Abstract
The goal of this work was to evaluate the efficiency of mast cell identification by different histochemical and immunohistochemical methods and to determine the features of their localization in the human pineal gland. Mast cells were found to be an essential component of the human pineal gland at any age. According to our data, the number of mast cells in the pineal gland increased with age. Mast cells were mostly located in the pineal stroma and did not tend to associate with concrements, cysts, or melanin accumulations. Mast cells in the pineal gland were predominantly non-degranulating, which indicated their inactive state. The detectability of mast cells in the pineal gland depended significantly on the used staining technique. The largest number of mast cells was revealed by tryptase immunohistochemical assay, which should be used to accurately determine the population of mast cells of the pineal gland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The pineal gland (or epiphysis cerebri), an endocrine organ located in the epithalamus, serves as the primary source of melatonin. The levels of melatonin secretion into the bloodstream exhibit periodic oscillations (with a nocturnal maximum) that follow the intensity of the circadian rhythm signals generated by the suprachiasmatic nuclei of the hypothalamus. Melatonin has a pronounced effect on the activity of central and peripheral endocrine organs, and the circadian oscillations in melatonin blood levels determine the corresponding rhythm in the functioning of the endocrine, immune, and other systems [1]. In addition to controlling circadian rhythms of different physiological functions, melatonin plays an important role in the aging. A decrease in the nocturnal peak of melatonin secretion and diminished populations of nonpineal melatonin-secreting cells were observed in natural and accelerated aging [9, 25]. Rats with experimental pinealectomy were found to have a decreased life expectancy [32], while the administration of melatonin or pineal gland peptides prevented or delayed aging and increased longevity [3, 16, 31]. These observations clearly implicate the pineal gland in the regulation of senescence and show that melatonin is a marker of integrated aging processes [7].
The principal cell populations of the pineal gland are melatonin-producing pinealocytes and glial cells. Along with these two dominant cell types, the pineal gland also contains mastocytes: cells of hematogenous origin that are involved in the immune response, as well as in the regulation of local hemodynamics and tissue integrity [10, 29]. Traditionally, mast cells are detected in histological preparations with aniline dyes [, –, –, , , ], which produce metachromatic mastocyte staining. It was reported that the rate of mast cell detection depended significantly on the fixative used, the duration of fixing and staining, the dye pH, and other parameters of histological treatment, as well as on the mast cell type and maturity [12, 19, 28, 34]. Insufficient reliability of the techniques of mast cell detection leads to considerable variation in the results reported by different authors and calls for improvement of the existing methods.
Although the presence of mast cells in the pineal gland was described more than a century ago [20], their functional significance in this organ still has not been established, and the patterns of their occurrence and distribution, as well as their histochemical characteristics, are not quite clear. In this context, the goal of our work was to determine mast cells in the human pineal gland by different histochemical techniques and to investigate their distribution patterns.
MATERIALS AND METHODS
The study was performed in human pineal gland specimens (n = 6) from subjects 17 to 68 years old. The specimens were provided by the archive of the Department of General and Special Morphology of the Institute of Experimental Medicine. The tissues were fixed in alcohol–formaline, dehydrated, embedded in paraffin by conventional techniques, and cut into 6- to 7‑μm-thick sections. To assess the presence and location of mast cells, the preparations were stained with toluidine blue (Lab Point Histology, Russia) or Alcian blue (BioVitrum, Russia) and counterstained with 0.5% Nuclear Fast Red solution (Sigma-Aldrich, United States). In addition, immunohistochemical (IHC) assay was performed with monoclonal mouse antibodies to human mast cell tryptase (clone AA1, BioGenex, United States) in the manufacturer’s dilution. The MACH2 Universal HRP Polymer Kit for mouse and rabbit (Biocare Medical, United States) was used as secondary antibodies. The products of the IHC reaction were visualized with 3'3-diaminobenzidine (DAB+ kit, Dako, Denmark). After the IHC assay, some sections were counterstained with 0.2% aqueous solution of Astra blue (Merck, Germany). The preparations were analyzed and photographed with a Leica DM750 microscope and an ICC50 digital camera (Leica, Germany).
For each case, the mast cells were counted in the preparations stained with toluidine blue, Alcian blue and IHC assay. The number of mastocytes was determined in three random fields of vision at magnification ×40 to calculate their number per square millimeter of specimen area. The measurements were performed with the ImageJ software (NIH, United States). Pairwise correlation was analyzed with Statistica 6.0 (StatSoft, United States).
RESULTS AND DISCUSSION
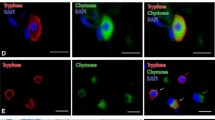
Microscopic investigation showed that all of the studied human pineal gland specimens were well preserved and exhibited a characteristic gland structure with numerous pinealocytes in the parenchyma of lobules separated with septae. Both toluidine blue and Alcian blue staining, as well as immunostaining for tryptase-detected mast cells in all studied specimens (Fig. 1) and the number of mast cells, varied considerably among the pineal gland specimens, irrespectively of the staining technique. The rate of mast cell detection differed significantly depending on the staining technique used. Immunostaining for tryptase revealed the highest number of mast cells, while staining with Alcian blue and toluidine blue detected two and five times fewer mast cells, respectively (Table). Moreover, an analysis of the correlation between the results obtained with different staining techniques in the same specimen showed that the results of immunostaining for tryptase correlated significantly with the data obtained with Alcian blue (r = 0.89, P = 0.01) but not with the data of toluidine blue staining (r = 0.54, P = 0.26).
In an overwhelming majority of cases, the mast cells exhibited a characteristic granular content of the cytoplasm (Fig. 1a); in the course of degranulation, only a few mast cells with stained granules located near the cell outside the cytoplasm could be detected (Fig. 1c). Cytoplasm granulation was clearly visible in the IHC tryptase assay and with toluidine blue staining but not with Alcian blue staining (Fig. 1b). Mast cells were found both in the stroma (capsule and septae) and in the parenchyma among pinealocytes; they were often located in vicinity of blood vessels (Fig. 2).
All of the studied pineal gland specimens featured cysts, melanin agglomerates, and concrements. In some cases, it could be seen that the cysts were lined with glial cells. The amounts of melanin varied considerably among different pineal gland specimens; it was usually distributed in the extracellular space, but it also was frequently observed in elongated structures that resembled stromal-cell projections. Stratified concrements of different shapes and sizes were usually located individually (Fig. 3), sometimes occurring in small groups. We did not observe any significant association between the location of mast cells in the pineal gland and cysts, concrements, or melanin.
Analysis of the correlation between the number of mast cells in the pineal gland as assessed by immunostaining for tryptase and the subjects’ age showed a trend toward a positive correlation; however, it did not reach the significance threshold (r = 0.61, P = 0.198).
Our data suggest that mast cells are an essential component of the human pineal gland, since they were detected in all specimens obtained from subjects of different ages. The efficiency of mast cell detection depended critically on the staining technique used. The highest mastocyte detection rate was achieved with immunostaining for tryptase; it significantly exceeded the efficiency of staining with Alcian blue and especially with toluidine blue. The results obtained with IHC assay exhibited a strong correlation with the data from Alcian blue staining but not with the results of toluidine blue staining.
The same relative efficiency of these techniques of mast cell detection was observed in the study of the human choroid plexus [14]. In the study by Maślińska et al. [27], the rate of mast cell detection in the human pineal gland also was the highest with tryptase immunostaining. At the same time, in a number of peripheral organs of humans and laboratory animals (e.g., the skin and the organs of the digestive system), mast-cell detection by toluidine blue staining was at least as efficient as by tryptase immunoassay, whereas the efficiency of Alcian blue staining was the lowest [5, 18]. These controversial data imply that, for reliable mast-cell research, it is necessary to determine the most appropriate technique for their detection in a given organ.
The data on mast cell degranulation are of considerable importance. Degranulation, i.e., the release of synthesized products from cytoplasmic granules into the surrounding tissue, is a sign of mast cell activation. We observed a low level of mast cell degranulation in the pineal gland, which agrees with the data from other studies [15] and suggests a low activity level (in contrast, for example, to mast cells of the choroid plexus, in which the share of degranulating mast cells can be as high as 60%) [14].
The pineal gland typically contains noticeable amounts of calcium deposits, and it was observed that calcification increases significantly in the course of aging [17]. Furthermore, it was reported that mast cells in the human pineal gland were predominantly located near calcium deposits [27]. It was not the goal of the present work to investigate calcified concrements, but the mast cells in all of the studied pineal gland specimens were randomly distributed in the stroma and were not associated with calcium deposits. Our observations agree with the data from other publications [15].
Studies of age-related changes in the pineal gland function showed that melatonin blood levels decrease and the patterns of their circadian fluctuation change with age, indicating that the functional activity of pinealocytes diminishes for reasons that have not yet been established [2, 35]. Decreased pinealocyte function is not associated with aging-related changes in the pineal gland structure, since these changes do not suggest pinealocyte degeneration (although the available data on the morphology of a senescent pineal gland are incomplete and inconsistent) [2, 4, 35]. Our own research on pineal mast cells showed that mastocytes were present in the pineal gland of elderly subjects; moreover, in our small sample, their number exhibited a noticeable increase with age (Table 1).
Back in 1979, J.J. Dropp observed that the number of mast cells in the human pineal gland decreased with age [21]. However, that work used staining with cresyl violet, which, similarly to the toluidine blue used in the present work, produces a metachromatic stain of mast cells. Our data indicate that this method is significantly less efficient in mast cell detection than immunostaining for tryptase and even than Alcian blue staining. Importantly, staining with toluidine blue in our work, in contrast to staining with Alcian blue and immunostaining for tryptase, did not detect any age-related changes in the mast cell numbers in the pineal gland. Apparently, mast cells could not be detected in the pineal gland of elderly subjects in [21] because of the use of this insufficiently sensitive method. However, the same method was nevertheless able to detect mast cells of most pineal gland specimens of younger subjects, which apparently suggests that the tinctorial properties of pineal mast cells change in the course of aging. This hypothesis is consistent with the data showing an aging-related decrease in the functional activity of mast cells and their sensitivity to activators [22, 23]. Accordingly, the mast cell populations could be expected to grow to compensate their diminished functional activity; in fact, both increased and decreased mast cell numbers were observed in different organs of elderly humans and animals [11, 13, 23, 24, 30]. Thus, further research concerning age-related changes in the number and properties of mast cells in the pineal gland is required.
The functions of mast cells in the pineal gland have not yet been determined. On the organism level, they are involved in a number of processes, including immune response, allergic reactions, tissue repair, inflammation, regulation of vascular permeability, and angiogenesis [10]. This multifunctionality of mast cells is due to their ability to synthesize a broad variety of biologically active compounds that are stored within cytoplasmic granules. These include biogenic amines (histamine, serotonin), cytokines, growth factors, and mast cell-specific proteases (tryptase and chymase), as well as nonspecific proteases (catepsin G, renin, and caspase 3), prostaglandines, leukotrienes, neuropeptides (vasointestinal peptide, gonadotropin-releasing hormone [6, 33]), and, which is particularly important in the context of our study, melatonin [8, 26]. However, melatonin production by pineal mast cells has never been observed so far, and the nature of their relationships with pinealocytes and glial cells remains unknown. It is likely that the presence of an immunocompetent mast cell population in the pineal gland somehow helps this organ to participate in the regulation of the entire neuroimmunoendocrine system.
CONCLUSIONS
This work showed that mast cells are an essential component of the human pineal gland, irrespective of the subject’s age. Our data suggest that the population of mast cells in the pineal gland increases with age. Mast cells were predominantly located in the pineal gland stroma and did not exhibit significant association with calcium deposits, cysts, or melanin aggregates. An overwhelming majority of mast cells in the pineal gland were non-degranulating, which indicates their inactive state. The rate of mast cell detection in the pineal gland varied considerably depending on the used staining techniques. The highest number of mast cells was revealed with immunostaining for tryptase; therefore, this technique can be recommended for accurate assessment of mast cell populations in the pineal gland.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. The specimens were provided by the archive of the Department of General and Special Morphology of the Institute of Experimental Medicine. The biological materials were archived in agreement with the ethical guidelines, as confirmed by conclusion no. 58-9/1-684 of the Ethics Committee of the Institute from December 11, 2009.
REFERENCES
Anisimov, V.N., Epiphysis and melatonin production, in Melatonin v norme i patologii (Melatonin in Normal and Pathological States), Komarov, F.I. et al., Eds., Moscow, 2004, pp. 7–19.
Anisimov, V.N., Age-related changes in the pineal gland, in Melatonin v norme i patologii (Melatonin in Normal and Pathological States), Komarov, F.I. et al., Eds., Moscow, 2004, pp. 20–33.
Anisimov, V.N., The effect of melatonin on aging, in Melatonin v norme i patologii (Melatonin in Normal and Pathological States), Komarov, F.I. et al., Eds., Moscow, 2004, pp. 223–236.
Anisimov, V.N., Epiphysis, biorhythms, and aging, Usp. Fiziol. Nauk, 2008, vol. 39, no. 4, pp. 40–65.
Atyashkin, D.A., Burtseva, A.S., and Sokolov, D.A., Evaluation of the effectiveness of mast cells detection in Mongolian gerbils jejunum mucosa using histochemical methods, Zh. Anat. Gistopatol., 2016, vol. 5, no. 4, pp. 85–89.
Bykov, V.L., Secretory mechanisms and secretory products of mast cells, Morfologiya, 1999, vol. 115, no. 2, pp. 72–79.
Zuev, V.A., Trifonov, N.I., Lin’kova, N.S., and Kvetnaya, T.V., Melatonin as a molecular marker of age pathology, Usp. Gerontol., 2017, vol. 30, no. 1, pp. 62–69.
Kvetnoi, I.M. and Ingel’, I.E., Hormonal function of nonendocrine cells: role of new biological phenomenon in the regulation of homeostasis, Bull. Exp. Biol. Med., 2000, vol. 130, no. 5, pp. 1027–1030.
Knyaz’kin, I.V., Role of extrapineal melatonin in the accelerated and premature aging in rats, Usp. Gerontol., 2008, vol. 21, no. 1, pp. 80–82.
Kutukova, N.A. and Nazarov, P.G., Role of mast cells in inflammation, tissue recovery, and fibrosis development, Tsitokiny Vospalenie, 2014, vol. 13, no. 2, pp. 11–20.
Kutukova, N.A., Nazarov, P.G., Kudryavtseva, G.V., and Shishkin, V.I., Mast cells and aging, Adv. Gerontol., 2017, vol. 7, no. 1, pp. 68–75.
Lillie, R.D., Histopathologic Technic and Practical Histochemistry, New York: McGraw-Hill, 1965, 3rd ed.
Turygin, V.V., Babik, T.M., and Boyakov, A.A., Characteristics of mast cells of the choroid plexus of the human brain ventricles during aging, Morfologiya, 2004, vol. 126, no. 6, pp. 61–62.
Fedorova, E.A., Grigorev, I.P., Syrtsova, M.A., et al., Identification of morphological features of mast cell degranulation in the choroid plexus of the human brain using various methods of staining and immunohistochemistry, Morfologiya, 2018, vol. 153, no. 2, pp. 70–75.
Yuneman, O.A., Morphological organization of the epiphysis and choroid plexus of the third ventricle of the human brain, Morfol. Ved., 2012, no. 3, pp. 97–100.
Anisimov, V.N. and Khavinson, V.Kh., Pineal peptides as modulators of aging, in Aging Interventions and Therapies, Rattan, S.I.S., Ed., Singapore: World Scientific, 2005, pp. 127–146.
Antić, S., Jovanović, I., Stefanović, N., et al., Morphology and histochemical characteristics of human pineal gland acervuli during the aging, Facta Univ., Ser.: Med. Biol., 2004, vol. 11, no. 2, pp. 63–68.
Atiakshin, D., Samoilova, V., Buchwalow, I., et al., Characterization of mast cell populations using different methods for their identification, Histochem. Cell Biol., 2017, vol. 147, no. 6, pp. 683–694.
Beil, W.J., Schulz, M., McEuen, A.R., et al., Number, fixation properties, dye–binding and protease expression of duodenal mast cells: comparisons between healthy subjects and patients with gastritis or Crohn’s disease, Histochem. J., 1997, vol. 29, no. 10, pp. 759–773.
Costantini, G., Interno ad alcune particolarità di struttura della glandola pineale, Pathologica, 1910, vol. 2, pp. 439–441.
Dropp, J.J., Mast cells in the human brain, Acta Anat., 1979, vol. 105, no. 4, pp. 505–513.
Gashev, A.A. and Chatterjee, V., Aged lymphatic contractility: recent answers and new questions, Lymphatic Res. Biol., 2013, vol. 11, no. 1, pp. 2–13.
Gomez, C.R., Nomellini, V., Faunce, D.E., and Kovacs, E.J., Innate immunity and aging, Exp. Gerontol., 2008, vol. 43, no. 8, pp. 718–728.
Hart, P.H., Grimbaldeston, M.A., Hosszu, E.K., et al., Age-related changes in dermal mast cell prevalence in BALB/c mice: functional importance and correlation with dermal mast cell expression of kit, Immunology, 1999, vol. 98, no. 3, pp. 352–356.
Kvetnoy, I.M., Extrapineal melatonin: location and role within diffuse neuroendocrine system, Histochem. J., 1999, vol. 31, no. 1, pp. 1–12.
Maldonado, M.D., Mora-Santos, M., Naji, L., et al., Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation, Pharmacol. Res., 2010, vol. 62, no. 3, pp. 282–287.
Maślińska, D., Laure-Kamionowska, M., Deręgowski, K., and Maśliński, S., Association of mast cells with calcification in the human pineal gland, Folia Neuropathol., 2010, vol. 48, no. 4, pp. 276–282.
Mayrhofer, G., Fixation and staining of granules in mucosal mast cells and intraepithelial lymphocytes in the rat jejunum, with special reference to the relationship between the acid glycosaminoglycans in the two cell types, Histochem. J., 1980, vol. 12, no. 5, pp. 513–526.
Metcalfe, D.D., Baram, D., and Mekori, Y.A., Mast cells, Physiol. Rev., 1997, vol. 77, no. 4, pp. 1033–1079.
Nguyen, M., Pace, A.J., and Koller, B.H., Age-induced reprogramming of mast cell degranulation, J. Immunol., 2005, vol. 175, no. 9, pp. 5701–5707.
Pierpaoli, W., Dall’Ara, A., Pedrini, E., and Regelson, W., The pineal control of aging: the effects of melatonin and pineal grafting on the survival of older mice, Ann. N.Y. Acad. Sci., 1991, vol. 621, pp. 291–313.
Reiter, R.J., Tan, D.-X., and Kim, S.J., Augmentation of indices of oxidative damage in life-long melatonin-deficient rats, Mech. Ageing Dev., 1999, vol. 110, pp. 157–173.
Wernersson, S. and Pejler, G., Mast cell secretory granules: armed for battle, Nat. Rev. Immunol., 2014, vol. 14, no. 7, pp. 478–494.
Wingren, U. and Enerbäck, L., Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan, Histochem. J., 1983, vol. 15, no. 6, pp. 571–582.
Wu, Y.H. and Swaab, D.F., The human pineal gland and melatonin in aging and Alzheimer’s disease, J. Pineal Res., 2005, vol. 38, no. 3, pp. 145–152.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Timchenko
Rights and permissions
About this article
Cite this article
Fedorova, E.A., Sufieva, D.A., Grigorev, I.P. et al. Mast Cells of the Human Pineal Gland. Adv Gerontol 9, 62–66 (2019). https://doi.org/10.1134/S2079057019010053
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057019010053