani T et al (2008) Identity and distribution of immunoreactive adenohypophysial cells in the pituitary during the life cycle of sea lampreys, Petromyzon marinus. Gen Comp Endocrinol 155:403–412. https://doi.org/10.1016/j.ygcen.2007.07.012
Pang PK, Schreibman MP, Balbontin F, Pang RK (1978) Prolactin and pituitary control of calcium regulation in the killifish, Fundulus heteroclitus. Gen Comp Endocrinol 36(2):306–316. https://doi.org/10.1016/0016-6480(78)90037-0
Parks SK, Tresguerres M, Goss GG (2007) Interactions between Na+ channels and Na+-HCO3- cotransporters in the freshwater fish gill MR cell: a model for transepithelial Na+ uptake. Am J Physiol Cell Physiol 292(2):C935–C944. https://doi.org/10.1152/ajpcell.00604.2005
Pavlovic D, Fuller W, Shattock MJ (2013) Novel regulation of cardiac Na pump via phospholemman. J Mol Cell Cardiol 61:83–93. https://doi.org/10.1016/j.yjmcc.2013.05.002
Pelis RM, McCormick SD (2001) Effects of growth hormone and cortisol on Na+–K+–2Cl− cotransporter localization and abundance in the gills of Atlantic salmon. Gen Comp Endocrinol 124:134–143. https://doi.org/10.1006/gcen.2001.7703
Pérez-Rius C, Gaitán-Peñas H, Estévez R, Barrallo-Gimeno A (2015) Identification and characterization of the zebrafish ClC-2 chloride channel orthologs. Pflugers Arch 467(8):1769–1781. https://doi.org/10.1007/s00424-014-1614-z
Perry SF, Goss GG, Fenwick JC (1992) Interrelationships between gill chloride cell morphology and calcium uptake in freshwater teleosts. Fish Physiol Biochem 10(4):327–337. https://doi.org/10.1007/BF00004482
Peter MCS, Lock RA, Wendelaar Bonga SE (2000) Evidence for an osmoregulatory role of thyroid hormones in the freshwater Mozambique tilapia Oreochromis mossambicus. Gen Comp Endocrinol 120(2):157–167. https://doi.org/10.1006/gcen.2000.7542
Pickford GE (1953) A study of the hypophysectomized male Fundulus heteroclitus (Linn.) Bull Bingham. Oceanogr Collect 14(2):5–45
Pickford GE, Atz JW (1957) The physiology of the pituitary gland of fishes. New York Zoological Society, New York
Pickford GE, Phillips JG (1959) Prolactin, a factor in promoting survival of hypophysectomized killifish in fresh water. Science 130:454–455. https://doi.org/10.1126/science.130.3373.454
Pickford GE, Griffith RW, Torretti J et al (1970a) Branchial reduction and renal stimulation of (Na+, K+)-ATPase by prolactin in hypophysectomized killifish in fresh water. Nature 228(5269):378–379. https://doi.org/10.1038/228378a0
Pickford GE, Pang PKT, Weinstein E et al (1970b) The response of the hypophysectomized cyprinodont, Fundulus heteroclitus, to replacement therapy with cortisol: effects on blood serum and sodium-potassiurn activated adenosine triphosphatase in the gills, kidney, and intestinal mucosa. Gen Comp Endocrinol 14:524–534. https://doi.org/10.1016/0016-6480(70)90036-5
Pierce AL, Fox BK, Davis LK et al (2007) Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia: tissue specific expression and differential regulation by salinity and fasting. Gen Comp Endocrinol 154(1–3):31–40. https://doi.org/10.1016/j.ygcen.2007.06.023
Pierce AL, Breves JP, Moriyama S et al (2011) Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: effects of insulin and cortisol on GH sensitivity. J Endocrinol 211(2):201–210. https://doi.org/10.1530/JOE-10-0456
Pisam M, Auperin B, Prunet P et al (1993) Effects of prolactin on alpha and beta chloride cells in the gill epithelium of the saltwater adapted tilapia “Oreochromis niloticus”. Anat Rec 235(2):275–284. https://doi.org/10.1002/ar.1092350211
Poppinga J, Kittilson J, McCormick SD, Sheridan MA (2007) Effects of somatostatin on the growth hormone-insulin-like growth factor axis and seawater adaptation of rainbow trout (Oncorhynchus mykiss). Aquaculture 273:312–319. https://doi.org/10.1016/j.aquaculture.2007.10.021
Potts WTW, Evans DH (1966) The effects of hypophysectomy and bovine prolactin on salt fluxes in fresh-water-adapted Fundulus heteroclitus. Biol Bull 131:362–368
Prunet P, Auperin B (1994) Prolactin receptors. In: Sherwood NM, Hew CL (eds) Fish physiology, vol 13. Molecular Endocrinology of Fish. Academic Press, New York, pp 367–391
Prunet P, Boeuf G, Bolton JP, Young G (1989) Smoltification and seawater adaptation in Atlantic salmon (Salmo salar): plasma prolactin, growth hormone, and thyroid hormones. Gen Comp Endocrinol 74:355–364. https://doi.org/10.1016/S0016-6480(89)80031-0
Prunet P, Pisam M, Claireaux JP et al (1994) Effects of growth hormone on gill chloride cells in juvenile Atlantic salmon (Salmo salar). Am J Physiol Regul Integr Comp Physiol 266:R850–R857. https://doi.org/10.1152/ajpregu.1994.266.3.R850
Prunet P, Sturm A, Milla S (2006) Multiple corticosteroid receptors in fish: from old ideas to new concepts. Gen Comp Endocrinol 147:17–23. https://doi.org/10.1016/j.ygcen.2006.01.015
Rai S, Szeitz A, Roberts BW et al (2015) A putative corticosteroid hormone in Pacific lamprey, Entosphenus tridentatus. Gen Comp Endocrinol 212:178–184. https://doi.org/10.1016/j.ygcen.2014.06.019
Refstie T (1982) The effect of feeding thyroid hormones on saltwater tolerance and growth rate of Atlantic salmon. Can J Zool 60(11):2706–2712. https://doi.org/10.1139/z82-346
Reindl KM, Sheridan MA (2012) Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp Biochem Physiol A 163(3–4):231–245. https://doi.org/10.1016/j.cbpa.2012.08.003
Reinecke M, Schmid A, Ermatinger R, Loffing-Cueni D (1997) Insulin-like growth factor I in the teleost Oreochromis mossambicus, the tilapia: gene sequence, tissue expression, and cellular localization. Endocrinology 138(9):3613–3619. https://doi.org/10.1210/endo.138.9.5375
Reis-Santos P, McCormick SD, Wilson JM (2008) Ionoregulatory changes during metamorphosis and salinity exposure of juvenile sea lamprey (Petromyzon marinus L.). J Exp Biol 211:978–988. https://doi.org/10.1242/jeb.014423
Ren J, Chung-Davidson YW, Yeh CY et al (2015) Genome-wide analysis of the ATP-binding cassette (ABC) transporter gene family in sea lamprey and Japanese lamprey. BMC Genom 16(1):436. https://doi.org/10.1186/s12864-015-1677-z
Rhee JS, Kim RO, Seo JS et al (2010) Effects of salinity and endocrine-disrupting chemicals on expression of prolactin and prolactin receptor genes in the euryhaline hermaphroditic fish, Kryptolebias marmoratus. Comp Biochem Physiol C 152(4):413–423. https://doi.org/10.1016/j.cbpc.2010.07.001
Richards JG, Semple JW, Bystriansky JS, Schulte PM (2003) Na+/K+-ATPase alpha-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J Exp Biol 206(Pt 24):4475–4486. https://doi.org/10.1242/jeb.00701
Rouzic PL, Sandra O, Grosclaude J et al (2002) Evidence of rainbow trout prolactin interaction with its receptor through unstable homodimerisation. Mol Cell Endocrinol 172(1–2):105–113. https://doi.org/10.1016/s0303-7207(00)00377-4
Ruhr IM, Wood CM, Schauer KL et al (2020) Is aquaporin-3 involved in water-permeability changes in the killifish during hypoxia and normoxic recovery, in freshwater or seawater? J Exp Zool A 333(7):511–525. https://doi.org/10.1002/jez.2393
Saito K, Nakamura N, Ito Y et al (2010) Identification of zebrafish Fxyd11a protein that is highly expressed in ion-transporting epithelium of the gill and skin and its possible role in ion homeostasis. Front Physiol 1:129. https://doi.org/10.3389/fphys.2010.00129
Sakamoto T, Hirano T (1991) Growth hormone receptors in the liver and osmoregulatory organs of rainbow trout: characterization and dynamics during adaptation to seawater. J Endocrinol 130:425–433. https://doi.org/10.1677/joe.0.1300425
Sakamoto T, McCormick SD (2006) Prolactin and growth hormone in fish osmoregulation. Gen Comp Endocrinol 147(1):24–30. https://doi.org/10.1016/j.ygcen.2005.10.008
Sakamoto T, Shepherd BS, Madsen SS et al (1997) Osmoregulatory actions of growth hormone and prolactin in an advanced teleost. Gen Comp Endocrinol 106(1):95–101. https://doi.org/10.1006/gcen.1996.6854
Santos CR, Ingleton PM, Cavaco JE et al (2001) Cloning, characterization, and tissue distribution of prolactin receptor in the sea bream (Sparus aurata). Gen Comp Endocrinol 121(1):32–47. https://doi.org/10.1006/gcen.2000.7553
Saunders RL, McCormick SD, Henderson EB et al (1985) The effect of orally administered 3,5,3′-triiodo-L-thyronine on growth and salinity tolerance of Atlantic salmon (Salmo salar L.). Aquaculture 45:143–156. https://doi.org/10.1016/0044-8486(85)90265-0
Schreiber AM, Specker JL (1999) Metamorphosis in the summer flounder, Paralichthys dentatus: thyroidal status influences salinity tolerance. J Exp Zool 284(4):414–424. https://doi.org/10.1002/(sici)1097-010x(19990901)284:4%3c414::aid-jez8%3e3.0.co;2-e
Schreiber AM, Specker JL (2000) Metamorphosis in the summer flounder, Paralichthys dentatus: thyroidal status influences gill mitochondria-rich cells. Gen Comp Endocrinol 117(2):238–250. https://doi.org/10.1006/gcen.1999.7407
Schultz ET, McCormick SD (2013) Euryhalinity in an evolutionary context. In: McCormick SD, Farrell AP, Brauner CJ (eds) Euryhaline fishes. Elsevier, New York, pp 477–529
Scott GR, Keir KR, Schulte PM (2005) Effects of spironolactone and RU486 on gene expression and cell proliferation after freshwater transfer in the euryhaline killifish. J Comp Physiol B 175:499–510. https://doi.org/10.1007/s00360-005-0014-2
Seale AP, Riley LG, Leedom TA et al (2002) Effects of environmental osmolality on release of prolactin, growth hormone and ACTH from the tilapia pituitary. Gen Comp Endocrinol 128:91–101. https://doi.org/10.1016/S0016-6480(02)00027-8
Seale AP, Watanabe S, Grau EG (2012) Osmoreception: perspectives on signal transduction and environmental modulation. Gen Comp Endocrinol 176(3):354–360. https://doi.org/10.1016/j.ygcen.2011.10.005
Seale AP, Pavlosky KK, Celino-Brady FT et al (2019) Systemic versus tissue-level prolactin signaling in a teleost during a tidal cycle. J Comp Physiol B 189(5):581–594. https://doi.org/10.1007/s00360-019-01233-9
Seale LA, Gilman CL, Zavacki AM et al (2021) Regulation of thyroid hormones and branchial iodothyronine deiodinases during freshwater acclimation in tilapia. Mol Cell Endocrinol 538:111450. https://doi.org/10.1016/j.mce.2021.111450
Shaughnessy CA, Breves JP (2021) Molecular mechanisms of Cl- transport in fishes: new insights and their evolutionary context. J Exp Zool A 335(2):207–216. https://doi.org/10.1002/jez.2428
Shaughnessy CA, McCormick SD (2020) Functional characterization and osmoregulatory role of the Na+-K+-2Cl- cotransporter in the gill of sea lamprey (Petromyzon marinus), a basal vertebrate. Am J Physiol Regul Integr Comp Physiol 318(1):R17–R29. https://doi.org/10.1152/ajpregu.00125.2019
Shaughnessy CA, Barany A, McCormick SD (2020) 11-Deoxycortisol controls hydromineral balance in the most basal osmoregulating vertebrate, sea lamprey (Petromyzon marinus). Sci Rep 10:12148. https://doi.org/10.1038/s41598-020-69061-4
Shimomura T, Nakajima T, Horikoshi M et al (2012) Relationships between gill Na+, K+-ATPase activity and endocrine and local insulin-like growth factor-I levels during smoltification of masu salmon (Oncorhynchus masou). Gen Comp Endocrinol 178:427–435. https://doi.org/10.1016/j.ygcen.2012.06.011
Shir-Mohammadi K, Perry SF (2020) Expression of ion transport genes in ionocytes isolated from larval zebrafish (Danio rerio) exposed to acidic or Na+-deficient water. Am J Physiol Regul Integr Comp Physiol 319(4):R412–R427. https://doi.org/10.1152/ajpregu.00095.2020
Shrimpton JM, McCormick SD (1998) Regulation of gill cytosolic corticosteroid receptors in juvenile Atlantic salmon: interaction effects of growth hormone with prolactin and triiodothyronine. Gen Comp Endocrinol 112(2):262–274. https://doi.org/10.1006/gcen.1998.7172
Shrimpton J, McCormick SD (1999) Responsiveness of gill Na+/K+-ATPase to cortisol is related to gill corticosteroid receptor concentration in juvenile rainbow trout. J Exp Biol 202:987–995. https://doi.org/10.1242/jeb.202.8.987
Shrimpton JM, Randall DJ (1994) Downregulation of corticosteroid receptors in gills of coho salmon due to stress and cortisol treatment. Am J Physiol Regul Integr Comp Physiol 267:R432-438. https://doi.org/10.1152/ajpregu.1994.267.2.R432
Shrimpton JM, Bernier NJ, Randall DJ (1994) Changes in cortisol dynamics in wild and hatchery-reared juvenile coho salmon (Oncorhynchus kisutch) during smoltification. Can J Fish Aquat Sci 51:2179–2187. https://doi.org/10.1139/f94-219
Singer TD, Finstad B, McCormick SD et al (2003) Interactive effects of cortisol treatment and ambient seawater challenge on gill Na+, K+-ATPase and CFTR expression in two strains of Atlantic salmon smolts. Aquaculture 222:15–28. https://doi.org/10.1016/S0044-8486(03)00099-1
Sloman KA, Desforges PR, Gilmour KM (2001) Evidence for a mineralocorticoid-like receptor linked to branchial chloride cell proliferation in freshwater rainbow trout. J Exp Biol 204:3953–3961. https://doi.org/10.1242/jeb.204.22.3953
Smith JJ, Kuraku S, Holt C et al (2013) Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet 45(4):415–421. https://doi.org/10.1038/ng.2568
Smith JJ, Timoshevskaya N, Ye C et al (2018) The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat Genet 50(2):270–277. https://doi.org/10.1038/s41588-017-0036-1
Specker JL, Kobuke L (1987) Seawater-acclimation and the thyroidal response to thyrotropin in juvenile coho salmon (Oncorhynchus kisutch). J Exp Zool 241:327–332. https://doi.org/10.1002/jez.1402410307
Specker JL, Schreck CB (1982) Changes in plasma corticosteroids during smoltification of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol 46:53–58. https://doi.org/10.1016/0016-6480(82)90162-9
Sturm A, Bury N, Dengreville L et al (2005) 11-Deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 146:47–55. https://doi.org/10.1210/en.2004-0128
Sugimoto A, Oka K, Sato R et al (2016) Corticosteroid and progesterone transactivation of mineralocorticoid receptors from Amur sturgeon and tropical gar. Biochem J 473:3655–3665. https://doi.org/10.1042/BCJ20160579
Takabe S, Inokuchi M, Yamaguchi Y, Hyodo S (2016) Distribution and dynamics of branchial ionocytes in houndshark reared in full-strength and diluted seawater environments. Comp Biochem Physiol A 198:22–32. https://doi.org/10.1016/j.cbpa.2016.03.019
Takahashi H, Sakamoto T (2013) The role of “mineralocorticoids” in teleost fish: relative importance of glucocorticoid signaling in the osmoregulation and “central” actions of mineralocorticoid receptor. Gen Comp Endocrinol 181:223–228. https://doi.org/10.1016/j.ygcen.2012.11.016
Takei Y (2021) The digestive tract as an essential organ for water acquisition in marine teleosts: lessons from euryhaline eels. Zool Lett 7(1):10. https://doi.org/10.1186/s40851-021-00175-x
Takei Y, McCormick SD (2013) Hormonal control of fish euryhalinity. In: McCormick SD, Brauner CJ, Farrell AP (eds) Fish physiology, vol 32. Euryhaline Fishes. Academic Press, Amsterdam, pp 69–123
Takei Y, Hiroi J, Takahashi H, Sakamoto T (2014) Diverse mechanisms for body fluid regulation in teleost fishes. Am J Physiol Regul Integr Comp Physiol 307(7):R778-792. https://doi.org/10.1152/ajpregu.00104.2014
Takvam M, Denker E, Gharbi N et al (2021) Sulfate homeostasis in Atlantic salmon is associated with differential regulation of salmonid-specific paralogs in gill and kidney. Physiol Rep 9(19):e15059. https://doi.org/10.14814/phy2.15059
Tang CH, Lee TH (2011) Ion-deficient environment induces the expression of basolateral chloride channel, ClC-3-like protein, in gill mitochondrion-rich cells for chloride uptake of the tilapia Oreochromis mossambicus. Physiol Biochem Zool 84(1):54–67. https://doi.org/10.1086/657161
Tingaud-Sequeira A, Calusinska M, Finn RN et al (2010) The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol Biol 10:38. https://doi.org/10.1186/1471-2148-10-38
Tipsmark CK (2018) Identification of FXYD protein genes in a teleost: tissue-specific expression and response to salinity change. Am J Physiol Regul Integr Comp Physiol 294(4):R1367-1378. https://doi.org/10.1152/ajpregu.00454.2007
Tipsmark CK, Madsen SS (2009) Distinct hormonal regulation of Na+, K+-atpase genes in the gill of Atlantic salmon (Salmo salar L.). J Endocrinol 203:301–310. https://doi.org/10.1677/JOE-09-0281
Tipsmark CK, Madsen SS, Seidelin M et al (2002) Dynamics of Na+, K+, 2Cl− cotransporter and Na+, K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J Exp Zool 293:106–118. https://doi.org/10.1002/jez.10118
Tipsmark CK, Baltzegar DA, Ozden O et al (2008a) Salinity regulates claudin mRNA and protein expression in the teleost gill. Am J Physiol Regul Integr Comp Physiol 294(3):R1004-1014. https://doi.org/10.1152/ajpregu.00112.2007
Tipsmark CK, Kiilerich P, Nilsen TO et al (2008b) Branchial expression patterns of claudin isoforms in Atlantic salmon during seawater acclimation and smoltification. Am J Physiol Regul Integr Comp Physiol 294(5):R1563-1574. https://doi.org/10.1152/ajpregu.00915.2007
Tipsmark CK, Jørgensen C, Engelund M et al (2009) Effects of cortisol, growth hormone and prolactin on gill claudin expression in Atlantic salmon. Gen Comp Endocrinol 163:270–277. https://doi.org/10.1016/j.ygcen.2009.04.020
Tipsmark CK, Sørensen KJ, Madsen SS (2010) Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J Exp Biol 213(3):368–379. https://doi.org/10.1242/jeb.034785
Tipsmark CK, Breves JP, Seale AP et al (2011) Switching of Na+, K+-ATPase isoforms by salinity and prolactin in the gill of a cichlid fish. J Endocrinol 209(2):237–244. https://doi.org/10.1530/JOE-10-0495
Tomy S, Chang YM, Chen YH et al (2009) Salinity effects on the expression of osmoregulatory genes in the euryhaline black porgy Acanthopagrus schlegeli. Gen Comp Endocrinol 161(1):123–132. https://doi.org/10.1016/j.ygcen.2008.12.003
Trayer V, Hwang PP, Prunet P, Thermes V (2013) Assessment of the role of cortisol and corticosteroid receptors in epidermal ionocyte development in the medaka (Oryzias latipes) embryos. Gen Comp Endocrinol 194:152–161. https://doi.org/10.1016/j.ygcen.2013.09.011
Tse WK, Au DW, Wong CK (2006) Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem Biophys Res Commun 346(4):1181–1190. https://doi.org/10.1016/j.bbrc.2006.06.028
Tse WK, Au DW, Wong CK (2007) Effect of osmotic shrinkage and hormones on the expression of Na+/H+ exchanger-1, Na+/K+/2Cl- cotransporter and Na+/K+-ATPase in gill pavement cells of freshwater adapted Japanese eel, Anguilla japonica. J Exp Biol 210(Pt 12):2113–2120. https://doi.org/10.1242/jeb.004101
Tseng YC, Yan JJ, Furukawa F et al (2022) Teleostean fishes may have developed an efficient Na+ uptake for adaptation to the freshwater system. Front Physiol 13:947958. https://doi.org/10.3389/fphys.2022.947958
Vialle RA, de Souza JES, Lopes KP et al (2018) Whole genome sequencing of the pirarucu (Arapaima gigas) supports independent emergence of major teleost clades. Genome Biol Evol 10(9):2366–2379. https://doi.org/10.1093/gbe/evy130
Wang PJ, Lin CH, Hwang HH, Lee TH (2008) Branchial FXYD protein expression in response to salinity change and its interaction with Na+/K+-ATPase of the euryhaline teleost Tetraodon nigroviridis. J Exp Biol 211(Pt 23):3750–3758. https://doi.org/10.1242/jeb.018440
Wang YF, Tseng YC, Yan JJ et al (2009) Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl uptake mechanism in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 296(5):R1650–R1660. https://doi.org/10.1152/ajpregu.00119.2009
Wang YF, Yan JJ, Tseng YC et al (2015) Molecular physiology of an extra-renal Cl− uptake mechanism for body fluid Cl− homeostasis. Int J Biol Sci 11(10):1190–1203. https://doi.org/10.7150/ijbs.11737
Watanabe S, Kaneko T, Aida K (2005) Aquaporin-3 expressed in the basolateral membrane of gill chloride cells in Mozambique tilapia Oreochromis mossambicus adapted to freshwater and seawater. J Exp Biol 208(Pt 14):2673–2682. https://doi.org/10.1242/jeb.01684
Watanabe S, Itoh K, Kaneko T (2016) Prolactin and cortisol mediate the maintenance of hyperosmoregulatory ionocytes in gills of Mozambique tilapia: exploring with an improved gill incubation system. Gen Comp Endocrinol 232:151–159. https://doi.org/10.1016/j.ygcen.2016.04.024
Weisbart M, Chakraborti PK, Gallivan G, Eales JG (1987) Dynamics of cortisol receptor activity in the gills of the brook trout, Salvelinus fontinalis, during seawater adaptation. Gen Comp Endocrinol 68:440–448
Weng CF, Lee TH, Hwang PP (1997) Immune localization of prolactin receptor in the mitochondria-rich cells of the euryhaline teleost (Oreochromis mossambicus) gill. FEBS Lett 405(1):91–94. https://doi.org/10.1016/s0014-5793(97)00162-2
Whitehead A, Roach JL, Zhang S, Galvez F (2011) Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc Natl Acad Sci USA 108(15):6193–6198. https://doi.org/10.1073/pnas.1017542108
Wongdee K, Charoenphandhu N (2013) Regulation of epithelial calcium transport by prolactin: from fish to mammals. Gen Comp Endocrinol 181:235–240. https://doi.org/10.1016/j.ygcen.2012.07.006
Wright PA, Wood CM (2015) Regulation of ions, acid–base, and nitrogenous wastes in elasmobranchs. In: Shadwick RE, Farrell AP, Brauner CJ (eds) Fish physiology. Academic Press, pp 279–345
Wu CY, Lee TH, Tseng DY (2023) Mineralocorticoid receptor mediates cortisol regulation of ionocyte development in tilapia (Oreochromis mossambicus). Fishes 8(6):283. https://doi.org/10.3390/fishes8060283
Xu B, Miao H, Zhang P, Li D (1997) Osmoregulatory actions of growth hormone in juvenile tilapia (Oreochromis niloticus). Fish Physiol Biochem 17:295–301. https://doi.org/10.1023/A:1007750022878
Yada T, Miyamoto K, Miura G, Munakata A (2014) Seasonal changes in gene expression of corticoid receptors in anadromous and non-anadromous strains of rainbow trout Oncorhynchus mykiss. J Fish Biol 85:1263–1278. https://doi.org/10.1111/jfb.12521
Yamaguchi K, Hara Y, Tatsumi K et al (2020) Inference of a genome-wide protein coding gene set of the inshore hagfish Eptatretus burgeri. bioRxiv. https://doi.org/10.1101/2020.07.24.218818
Yamaguchi Y, Ikeba K, Yoshida MA, Takagi W (2023) Molecular basis of the unique osmoregulatory strategy in the inshore hagfish, Eptatretus burgeri. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00166.2023
Yan JJ, Hwang PP (2019) Novel discoveries in acid-base regulation and osmoregulation: a review of selected hormonal actions in zebrafish and medaka. Gen Comp Endocrinol 277:20–29. https://doi.org/10.1016/j.ygcen.2019.03.007
Yao K, Niu PD, Gac FL, Bail PYL (1991) Presence of specific growth hormone binding sites in rainbow trout (Oncorhynchus mykiss) tissues: characterization of the hepatic receptor. Gen Comp Endocrinol 81:72–82. https://doi.org/10.1016/0016-6480(91)90126-Q
Young G, Björnsson BT, Prunet P et al (1989) Smoltification and seawater adaptation in coho salmon (Oncorhynchus kisutch): plasma prolactin, growth hormone, thryroid hormones, and cortisol. Gen Comp Endocrinol 74:335–345. https://doi.org/10.1016/s0016-6480(89)80029-2
Yu D, Ren Y, Uesaka M et al (2023) Hagfish genome illuminates vertebrate whole genome duplications and their evolutionary consequences. bioRxiv. https://doi.org/10.1101/2023.04.08.536076
Zhou B, Kelly SP, Ianowski JP, Wood CM (2003) Effects of cortisol and prolactin on Na+ and Cl- transport in cultured branchial epithelia from FW rainbow trout. Am J Physiol Regul Integr Comp Physiol 285(6):R1305-1316. https://doi.org/10.1152/ajpregu.00704.2002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Breves, J.P., Shaughnessy, C.A. Endocrine control of gill ionocyte function in euryhaline fishes. J Comp Physiol B (2024). https://doi.org/10.1007/s00360-024-01555-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00360-024-01555-3