Abstract
The structural stability and physical properties of NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) mixed oxides, elaborated by conventional sol–gel process, have been investigated and obtained results show that substitution of nickel by cobalt at x = 0.5 enhances conductivity at room temperature; σ = 17.24 Ω−1 cm−1 coinciding with minimum activation energy (E a = 0.05 eV). Rietveld refinements of X-ray powder diffraction patterns at room temperature indicate that all compositions crystallize in a tetragonal system with I4/mmm space group and exhibit K2NiF4-type structure. Variations of a and c parameters display various behavior with increasing cobalt content. Changes in cell parameters are discussed in terms of crystal field theory. In addition, transition metal oxidation state is investigated on the basis of the Brown bond valence calculation. The deduced Global Instability Index (GII) value decreases when cobalt substance increases, indicating that the structure becomes more stable once cobalt is introduced. Oxygen stoichiometry of these compounds was determined from thermogravimetric analyses (TGA) followed by reduction in 5% H2 in N2 gas. Conductivity of NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) oxides was measured by an ac four-probe method. Oxygen vacancies are the possible ionic charge carriers. Specimens exhibit a semiconducting behavior in the whole range of temperature. The electrical transport mechanism agrees with an adiabatic small polaron hopping (ASPH) model.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Ruddlesden–Popper series displaying the K2NiF4-type structure are the central focus of researchers especially chemists and physicists as they present a wide range of chemical and physical properties. Indeed, they permeate diverse fields as superconductivity [1], magneto resistance [2, 3], catalysis [4], and mixed ionic–electronic conductivity [5].
In particular, several authors have been attracted by the possible application of Ln2MO4 compounds (Ln = rare earth; M = Co, Ni) such as oxygen membrane or electrode in solid oxide fuel cells (SOFCs) in addition to solid oxide electrolysis cell (SOEC) devices [6,7,8]. Furthermore, doping these compounds with alkaline earth and transition metal can modulate not only the oxygen content in the system but also the average oxidation state of the transition metal ion [9, 10].
The majority of investigations are devoted to lanthanum compounds, only few were interested in neodymium rare earth. In fact, Moritomo et al. had explored La2 − x Sr x CoO4 (0.4 ≤ x ≤ 1.0) solid solution with a mixed valence of cobalt ion (Co2+/Co3+) and found steep decreases in electrical resistivity with an increase in the amount of Sr beyond the value 0.7 [11, 12]. Next to these attempts, Kharton et al. reported that the conductivity of layered La2Ni1 − x Co x O4 + δ is predominantly p-type electronic within the stability domain (x = 0.1–0.2) phase and occurs via small polaron mechanism, which is indicated by temperature-activated hole mobility and p(O2) dependencies of electrical properties [13].
Over the last decade, great interest has been paid to neodymium oxides Nd2 − x Sr x CoO4 with the K2NiF4-type structure for the discovery of magneto resistance and superconductivity [14, 15]. D. Grandjean et al. [16] have studied the effect of cobalt substitution for copper in the parent compound NdSrCoO4, and highlighted that this doping produces quite dissimilar oxide coordination environments for the dopant and copper ion which may change spin state.
A previous study performed by H. Chaker et al. [17] focused on the effect of copper substitution in NdSrNiO4 which showed a metal-semiconductor transition at ~190 K [18]. Structural and conductivity results for the composition NdSrNi0.8Cu0.2O4 reveal that this phase is a possible candidate for integrated devices like, for example, bottom electrode in parallel plate ferroelectric capacitors. At the second stage of this study, we used this electrode to epitaxial growth for a functional oxide, namely the Aurivillius phase ferroelectric [19].
As we got more and more interested in our lab in mixed oxides based on neodymium, erbium, and dysprosium rare earths, we have already reported in previous works the obtained results after substitution of nickel for copper and chromium [20,21,22,23,24,25,26].
Within this framework, our present work is an extension to our previous studies on neodymium nickelate-based mixed oxide. It aims to investigate the effect of cobalt incorporation in the parent compound NdSrNiO4 on the structural features and the ability to accommodate oxygen non-stoichiometry. We suspect that these new functional K2NiF4-type oxides should form a very interesting family of materials: their physical properties and the possibility to combine them with a ferroelectric oxide in heterostructures by epitaxy open a way for fabricating innovating and high-performance components for applications in microelectronic and as electrode in SOFCs operating at intermediate temperatures.
For this reason, we have synthesized new compounds in the NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) system by sol–gel method. We report here some preliminary results of powder X-ray diffraction, thermogravimetric analysis, bond valence calculation, and resistivity measurements.
Materials and methods
Polycrystalline samples with particular compositions (x = 0.1, 0.3, 0.5, 0.7 and 0.9) have been synthesized in the solid solutions NdSrNi1 − x Co x O4 ± δ from powders Nd2O3 (Aldrich, 99.99%), SrCO3, NiO, and Co3O4 (Aldrich, 99.99%) by the sol–gel method in the desired mole ratio in the reaction:
Neodimium oxide, strontium carbonate, nickel, and cobalt oxides were initially independently dissolved in a minimum quantity of concentrated chlorid acid with magnetic stirring. After intimate mixture of these solutions with distilled water, citric acid C6H8O7·H2O and ethylene glycol C2H6O2 were then added. The obtained sol was left on a hot plate with constant stirring for approximately 5 h at 80 °C until it gelled and changed the color from transparent purple to green. After that, the formed pale green gel was treated by further heating, first at 200 °C for approximately 24 h then at 450 °C in a muffle furnace. The resulting powder was ground before it was returned to the furnace in air at 600 °C for 48 h. The calcined mixtures were subsequently pressed into pellets (13-mm diameter and 2-mm thickness under 10 t/cm2) and annealed in a tube furnace at 1150 °C in a flow of oxygen and cooled to room temperature at a rate of 100°/h for 3 days with intermediate regrinding and repelleting until no further reaction was established by powder X-ray diffraction.
Powder X-ray diffraction data for the six samples (x = 0.1, 0.3, 0.5, 0.7 and 0.9) were collected at room temperature using a Bruker D8 Advance, with a monochromatic CuKα1 radiation (λ = 1.54056 Å). Data were collected with a 0.0105° step 2θ width and ≈ 2-s counting time per point over a 2θ range from 10 to 120°. The whole pattern profile refinements were carried out with the FullProf program [27].
The morphological features and grain size of the particles were studied by scanning electron microscopy (SEM) on a JSM-6400 apparatus working at 20 kV at room temperature. For this reason, the samples were ground, deposited, and then metalized. Compositional analyses were performed by recording the energy dispersive X-ray analysis (EDX).
The total oxygen content of the samples was determined by hydrogen reduction in a TGDTA 92-SETARAM thermogravimetric analyzer (TGA). The material was heated in an alumina pan under flowing dried 5% H2 in N2 gas (1.5 l/h) from room temperature up to 1100 °C at a rate of 10 °C/min.
The temperature dependence of dc resistivity ρ(T) was measured by the conventional four-probe method under high vacuum conditions using the van der Pauw method [28]. The latter was implemented through cooling down the samples from 390 to 20 K with a rate of 1 K/min using the Cryodyne Industries Refrigerator, model 22.
Results and discussion
Structural study
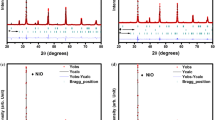
Departing from the superposition of the patterns of all prepared compositions in the NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) system, it is deduced that the corresponding compounds are structurally identical and no evidence for a structural phase transition was detected when varying the composition of the cobalt. In fact, auto-indexing of the whole pattern profile of the X-ray peaks for all compositions led to only one solution with all lines indexed and a good figure of merit. Profile analysis has been performed on the basis of a tetragonal system with space group I4/mmm. The peak shape was described by a pseudo-Voigt function (Thomson–Cox–Hasting (TCH)).
For each diffraction pattern, a zero-point shift and unit cell parameters were refined in addition to profile parameters. The background was fitted with a linear interpolation between 24 chosen points. The lattice parameters deduced from the whole pattern profile refinement (“pattern matching” mode in FullProf) of the X-ray data are presented in Table 1.
Figure 1 portrays the variation of the lattice parameters as a function of x in NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) compounds (results for x = 0 have been reported from a preceding research [17]). As it can be observed in this figure, the c parameter, at the first time, increases clearly with doping cobalt but subsequently, it decreases remarkably. On the other side, a parameter decreases slightly then remains unchangeable.
To explain these evolutions, we need to take into account the Jahn–Teller effect in (Ni/Co)O6 octahedron. In fact, the fractional substitution of Ni3+ adopting \( {t}_{2 g}^6{d}_{x^2-{y}^2}^1{d}_{z^2}^0 \) configuration in the NdSrNiO4 parent compound [17] by Co2+, which usually exhibits \( {t}_{2 g}^6{d}_{z^2}^1{d}_{x^2-{y}^2}^0 \) configuration in low spin state as declared in tetragonal symmetry, causes an increase only of c parameter owing to the increase in electron quantity in the \( \overrightarrow{c} \) direction. Moreover, the slight decrease in a parameter is due to the deficiency of one electron in \( \overrightarrow{a} \) and \( \overrightarrow{b} \) directions compared to Ni3+ (change in \( {e}_g^1 \) configuration: \( {d}_{z^2} \) orbital is occupied instead of \( {d}_{x^2-{y}^2} \)).
Furthermore, for a higher percentage of cobalt than nickel, parameter c decreases dramatically. This results from the fact that Co2+ (0.65 Å) oxidizes to smaller Co3+ (0.545 Å) ion for low spin state in sixfold coordination [29]. This finding is in good agreement with the statement of (+III) oxidation for nickel and the mixed valence (+II/+III) for cobalt. The unit cell volume, shown in Fig. 2, decreases with x in the series NdSrNi1 − x Co x O4 ± δ (0.0 ≤ x ≤ 0.9), but the reduction rate proves to be significant for x ≥ 0.5.
In order to ascertain the cobalt content in the prepared materials, semiquantitative analysis using EDX was carried out. These measurements prove the presence of all the chemical elements with their stoichiometric proportions. The results of the analysis are largely summarized in Table 2.
A general tendency was observed through SEM. In fact, the particle size extended and the porosity, as a matter of fact, diminished when we have progressively introduced cobalt instead of nickel. Consequently, the powder became more and more dense and crystallized with homogenous distribution. Figure 3 displays SEM micrographs of the six samples with the same scale.
Identifying tolerance factor (t) for layered oxide having K2NiF4 structure is established by \( t=\frac{\mathrm{A}-\mathrm{O}}{\sqrt{2}\left(\mathrm{B}-\mathrm{O}\right)} \), where A–O and B–O are the metal–oxygen bond lengths of A-site and B-site cation, respectively. Referring to Goldschmidt [30], K2NiF4-type structure can be formed when (0.85 < t < 1.05). On this basis, the tolerance factor was calculated using tabulated values of ionic radii [29]. These are presented in Table 1 for NdSrNi1 − x Co x O4 ± δ (0.0 ≤ x ≤ 0.9) phases. Within this context, the tolerance factor values of these materials reside in the range 0.912 ≤ t ≤ 0.945 for which the tetragonal distortion is favored [31].
The structural refinement of each composition in NdSrNi1 − x Co x O4 ± δ family was performed in the tetragonal system (I4/mmm space group) beginning with atomic positions taken from NdSrNiO4 [32], which involves Nd3+ and Sr2+ ions that were disorganized over the ninefold coordinate 4e sites with their ratio according to the nominal composition (Table 2). The Ni and Co ions are situated at the 2a site, and the O1, O2 atoms are placed at (0, ½, 0) in site 4c and at (0, 0, z) in site 4e, respectively. At the final stage, good reliability factors are obtained as a result to refinement of all parameters, i.e., atomic coordinates, global atomic thermal displacement, and preferential orientation along the [001]. To illustrate these notions, the corresponding refined parameters of the NdSrNi0.5Co0.5O4 − δ compound are presented in Table 3 and the final fit is displayed in Fig. 4. Projection of the NdSrNi0.5Co0.5O4 − δ structure is portrayed in Fig. 5, and some corresponding inter-atomic distances are given in Table 4. Consequently, Ni/Co atoms shape a distorted octahedral where the axial (Ni/Co)–O1 band in the ab plane is shorter than the apical (Ni/Co)–O2 band along the c direction (Fig. 6). The coordination environments for strontium cation are highlighted also in Fig. 6.
Under this situation, metal–oxygen bonds are strained and the extension of these strains can be deduced using BVM (bond valence method) expressed by S ij = exp[(R 0 − R ij/B)], where R 0 and B (constant = 0.37) are the experimentally given parameters and R ij is the bond length of the cation–anion pair. Referring to Brown [33], the sum of the bond valence around an ion has to be equal to the formal valence V i of these ions \( \left({V}_{\mathrm{i}}=\sum_{\mathrm{i}\mathrm{j}}{S}_{\mathrm{i}\mathrm{j}}\right) \). It is called VSR (valence sum rule). The deviation observed between these two values can be attributed to instabilities of the structure. The root mean square of deviations for all atoms is named global instability index and expressed as [34] \( \mathrm{GII}=\sqrt{\frac{\sum_{i=1}^N\left\{{\left(\sum_{\mathrm{j}}{S}_{i\mathrm{j}}-{V}_i\right)}^2\right\}}{N}} \), where N is the number of atoms in the asymmetric unit. Sánchez-Andújar M. and Señarı́s-Rodrı́guez MA have investigated the Global Instability Index (GII) of K2NiF4-type LnSrCoO4 (Ln = La, Nd, and Gd) [35] and demonstrated that GII decreases with the increase of the ionic radius of the rare earth ion and/or the doping level.
In the present work, the following R 0 values are used for subsequent calculations: 1.637 Å for Co3+–O2− bond, 1.686 Å for Ni3+–O2− bond, 2.105 Å for Nd3+–O2− bond, and 2.118 Å for Sr2+–O2− bond [36,37,38]. Computed bond valence sum by FullProf for cobalt atom concerning all compositions resting on Brown theory for oxygen deficiency on the z = 0 plane is recapitulated in Table 5. The GII of the NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) compounds illustrated also in Table 5 denotes that the crystal structure becomes more and more stable when we substitute nickel for cobalt.
Thermogravimetric analysis and oxygen stoichiometry determination
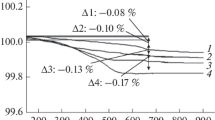
In order to investigate the variation in the oxygen deficiency as a function of cobalt proportion, we have performed thermogravimetric analysis in hydrogen/nitrogen atmosphere. A common aspect in the reduction curves for all compositions was the observation of a step-like behavior. These types of wide plateaus in the weight loss curves which appear throughout the reduction process are generally related to the stabilization of a number of phases with different oxygen stoichiometry [39]. Departing from the weight loss values, oxygen contents are deduced for all compositions taking into account Nd2O3, SrO, and metallic Ni and Co as final products. Figure 7 shows the relative weight loss curve for NdSrNi0.9Co0.1O4 − δ and NdSrNi0.7Co0.3O4 − δ which exhibit similar behavior. As it can be seen, the reduction phenomenon comprises three steps. The first process corresponds to the reduction of Ni3+ to Ni2+. The second one stands for the reduction of Co3+ to Co2+. The third plateau in this weight loss curve corresponds to reduction of Ni2+ and Co2+ into metallic nickel and cobalt without any distinction between them. Within the same framework, for both cases where x = 0.7 and x = 0.9, close results are recorded and presented in Fig. 8. In this specimen, the reduction phenomenon occurs only with two steps bringing about two plateaus. The first one for ≈400 ≤ T(C°) ≤ 550, which stands for reduction of the majority of cobalt cation from Co3+ to Co2+ and minority of nickel from Ni3+ to Ni2+. A second wide plateau is observed at T(C°) ≥ 750 and corresponds to the complete reduction of cobalt and nickel ions to metal. Referring to Table 6, oxygen content for the synthesized phases proves to be consistently under 4.00 atoms per formula unit and seems to be essentially dependent on the cobalt content. In fact, oxygen content diminishes up to x = 0.3 then rises with the increase of cobalt content. This behavior confirms the presence of Co3+ in compounds having a percentage of cobalt more or equal to 50%.
Electrical transport properties
The electrical resistivity measurements of NdSrNi1 − x Co x O4 ± δ (0.1 ≤ x ≤ 0.9) demonstrate that all the members of the solid solution are semiconducting between 20 and 390 K. In this common feature, the conductivity may be accounted for on the basis of two different models.
Firstly, Emin–Holstein theory of adiabatic small polaron hopping model (ASPH) [40], which is expressed as
where E a is the activation energy for hopping conduction and B is the residual resistivity. Figure 9 portrays the corresponding results for the ASPH model, where the straight line is a fit to Eq. (1). The activation energy E a is deduced from the fit of ln (ρ/T) versus 1/T curve.
Temperature dependence of ρ for x= 0.1, 0.3, 0.5, 0.7 and 0.9 in NdSrNi Co O. The inset (a) show ln(ρ /T) versus 1/T plots in the high-temperature region. full line is the fit to equation: ρ (T) = BT exp(Ea/kBT). The temperature dependence of the conductivity σ in scales of ln(σ) as a function of (1/T1/4) is also shown in the inset (b) with their fits (full lines) using the variable range hopping (VRH) model
Secondly, Mott et al. [41] have established the variable range hopping (VRH) mechanism, expressed by the following equation:
where ρ 0 is considered as a constant which depends on electron–phonon interaction, though it is slightly affected by temperature [42]. The characteristic VRH temperature is noted T 0 = 16.α 3/k β.N(E F) where N(E F) is the density of states at the Fermi level. As a matter of fact, the T 0 value is calculated from the slope of the plot ln(σ) versus T −1/4 (Fig. 9). Referring to [40], constant α was taken as 2.22 nm−1. Table 7 illustrates the computed values of T 0, N(E F), and E a based on these two models. At this stage of analysis, it is worth noting that our results obtained for the semiconducting phase can be described quite well according to both models but the first one (ASPH) provides the best squared linear correlation coefficients (R 2). To conclude, we would assert that the transport behavior is dominated by the adiabatic small polaron hopping mechanism.
In this work and in related references [43,44,45], it is inferred that the obtained values of activation energy for NdSrNi1 − x Co x O4 ± δ (x = 0.1, 0.3, and 0.5) compounds (Table 7) are lower than that for NdSrCoO4 [43]. Summing up these results, it can be concluded that after the incorporation of nickel and cobalt in the same site, we obtain a semiconductor material with conductivity in the range 0.3–17.24 Ω−1 cm−1 and low activation energy. The same result is found by several authors, for instance for the NdSrNi0.5Cr0.5O4 ± δ compound, the σ RT = 8.86 Ω−1 m−1; E a = 0.09 eV [25], for Nd1.8Sr0.2Ni0.6Cu0.4O4 ± δ the σ 640°C = 42.7 (3) S cm−1; E a = 0.07 eV [46] and for NdSrNi0.8Cu0.2O4 ± δ the σ RT = 1.13 Ω−1 cm−1; E a = 0.06 eV [17].
Based on the outcome of our investigation, it is possible to conclude that NdSrNi1 − x Co x O4 ± δ compounds having low activation energy (in the range 0.04–0.09) can be used as mixed ionic–electronic conductors (MIECs) [46].
The room temperature resistivity (ρ RT) values as a function of cobalt content x are presented in Fig. 10. As it can be observed, the ρ RT of the samples is constant up to x ≈ 0.5, then increases rapidly with x. It is significant to note that room temperature resistivity exhibits an increase each time x increases, which is different from the evolution in the formal valence of cobalt. Referring to Brian et al. [18], the half-filled \( {\sigma}_{x^2-{y}^2}^{\ast } \) band is responsible for the metallic behavior of NdSrNiO4 compound. In the present research, when substituting Ni3+ for Co2+/Co3+ (electronic configuration \( {t}_{2 g}^6{d}_{z^2}^1{d}_{x^2-{y}^2}^0/{t}_{2 g}^6{d}_{z^2}^0{d}_{x^2-{y}^2}^0 \)), the half-filled \( {\sigma}_{x^2-{y}^2}^{\ast } \) band changes gradually to a half-filled \( {\sigma}_{z^2}^{\ast } \) band due to the presence of Co2+ cation. On the other side, when we have compositions corresponding to a higher percentage of cobalt than that of nickel (x ˃ 0.5), the resistivity increases remarkably with the incorporation of the diamagnetic Co3+ (\( {t}_{2 g}^6 \)) cation.
On the basis of the electrical resistivity (ρ 300K = 0.058 Ω cm in bulk form) and the structural and microstructural characteristics of the NdSrNi0.5Co0.5O4 ± δ material, and at least, it can be successfully deposited in thin film and used as a bottom electrode suitable for the epitaxial regrowth of functional ferroelectric-based parallel plate capacitors. Our material has great potential for other applications as an electrode in solid oxide fuel cells (SOFCs) operating at intermediate temperatures.
Conclusion
Cobalt can be successfully substituted for nickel in the parent compound NdSrNiO4. Refinements of X-ray diffraction data for all compositions (x = 0.1, 0.3, 0.5, 0.7, 0.9) show that these phases crystallize in the tetragonal K2NiF4-type structure (I4/mmm space group with z = 2). Oxygen content deduced from thermogravimetric analysis (TGA) under reducing atmosphere displays substantial deviations from the ideal values after the incorporation of cobalt in the structure. These oxygen vacancies are the possible ionic charge carriers. The a and c unit cell parameters values exhibit certain variations with increasing cobalt content. This behavior is deduced from Jahn–Teller distortions as well as the presence of the mixed valence of Co2+/Co3+ ions. Bond valence calculations show that cobalt has an oxidation state between two and three. The GII of NdSrNi1 − x Co x O4 − δ (0.1 ≤ x ≤ 0.9) compounds indicates that the crystal structure becomes more stable when we substitute nickel for cobalt. The transport mechanism in NdSrNi1 − x Co x O4 − δ (0.1 ≤ x ≤ 0.9) solid solution has been investigated and shows an adiabatic small polaron hopping model. Departing the activation energy that has been undertaken, it is possible to conclude that NdSrNi1 − x Co x O4 ± δ (x = 0.1, 0.3, and 0.5) compounds are semiconductors with low activation energy. This paper has clearly demonstrated that the composition NdSrNi0.5Co0.5O4 ± δ exhibits the most interesting physical properties: maximum conductivity at room temperature σ = 17.24 Ω−1 cm−1 is coinciding with minimum activation energy (E a = 0.05 eV). These findings are of direct practical relevance in SOFCs and plate capacitors (bottom electrode).
References
Hohlwein D, Hoser A, Sonntag R, Prandl W, Schafer W, Kiemel R, Kemmler-Sack S, Hewat AW (1989) Structural changes in superconducting La1.8Sr0.2CuO4 by alloying copper with cobalt. Phys B Condens Matter 156-157:893–896
Zaghrioui M, Giovannelli F, Poirot N, Brouri D, Laffez I (2004) Anomalies in magnetic susceptibility of nonstoichiometric Nd2NiO4 + δ (δ = 0.049, 0.065, 0.077, 0.234). J Solid State Chem 177:3351–3358
Matsuura T, Tabuchi J, Mizusaki J, Yamauchi S, Fueki K (1988) Electrical properties of La2−x Sr x CoO4—II: models and analysis of the relationship between cobalt 3d electron state and structural, electrical and magnetic properties. J Phys Chem Solids 49:1409–1418
Luo L, Shao G, Duan Z (2005) Catalytic oxidation properties and characterization of LaSrCo0.9B′0.1O4 (B′ = Mn, Fe, Ni, Cu) mixed oxides. Turkish J Chem 29:597–605
Iguchi E, Nakatsugawa H, Futakuchi K (1998) Polaronic conduction in La2 − x Sr x CoO4 (0.25≤x≤1.10) below room temperature. J Solid State Chem 139:176–184
Ferchaud C, Grenier JC, Zhang-Steenwinkel Y, Van Tuel MMA, Van Berkel FPF, Bassat JM (2011) High performance praseodymium nickelate oxide cathode for low temperature solid oxide fuel cell. J Power Sources 196:1872–1879
Chauveau F, Mougin J, Mauvy F, Bassat JM, Grenier JC (2011) Development and operation of alternative oxygen electrode materials for hydrogen production by high temperature steam electrolysis. Int J Hydrog Energy 36:7785–7790
Grimaud A, Mauvy F, Bassat JM, Fourcade S, Marrony M, Grenier JC (2012) Hydration and transport properties of the Pr2 − x Sr x NiO4 + δ compounds as H+-SOFC cathodes. J Mater Chem 22:16017–16025
Patrakeev MV, Naumovich EN, Kharton VV, Yaremchenko AA, Tsipis EV, Nunez P, Frade JR (2005) Oxygen nonstoichiometry and electron-hole transport in La2Ni0.9Co0.1O4+δ. J. Solid State Ionics 176:179–188
Vashook VV, Ullmann H, Olshevskaya OP, Kulik VP, Lukashevich VE, Kokhanovskij LV (2000) Composition and electrical conductivity of some cobaltates of the type La2 − x Sr x CoO4.5 − x/2 ± δ. J Solid State Ionics 138:99–104
Moritomo Y, Higashi K, Matsuda K, Nakamura A (1997) Spin-state transition in layered perovskite cobalt oxides: La2 − x Sr x CoO4 (0.4⩽x⩽1.0). Phys Rev B 55:14725
Shimada Y, Miyasaka S, Kumai R, Tokura Y (2006) Semiconducting ferromagnetic states in La1 − x Sr1 + x CoO4. Phys Rev B 73:134424
Kharton VV, Yaremchenko AA, Shaula AL, Patrakeev MV, Naumovich EN, Logvinovich DI, Frade JR, Marques FMB (2004) Transport properties and stability of Ni-containing mixed conductors with perovskite and K2NiF4-type structure. J Solid State Chem 177:26–37
Huang S, Ruan K, Lv Z, Wu H, Pang Z, Cao L, Li X (2006) Evidence for spin-glass states and Griffiths singularities in Nd0.75Sr1.25CoO4. J Phys Condens Matter 18:7135–7144
Huang S, Ruan K, Lv Z, Zhuang L, Wei P, Wu H, Li M, Zhang J, Chai Y, Yang H, Cao L, Li X (2006) Magnetic and transport properties in layered Nd1 − x Sr1 + x CoO4. Phys rev B 73:94431
Grandjean D, Weller MT (1993) Structure and oxygen stoichiometry in complex neodymium strontium cobalt copper oxides: [NdSrCo1 − x Cu x O4 − y ]. J Mat Res Bull 28:685–692
Chaker H, Roisnel T, Potel M, Ben Hassen R (2004) Structural and electrical changes in NdSrNiO4 − δ by substitute nickel with copper. J Solid State Chem 177:4067–4072
Arbuckle BW, Ramanujachary KV, Zhang Z, Greenblatt M (1990) Investigations on the structural, electrical, and magnetic properties of Nd2 − x Sr x NiO4 + δ. J Solid State Chem 88:278–290
Chaker H, Députier S, Guizouarn T, Ben Hassen R, Perrin A, Guilloux-Viry M (2009) NdSrNi0.8Cu0.2O4 − δ thin films epitaxially grown by pulsed laser deposition on LaAlO3 and SrTiO3: a potential electrode for epitaxial regrowth of perovskite structure-based oxides. J Cryst Growth 311:2746–2752
Chaker H, Roisnel T, Cador O, Amami M, Ben Hassen R (2006) Neutron powder diffraction studies of NdSrNi1 − x Cu x O4 − δ, 0 ≤ x ≤ 1 and magnetic properties. J Solid State Sci 8:142–148
Chaker H, Roisnel T, Ceretti M, Ben Hassen R (2007) The synthesis, structural characterization and magnetic properties of compounds in the Ln2O3-SrO-NiO-CuO system for Ln = La, Nd, Gd, Dy, Ho and Er. J Alloys Comp 431:16–22
Hamdi S, Ouni S, Chaker H, Ben Hassen R (2012) Synthesis, structural and electrical characterizations of Er0.33Sr1.67Ni0.8Cu0.2O4 − δ. J Powder Diffract 27:252–255
Hamdi S, Ouni S, Chaker H, Rohlicek J, Ben Hassen R (2011) Synthesis, structural and electrical characterizations of DySr5Ni2.4Cu0.6O12 − δ. J Solid State Chem 184(11):2897–2901
Chaker H, Roisnel T, Ceretti M, Ben Hassen R (2010) Rietveld refinement of X-ray powder data and bond valence calculations of NdSrNi0.5Cr0.5O4 − δ compound. J Powder Diffract 25:241–246
Jammali M, Chaker H, Cherif K, Ben Hassen R (2010) Investigation on the structural and electrical properties of NdSrNi1 − x Cr x O4 + δ (0.1 ≤ x ≤ 0.9) system. J Solid State Chem 183:1194–1199
Jammali M, Ben Hassen R, Rohlicek J (2012) Structural and electrical properties of Nd1.7Ba0.3Ni0.9Cr0.1O4 + δ compound. J Powder Diffract 27:184–188
Rodriguez-Carvajal J (2001) Recent developments of the program FULLPROF. IUCr-CPD. News Lett 26:12
Van der Pauw LJ (1958) A method of measuring specific resistivity and hall effect of discs of arbitrary shape. Philips Res Rep 13:1–9
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767
Goldschmidt VM, Oslo A (1926) I Mater Nat 2:7
Ganguly P, Rao CN (1984) Crystal chemistry and magnetic properties of layered metal oxides possessing the K2NiF4 or related structures. J Solid State Chem 53:193–216
Takeda Y, Nishijima M, Imanishi N, Kanno R, Yamamoto O, Takano M (1992) Crystal chemistry and transport properties of Nd2 − x A x NiO4 (A = Ca, Sr, or Ba, 0 ≤ x ≤ 1.4). J Solid State Chem 96:72–83
Altermalt D, Brown ID (1985) The Automatic Searching for Chemical Bonds in Inorganic Crystal Structures. Acta Cryst B 41:240–244
Salinas-Sánchez A, García-Muñoz JL, Rodríguez-Carvajal J, Saez-Puche R, Martínez JL (1992) Structural characterization of R2 BaCuO5 (R = Y, Lu, Yb, Tm, Er, Ho, Dy, Gd, Eu and Sm) oxides by X-ray and neutron diffraction. J Solid State Chem 100:201–211
Sánchez-Andújar M, Señarís-Rodríguez MA (2004) Synthesis, structure and microstructure of the layered compounds Ln1 − x Sr1 + x CoO4 (Ln: La, Nd and Gd). J Solid State Sci 6:21–27
Brown ID, Altermatt D (1985) Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr B41:244–247
Wood RM, Palenik GJ (1998) Bond valence sums in coordination chemistry. A simple method for calculating the oxidation state of cobalt in complexes containing only Co−O bonds. Inorg Chem 37:4149–4151
Rodriguez-Carvajal J Private communication
Lewandowski JT, Beyerlein RA, Longo JM, Mccauley RA (1986) Nonstoichiometric K2NiF4-type phases in the lanthanum-cobalt-oxygen system. J Amer Ceram Soc 69:699–703
Chaikin PM, Beni G (1976) Thermopower in the correlated hopping regime. Phys Rev B 13:647–651
Mott N (1993) Conduction in non-crystalline materials. Clarendon, Oxford, pp 17–23
Laiho R, Lisunov KG, Lähderanta E, Stamov VN, Zakhvalinskii VS (2001) Variable range hopping conductivity in La1 − x Ca x MnO3. J Phys Condens Matter 13:1233–1246
Ang R, Sun YP, Luo X, Hao CY, Song WH (2008) Studies of structural, magnetic, electrical and thermal properties in layered perovskite cobaltite SrLnCoO4 (Ln = La, Ce, Pr, Nd, Eu, Gd and Tb). J Phys D App Phys 41(4). doi:10.1088/0022-3727/41/4/045404
Taguchi H, Nakade K, Hirota K (2007) Synthesis and characterization of K2NiF4-type CaLnCoO4 (Ln = Sm and Gd). Mater Res Bull 42:649–656
Taguchi H, Hirata K, Kido H, Takeda Y, Kato M, Hirota K (2009) Hopping conductivity of distorted K2NiF4-type (Ca1 + x Nd1 − x )CrO4. Solid State Sci 11:1222–1225
Khandale AP, Bansod MG, Bhoga SS (2015) Improved electrical and electrochemical performance of co-doped Nd1.8Sr0.2Ni1 − x Cu x O4 + δ. Solid State Ionics 276:127–135
Acknowledgements
The authors express sincere thanks to S. PAOFAI and J. ROCHERULLE for their technical assistance as well as their contribution in TGA. Collective and individual acknowledgements are also owed to T. GUIZOUARN for his constant support in terms of electrical measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaker, H., Raies, I., Chouket, A. et al. Chemical and physical characterizations of the n = 1 Ruddlesden–Popper phases: Nd2 − y Sr y Ni1 − x Co x O4 ± δ (y = 1 and 0.1 ≤ x ≤ 0.9). Ionics 23, 2229–2240 (2017). https://doi.org/10.1007/s11581-017-2167-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2167-x