Abstract
To create different oxygen nonstoichiometry in oxide samples of NdSr2Mn2O7 + δ, we have used a two-step method of synthesis, which makes it possible to achieve different oxygen nonstoichiometry of the samples. Oxygen nonstoichiometry is formed by two methods: quenching at certain temperatures and annealing at certain values of oxygen partial pressure. The value of oxygen nonstoichiometry is determined by the mass of oxygen emitted from the sample during the decomposition of samples of NdSr2Mn2O7 + δ to simple oxides. We have determined oxygen nonstoichiometry in NdSr2Mn2O7 + δ when recording external parameters such as pressure and temperature. The study of samples of NdSr2Mn2O7 + δ using thermogravimetry and X-rays revealed phase separation—two phases with the same cation composition, having different oxygen content. By example of a sample of NdSr2Mn2O7 + δ tempered from 1000°C and having oxygen nonstoichiometry of +0.09, we have proposed a crystallographic criterion of manifestation of phase separation in Ruddlesden–Popper (R–P) phases—a sharp change in the manganese-oxygen bond lengths. Measurements of the electrical dc resistance in the samples of NdSr2Mn2O7 have been performed in the temperature range of 5–300 K and magnetic fields up to 7 T using the standard 4-probe method. A magnetoresistive effect up to 400% was found in the studied oxides, which is nonlinearly dependent on their oxygen nonstoichiometry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Complex oxides of the composition Ln1 + xSr2 – xMn2O7 + δ, the so-called Ruddlesden–Popper phases (R-P), consisting of perovskite blocks in conjunction with such blocks as rock salt, have unique electrical, magnetic, and optical properties [1–7]. These properties make it possible to use similar materials in electronic devices, such as highly sensitive magnetic field sensors, high-density magnetic recording heads, and storage devices for large volumes of information [8]. Development of the conditions for the synthesis of layered complex oxides with desired physicochemical properties and investigation of the stability of these oxides when changing external parameters are an urgent task [9]. An attempt to obtain phases R–P \({{({\text{L}}{{{\text{n}}}_{{1 - x}}}{{{\text{A}}}_{x}})}_{{n + 1}}}{\text{M}}{{{\text{n}}}_{n}}{{{\text{O}}}_{{3n + 1}}}\) (n = 1, 2, 3) in air [7] was not successful. At the same time, it was possible to synthesize the compound \({\text{LaS}}{{{\text{r}}}_{2}}{\text{M}}{{{\text{n}}}_{{\text{2}}}}{{{\text{O}}}_{{7 \pm \delta }}}\) (n = 2) using a slow cooling technique to stabilize the structure and saturate it with oxygen [10, 11]. There are data in the literature on the effect of the conditions of synthesis and its duration on the structure and homogeneity of the fabricated samples of NdSr2Mn2O7 ± δ [8]. In compounds formed by lanthanides with large ionic radii, a fine phase separation was found, which affects the physical properties [11]. In [12], it was suggested that a key role in the manifestation of the colossal magnetoresistance effect is played by the phase separation, which is associated with different oxygen content in each phase. It was found that electronic properties of these phases are associated with various degrees of manganese oxidation and the size of the lanthanide ion [13]. А different degree of manganese oxidation can be due to either different content of the alkaline earth element (x value in the formula La1+xSr2 – xMn2O7 ± δ) or various contents of oxygen (the value δ in the formula). One can vary the oxygen content in the samples by changing the oxygen pressure in the synthesis of materials or by changing the temperature of the final annealing at a fixed oxygen (air) pressure. In this work, we used both methods of changing oxygen nonstoichiometry to obtain samples of NdSr2Mn2O7 ± δ showing a magnetoresistive effect.
The aim of the work is to reveal the dependence of structural and magnetic characteristics of samples on the change in their oxygen nonstoichiometry.
EXPERIMENTAL
The original sample of NdSr2Mn2O7 ± δ was obtained using ceramic synthesis in air from Nd2O3 of grade NdO-E, Mn2O3 with the purity ≥99%, and “very pure” SrCO3 at a temperature of 1400°C for 180 h, followed by cooling together with the furnace for 12 h. This made it possible to achieve the maximum oxygen saturation of the entire sample volume. To stabilize a certain oxygen nonstoichiometry, the sample was additionally annealed at temperatures of 900, 1000, 1100, and 1200°C in air for 72 h, followed by quenching onto a copper plate. The X-ray diffraction analysis (XRD) of the samples was performed using a Shimadzu XRD-7000 diffractometer in Cu Kα radiation in the range of angles 2θ = 20°–70° with a step of 0.03 and an exposure of 2 s. The parameters of the structure were calculated by the Rietveld method [14], using the EXPGUI software package [15]. The determination of absolute oxygen nonstoichiometry was carried out in a vacuum circulation system by the gravimetric method during decomposition of samples to simple oxides. To obtain oxygen pressures sufficient to decompose complex oxides to simple ones, hydrogen was used in the setup. In contact with the sample, hydrogen reacted with oxygen that was formed during the oxide decomposition, forming water vapor. The water vapor was removed using a trap with liquid nitrogen. The oxygen partial pressure was measured in atmospheres. The effect of oxygen pressure on the oxygen nonstoichiometry of the sample was studied at a research complex including a STA 449 F3 thermal analyzer (Jupiter) and isobaric additional attachment to it [16]. The sample was heated from room temperature to 900°C in the thermal analyzer at a rate of 10 deg/min. For the study, we used gas mixtures having a partial pressure of oxygen in the range –0.67 > log\({{P}_{{{{{\text{O}}}_{2}}}}}\) > –4, which were created using the isobaric device, followed by placing them into a thermal analyzer. The effect of oxygen nonstoichiometry of samples of NdSr2Mn2O7 ± δ on magnetoresistive properties was studied using a VSM CFS-9T-CVTI automated system (Cryogenic Ltd, UK). DC electrical resistance measurements were performed using the standard four-probe method in the temperature range of 5–300 K and magnetic fields up to 7 T. We used a Keithley K2400 as a current source, and the voltage was recorded using a Keithley K2182 nanovoltmeter. The samples for research had the form of rectangular parallelepipeds with geometric dimensions of 8 × 3 × 3 mm. The electrical contacts on the samples were made using indium-gallium paste and then soldered to the measuring cell with pure indium. In all experiments, the current was equal to 1 μA.
RESULTS AND DISCUSSION
The original sample of NdSr2Mn2O7 ± δ, annealed at 1400°С in air for 180 h and slowly cooled together with the furnace, had the following unit cell parameters: a = 3.8549(2) Å, c = 20.0954 (12) Å. Oxygen nonstoichiometry of the sample of NdSr2Mn2O7 ± δ was positive: δ = 0.22 ± 0.01. Table 1 shows the unit cell parameters of the samples annealed at different temperatures, quenched samples, and their oxygen nonstoichiometry.
The change in the mass of a sample with a change in temperature and partial pressure of oxygen was studied using a thermal analyzer equipped with an isobaric attachment. The thermogram in Fig. 1 shows the example of a sample tempered from 1000°C. Shown is the temperature dependence in the mass changes of the sample at various partial pressures of gas mixtures. One can note a steady trend of the mass loss with decrease in the pressure of oxygen. The change in mass of the sample ends at a temperature of 668°C, after which its stabilization is observed.
Such an abrupt change in the mass of the samples can be explained by the fact that at first the phase NdSr2Mn2O7 ± δ had one content of oxygen δ, and during heating, the samples lost oxygen when forming the phase with a lower δ. With decreasing oxygen pressure in the experiment, the mass loss of the samples increased, and the temperature of the onset of this process decreased from 635°С (sample 1) to 460°С (sample 4). After a temperature of 668°C, a phase formed with the lowest oxygen content, which subsequently remained stable, despite a change in temperature. The effect of oxygen pressure in the gaseous medium on the obtained process characteristics (mass loss, process onset temperature) indicates its redox nature. Changes in the oxidation state of manganese, calculated from the decrease in the mass of the sample, are shown in Fig. 2. Changes in the mass of the sample are due to oxygen release as a result of changing experimental conditions. In our opinion, the plateaus found in this work on the temperature dependences of the change in mass of the samples with a change in oxygen pressure (Fig. 1) are an argument in favor of the manifestation of the process of phase separation into two phases having different oxygen nonstoichiometry.
The change in the mass of a sample is a consequence of direct transition of oxygen into the gas phase; therefore the thermogravimetric method (using a thermal analyzer) registers it immediately. When a sufficiently large amount of oxygen is removed from the crystal lattice of the sample, the lattice distortion occurs as a result of the total effect. The distortion is characterized by a change in the lengths of the bonds between cations and oxygen, which is registered by high-temperature XRD analysis performed in the range of temperatures from room temperature to 1100°C. Using the results of processing the diffractometry data of the samples of NdSr2Mn2O7 ± δ, the structural parameters of the samples of NdSr2Mn2O7.09 were calculated by the Ritveld method. The achieved reliability factors are R(F 2)BR = 1.65%, ωRp = 6.6%, Rp = 5.2%.
In the structure of NdSr2Mn2O7 ± δ, manganese atoms are surrounded by six oxygen atoms. Four of them are located in the Mn–O plane (they are indicated as O3); one is above the plane (indicated as O1); another one is under the plane (O2), forming the octahedron MnO6 [17]. Figure 3 plots the temperature dependences of the Mn–O1 and Mn–O2 bond lengths, which form the height of the MnO6 octahedron of the sample of NdSr2Mn2O7.09. One can notice a sharp increase in the Mn–O1 bond length and a decrease in the Mn–O2 bond length in the temperature range of 220–300°C, which causes the octahedron MnO6 to stretch along the c axis. This process is accompanied by the appearance of a peak on the DSC curve in the same temperature range (see the inset in Fig. 3). A further increase in temperature causes the compression of MnO6 octahedron and a decrease in the Mn–O1 distance, as well as an increase in the bond length of Mn–O2. The minimum difference between the bond lengths of Mn–O1 and Mn–O2 is observed at a temperature of about 700°С, which is consistent with the end of the change in mass of the sample on the TG curve (Fig. 1). This temperature corresponds to the stabilization of the phase with a minimum oxygen content. It was found that, in the temperature range of 250–300°C, the lengths of bonds of manganese cations to oxygen change in the compound NdSr2Mn2O7, which causes distortion of MnO6 octahedra. In this case, no changes in the space group of the complex oxide are observed.
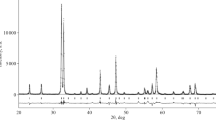
To confirm the assumption that there are two phases in the system that have different oxygen nonstoichiometry, the X-ray spectrum of sample NdSr2Mn2O7 ± δ was processed by the Rietveld method. The resultant spectrum showed the presence of two phases of the same cationic composition at a temperature of 300°C (Fig. 4). The lattice parameters of these two phases differ slightly: for the first phase, we obtained a = 3.8585(2) Å, c = 20.0364(4) Å, and for the second phase, a = 3.8473(1) Å, c = 20.0108(3) Å. These data are consistent with the data in [11], according to which two phases with equal cationic composition but with different oxygen content are present in LaSr2Mn2O7, as confirmed by neutron diffraction.
Thus, sharp changes in manganese–oxygen bond lengths found at certain temperatures can be considered as the crystallographic criterion for the appearance of phase separation. Additional evidence in favor of the phase separation version is the interval of oxygen nonstoichiometry of the sample that we identified, in which the equilibrium partial oxygen pressure is constant at a fixed temperature (Figs. 5a, 5b). It was found that, when oxygen is removed from the sample of NdSr2Mn2O7 ± δ, the partial pressure of oxygen at a temperature of 796°С remains constant. А thermodynamically equilibrium two-phase region in this range of oxygen content likely exists in the sample. And in this region, phases coexist with the same cationic composition but different oxygen nonstoichiometry and distribution of oxygen defects over different positions of the crystal structure. In Fig. 5, this region is seen as a plateau at oxygen pressure log\({{P}_{{{{{\text{O}}}_{2}}}}}\) = –14.3 and a temperature of 796°С. When oxygen is removed from the sample in this range of oxygen content, the amount of the phase enriched in oxygen decreases, and the amount of the phase with a lower oxygen content increases. The oxygen pressure in the system as a whole does not change. Within the limits of the detected range of oxygen content, the average valence of manganese in the sample varies in the range from +3.0 to +3.57. A sample obtained by slow cooling in air and initially having the stoichiometry of NdSr2Mn2O7.22 retains its structure when the oxygen pressure in the system decreases, releasing superstoichiometric oxygen. When the oxygen index reaches 7.03, thermodynamic equilibrium occurs in the system between the gas and solid phases. The oxygen pressure in the system as a whole does not change. When the oxygen content in the sample is lower than δ ≤ 6.42, the process of its dissociation is observed according to the scheme NdSr2Mn2O7 ± δ → Sr2MnO4 + NdMnO3 + О2↑.
To study the effect of oxygen nonstoichiometry on the physical properties of samples of NdSr2Mn2O7 ± δ, the temperature dependences of the electrical resistance were measured. The data were obtained in the cooling mode of the samples at a rate of 0.45 K/min in zero magnetic field and in the presence of a magnetic field of 7 T. The magnetoresistance of the samples was calculated using the known relation
where RB and R0 are the electrical resistances of the material in the magnetic field and in the absence of it.
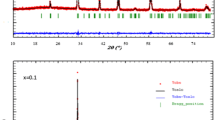
In the temperature range under study, the electrical resistance of samples of NdSr2Mn2O7 ± δ demonstrates the character of conductivity inherent to semiconductor materials. Samples quenched from temperatures of 900, 1000, 1100, and 1200°С and, therefore, having different oxygen nonstoichiometry showed a strong negative magnetoresistive effect below 200–250 K [18]. The maximum value of the effect at a temperature of 5 K is demonstrated by a sample quenched from the temperature of 1200°C and having an oxygen content closest to the stoichiometric one: δ = +0.03 (Fig. 6).
The use of various heat treatment modes of the samples causes the formation of defect in their structure, leading to a certain oxygen nonstoichiometry. Variation of the oxygen index in the samples of NdSr2Mn2O7 ± δ leads to a change in their electronic transport properties. One can note the monotonic dependence of the magnetoresistive effect with a decrease in oxygen nonstoichiometry (Fig. 7), which is possible owing to the formation of ferromagnetic clusters determining the magnetic behavior of samples [18].
CONCLUSIONS
The formation of various oxygen nonstoichiometry in oxide samples of NdSr2Mn2O7 ± δ was carried out by two methods: two-stage synthesis and exposure at a fixed reduced pressure of oxygen in a vacuum circulation system.
The presence of phase separation is shown—coexistence of two thermodynamically equilibrium phases with the same cationic composition, but with different oxygen nonstoichiometry.
Two anomalies were found in the temperature dependence of the Mn–O1 and Mn–O2 bond lengths. They indicate the structural adjustment in the complex oxide NdSr2Mn2O7.09 with increasing temperature and the changes in oxygen nonstoichiometry.
The concentration range of oxygen content in NdSr2Mn2O7 ± δ in which the sample retains its structure under isothermal conditions was determined. A sample with an oxygen index less than 6.42 dissociates into Sr2MnO4 and NdMnO3 phases with the release of oxygen. A strong magnetoresistive effect was found in oxides NdSr2Mn2O7 ± δ. The effect monotonically increases with decreasing temperature owing to a cluster nature of magnetic structure of materials.
REFERENCES
Battle, P.D., Blundell, S.J., Green, M.A., Hayes, W., Honold, M., Klehe, A.K., Laskey, N.S., Millburn, J.E., Murphy, L., and Rosseinsky, M.J., Colossal magnetoresistance in Sr2 –xNd1 +xMn2O7 (x = 0.0, 0.1), J. Phys.: Condens. Matter, 1996, vol. 8, no. 32, pp. 427–434.
Coldea, A.I., Spring, L.E., Blundell, S.J., Singleton, J., and Hayes, W., Magnetotransport studies on the Ruddlesden Popper phases Sr2RMn2O7 (R = Nd, Pr, Ho, Y) and Sr2 – xNd1 + xMn2O7 (x = 0, 0.1, 0.2, 0.5), J. Phys.: Condens. Matter, 1999, vol. 11, pp. 9053–9072.
Seshardri, R., Maignan, A., Hervieu, M., Nguyen, N., and Raveau, B., Complex magnetotransport in LaSr2Mn2O7, Solid State Commun., 1997, vol. 101, no. 6, pp. 453–457.
Argyriou, D.N, Bordallo, H.N., Campbell, B J., Cheetham, A.K., Cox, D.E., Gardner, J.S., Hanif, K., Dos Santos, A., and Strouse, G.F., Charge ordering and phase competition the layered perovskite LaSr2Mn2O7, Phys. Rev. B, 2000, vol. 61, no. 22, pp. 15269–15276.
Takata, M., Nishibori, E., Kato, K., Sakata, M., and Morimoto, Y., Direct observation of orbital order in manganites by MEM charge-density study, J. Phys. Soc. Jpn., 1999, vol. 68, no. 7, pp. 2190–2193.
Zhang, J., Wang, F., Zhang, P., Sun, X., and Yan, Q., Magnetic and electric properties of layered perovskites Nd2 – 2xSr1 + 2xMn2O7 (x = 0.3–0.5), J. Magn. Magn. Mater., 1998, vol. 190, pp. 166–170.
Majewski, P., Benne, D., Epple, L., and Aldinger, F., Phase equilibria in the system La2O3–SrO–Mn3O4 in air, Int. J. Inorg. Mater., 2001, vol. 3, no. 8, pp. 1257–1259.
Battle, P.D., Green, M.A., Laskey, N.S., Millburn, J.E., Murphy, L., Rosseinsky, M.J., Sullivan, S.P., and Vente, J.F., Layered Ruddlesden–Popper manganese oxides: synthesis and cationic ordering, Chem. Mater., 1997, vol. 9, pp. 552–559.
Missyul, A.B., Zvereva, I.A., and Palstra, T.T.M., The formation of the complex manganites LnSr2Mn2O7 (Ln = La, Nd, Gd), Mater. Res. Bull., 2012, vol. 47, no. 12, pp. 4156–4160.
Yankin, A.M., Fedorova, O.M., Zvereva, I.A., Titova, S.G., and Balakirev, V.F., Phase formation during synthesis of the LaSr2Mn2O7 compound, Glass Phys. Chem., 2006, vol. 32, no. 5, pp. 574–578.
Battle, P.D., Cox, D.E., Green, M.A., Millburn, J.E., Spring, L.E., Radaelli, P.G., Rosseinsky, M.J., and Vente, J.F., Antiferromagnetism, ferromagnetism, and phase separation in the GMR system Sr2 – xLa1 + xMn2O7, Chem. Mater., 1997, vol. 9, pp. 1042–1049.
Nagaev, E.L., Colossal Magnetoresistance and Phase Separation in Magnetic Semiconductors, Singapore: World Scientific, 2002.
Battle, P.D., Green, M.A., Laskey, N.S., Kasmir, N., Millburn, J.E., Spring, L.E., Sullivan, S.P., Rosseinsky, M.J., and Vente, J.F., Control of electronic properties by lanthanide size and manganese oxidation state in MnIII/MnIV Ruddlesden–Popper phases Ln2 – xSr1 + xMn2O7, J. Mater. Chem., 1997, vol. 7, pp. 977–988.
Larson A.C. and von Dreele R.B., General Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR 86-748, Los-Alamos, 2004.
Toby, B.H., EXPGUI, a graphical user interface for GSAS,J. Appl. Crystallogr., 2001, vol. 34, pp. 210–213.
Yankin, A.M. and Vedmid’, L.B., RF Patent 88452, 2009.
Ruddlesden, S.N. and Popper, P., The compound Sr3Ti2O7 and its structure, Acta Cryst., 1958, vol. 11, pp. 54–55.
Uporov, S.A., Mitrofanov, V.Ya., Fedorova, O.M., and Yankin, A.M., Influence of thermal processing on magnetotransport characteristics of NdSr2Mn2O7 + δ, Mater. Res. Bull., 2015, vol. 67, pp. 201–206.
ACKNOWLEDGMENTS
This work was carried out according to the State Assignment of the Institute of Metallurgy (Ural Branch of the Russian Academy of Sciences) within the framework of the Program of Fundamental Research of State Academies using the equipment of the Ural-M Central Scientific and Commercial Center.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by G. Dedkov
Rights and permissions
About this article
Cite this article
Fedorova, O.M., Vedmid’, L.B. & Uporov, S.A. Effect of Oxygen Nonstoichiometry on Phase Separation, Structure, and Magnetic Properties of the Complex Oxide NdSr2Mn2O7 . Inorg. Mater. Appl. Res. 11, 1065–1070 (2020). https://doi.org/10.1134/S207511332005010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207511332005010X