Abstract
The radial increment of Abies sibirica Ledeb. and mortality dynamics of fir stands in the mountain forests of the Eastern Sayan (Stolby State Nature Reserve) have been analyzed. The unprecedented decline in fir stands is caused by water stress due to an increase in air temperature and synergy with the impact of the Polygraphus proximus Blandford. This xylophage was not previously observed in the Abies sibirica range. In the initial phase of climate warming, an increase in radial increment was observed, which was replaced by a depression in 1985–2017. The declining of fir trees was preceded by an increase in the relationship between growth index and SPEI, as well as between the growth index and root-zone moisture content. At the same time, the growth index of the declining cohort was more closely associated with the root zone moisture content (r2 = 0.56) and SPEI (r2 = 0.74) than the growth index of the surviving cohort (r2 = 0.15 and r2 = 0.39, respectively). The decline in fir began in the 2000s, when Polygraphus proximus Blandford attacked trees Abies sibirica. During this period, dead stands were localized mainly on the relief elements with the highest probability of water stress (steep slopes located in a “rain shadow”). By 2017, the decline spread throughout the entire territory of fir stands, which led to the mortality of ~75% of fir stands. During the period of tree decline, a close relationship was observed between growth index and fir mortality (r = –0.79). The surviving trees grew under conditions of a higher root-zone humidity (compared to declining trees). The proximity of growth-index trajectories of cohorts of declining and surviving trees, which indicates the probability of mortality of surviving trees in the context of a predicted climate aridity increase, is noteworthy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the taiga forests of Siberia, the mortality of forest stands formed by the Siberian fir (Abies sibirica Ledeb.) and Siberian pine (Pinus sibirica Du Tour) has been increasing in the last decades. In Prebaikalia, in the Kuznetskii Alatau Mountains, as well as in the Eastern and Western Sayan, the condition of fir and pine forests is deteriorating (Kharuk et al. 2013; Kharuk et al., 2016а). The deterioration of conifers was observed not only in Siberia, but also in the “cold” forests of the boreal zone as a whole. In the United States, Pinus ponderosa Engel. stands are mortality (Logan et al., 2003; Millar and Stephenson, 2015). The mass mortality of fir (Picea abies L.) stands occurs in Eastern and Western Europe (Yousefpour et al., 2010; Martínez-Vilalta et al., 2012), as well as in Belorussia and the European part of Russia (Sazonov et al., 2013; Kharuk et al., 2016b). The climate-induced redistribution of woody plant species in the Boreal zone as a whole was predicted, including the reduction of fir and Siberian pine habitats in the southern taiga subzone (Anderegg et al., 2013; Kharuk et al., 2017). Probable cause of conifers mortality in the Boreal zone is the synergy of unfavorable climatic factors (mainly increasing aridity) and biotic effects (insect pests, fungal and bacterial phytopathogens) (Raffa et al., 2008; Kolb et al., 2016; Kharuk et al., 2017, 2013).
Climate warming contributed to insect pest activation in dark conifer forests of Siberia, both “traditional” (Dendrolimus sibiricus Tschetv.), and previously observed species (Polygraphus proximus Blandf.). The outbreak of mass reproduction of the Siberian silkworm in the Yenisei Siberia (2014–2018) covered a territory of more than 800 000 ha. It was peculiar due to the northward advance to the north of the previously observed border of the focal distribution of this insect (Kharuk et al., 2018a). Another insect, P. proximus, has become the most dangerous pest of fir in modern climatic conditions. This xylophage, previously known only in the Far East (Krivolutskaya, 1983), was discovered in 2006 in Moscow oblast (Chilakhsaeva, 2008) and in 2008 in Krasnoyarsk krai (Akulov and Mandel’shtam, 2012). Currently, P. proximus is widespread in most of the fir range in the southern taiga subzone (Krivets et al., 2015), which was facilitated by the weakening of stands by water stress due to the increasing aridity of the climate (Kharuk et al., 2018a).
This work aims to analyze the dynamics and causes of A. sibirica mortality in the mountain forests of the Eastern Sayan (Stolby State Nature Reserve). Forests of the reserve belong to the category of specially protected areas, which minimizes the anthropogenic impact on their living condition. The following aspects of the problem were analyzed:
(1) Spatiotemporal dynamics of fir stands mortality.
(2) Relation of the life condition and the fir radial increment with ecological and climatic variables.
(3) Modifying influence of relief (height above sea level, exposition and slope steepness) on the condition of stands.
(4) Influence of pest insects.
OBJECT OF RESEARCH
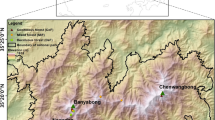
Close (closeness >0.3) dark conifer stands of the Stolby Nature Reserve, as well as similar in breed composition and growing conditions stands on the adjacent territory are investigated (Fig. 1). Stands are formed mainly by fir (75%) with the participation of Siberian pine and Siberian fir. The Stolby reserve, founded in 1925, is located in the system of the Eastern Sayan ridges and covers an area of 47 200 ha with absolute marks of 200–800 m above sea level. The low-mountain part is composed of limestone, sandstone, and clay; syenitic rocks are on the surface in the middle mountains.
The reserve territory is divided into two high-altitude belts differing in climatic conditions, vegetation, and soil cover, as well as orographic characteristics. The middle mountain belt with elevations from 500 to 800 m above sea level occupies more than 2/3 of the reserve area. It is dominated by dark conifer taiga; the soil cover is dominated by brown soils. In the low-mountain belt (elevations 200–500 m above the sea level), plant communities vary from light conifer and small-leaved forests to steppe meadows and soils vary from turf–podzol to chernozems (Tropina, 2015). The climate is sharply continental, with mean summer temperatures of 15.5°C, winter temperatures of 17.3°C below zero, and the mean annual temperature is 0.2°C below zero. The amount of summer precipitation is 230 mm, winter precipitation is 132 mm, and annual precipitation is 680 mm.
MATERIALS AND METHODS
Materials of remote sensing and ground surveys, data of dendrochronological analysis and forest inventory (Proekt organizatsii..., 2007), climatic variables, and GIS technologies were used in the work. Data on precipitation, temperature, and humidity of the root-inhabited layer was extracted from the databank CRU TS 4.01 (http://www.cru.uea.ac.uk/; resolution of 0.5° × 0.5 degree) and MERRA2 (https://gmao.gsfc. nasa.gov/reanalysis/MERRA-2; of 0.625° × 0.5 degree). The SPEI (difference between precipitation and potential evapotranspiration (Vicente-Serrano et al., 2010)) was calculated from data http://sac.csic.es/spei (resolution 0.5° × 0.5°). Mean monthly values of the root-layer moisture content (RLM, taken equal to 1.0 m) were extracted from the databank MERRA2 (available since 1980).
Remote sensing data include images of medium (Landsat, 30 m; http://glovis.usgs.gov) and high (WorldView, GeoEye; 0.41–0.46 m; www.google.com/ maps; www.bing.com/maps) permissions. The maps were generated based on an analysis of time series (N = 11) of the Landsat summer images (5, 7, 8). We used high-resolution images along with ground data for verifying forest stand maps. The images were corrected topographically (Riano et al., 2003) and processed in the Erdas Imagine software package (http://www.hexagongeospatial.com). For GIS analysis, we used DEM SRTMGL1 (resolution 30 m, https://lpdaac.usgs.gov); for statistical analysis we used Microsoft Excel and StatSoft Statistica (http://www.statsoft.ru).
Generating forest-stand maps. A series of maps covering the period from 1999 (before mortality stands) to 2017 was generated. We determined “healthy” and “dried” stands. This interpretation consisted of the following stages.
(1) Generating a dark conifer stand mask from a 1999 Landsat image. We used 24 training samples (1444 ± 217 pixels each). The accuracy of classifications was evaluated by Kappa (κ)-statistics. Omission and inclusion errors were 8% and 13% at κ = 0.79, which corresponds to a good level of classification accuracy.
(2) For the interpretation of mortality forest stands, we applied a normalized infrared index, NDII (1):
where NIR is the brightness in the near infrared range (851–879 nm) and SWIR is the brightness mid-infrared range (1566–1651 nm). The range of NDII values is ±1; the index is effective in assessing the condition of vegetation (Gu et al., 2007). In this work, the applicability of the NDII for classification of dried forest stands was evaluated according to the decoding of the picture Landsat-8 (2017). The resulting histogram of NDII values was consistent with normal distribution (χ2 = 6.48; p = 0,17); the dried stand was consistent with the NDII interval of 0.003–0,151 (mean median values of NDII = 0.077, σ = 0.037). To calibrate other Landsat images obtained at different dates and with different sensors (Landsat-5/TM, Landsat-7/ETM+), test points (N = 40) with relatively stable brightness characteristics (rocky and water surfaces, infrastructure) were used. Based on the data obtained, linear regression equations were compiled, linking the values of the NDII index of the reference image (Landsat-8, 2017) and NDII of the remaining images.
The accuracy of classification of the dried stands maps, estimated from a set of test plots (N = 255, size 42 ± 10 pixels), is given in Table 1 (satisfactory level of accuracy).
(3) In the analysis of spatial distribution of dried stands, the exposure was calculated by sectors of 45°; the steepness of the slopes was determined in an increment of one degree. Since the distribution of relief elements is unevenly distributed within the analyzed territory, we used the normalization (2):
where Kc(i) is normalization coefficient, c(i) us ith catefory of relief elements c, Ac(i)f is the square of the given relief elements within ith category of a topographic character c, and Ac(i)I is the square of the ith category of the topographic character c.
Fieldwork was conducted in the areas of fir stands mortality in the Stolby Nature Reserve and adjacent areas (Fig. 1). The temporary sample plot (SP; N = 9) was represented by a circular area (R = 9.8 m), where we determined forest type, species composition, height and diameter, described cover surface and soil characteristics, and relief characters (direction, slope, convexity/concavity of slope, elevation above sea level). Samples for dendrochronological analysis were selected at an elevation of 1.3 m in a random order on the territory of ~0.5 ha (centered in SP). In the surveyed stands closeness is 0.4–0.6; mean height and diameter of firs are 16 m and 20 cm and, for pines, 17.5 m and 36.0 cm, respectively. The mean age of fir is 85 years and that for pine is 115 years. Soil drainages moderate and well. Shrubs are represented by spirea (Spiraea.), currant (Ribes sp.), and rosehip (Rosa sp.). The ground cover is formed by sedge (Carex sp.), fern (Athyrium sp., Pteridium sp.), mosses, and lichens.
Dendrochronological analysis was performed based on a representative sample (N = 166) of fir trees. We extracted wood samples using an incremental drill. Measurements were carried out on the LINTAB 3 platform with an accuracy of 0.01 mm. The result was an absolute individual chronology for each tree (in millimeters). TSAP and COFECHA software were used to check the quality of cross dating (Holmes, 1983; Rinn, 1996). The mean interseries correlation coefficient was 0.48 for a cohort of living trees and 0.44 for dead ones. The population signal index (EPS) for both cohorts is 0.98. To eliminate the age trend, standardization was used using the ARSTAN program, which converts the time series of the width of annual rings to the time series of dimensionless indices (growth index, GI) with an average of 1.0 and a relatively constant dispersion (Speer, 2010).
RESULTS
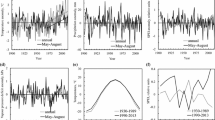
Dynamics of tree stands mortality. Mortality of fir stands was observed after 2005, sharply increasing in 2014–2017, covering ~75% of dark-conifer forest stands of the nature reserve (Figs. 1, 2). This phenomenon was preceded by an increase of the climate aridity (decrease in the dryness index of the SPEI and humidity of the root layer of RLM) and periodic droughts (Figs. 3b, 3c).
Dynamics of air temperature (a), dryness index SPEI ((b) June to August), precipitation (c), and humidity of the root layer (RLM) ((d) June to August). The drought in (b) and (c) correspond to dry years. Note: A decrease in the SPEI indicates an increase in aridity. The trends are significant (p < 0.05).
Mortality in the initial phase was observed predominantly on relatively steep (10°) drained slopes of the northeastern exposure, later embracing a large part of fir forests and extending to the lower slopes of less steepness; the shape of the azimuthal distribution of the mortality forest stands approaches that for conifers as a whole (Figs. 4a, 4b).
Dynamics of mortality declining trees. The fir growth index (GI) has been increasing since the mid-1950s, followed by a depression in the 1980s; the “change of sign” of the growth curve (breakpoint) occurred in 1983–1985. Later, after a slight increase in the GI in 2005–2010, there was a sharp drop in the GI with the division of trees into cohorts of “dried” and “living” and an increase in mortality of trees (Fig. 5).
In general, in the phase of growth (1955–1983), we have not identified significant correlations of the GI with climatic variables of the cohorts (Fig. 6), but in certain periods (the 1960s and 1977–1982) we observed the dependence of the GI of the drought index SPEI (Fig. 7). In the depression phase, the GI was negatively correlated with air temperature at the beginning of the growing season (May–June; Figs. 6, 7), which was observed against the background of low values of SPEI and humidity of the root layer (RLM) (Figs. 3b, 3d). Significant correlations between the GI and the parameters of water supply (SPEI and RLM) appeared in the late 1990s. The increment of the living tree cohort was associated with the value of SPEI, while the GI of the dried trees also depended on the RLM (Figs. 6, 7).
Correlations of the growth index (GI) of the cohorts of dried and surviving trees with air temperature (a, c), with the drought index SPEI (c, d), and with the moisture of the root layer (RLM) (a, c). The time interval is divided into periods of growth and depression of the GI (before and after 1984).
Maximum values of the GI correlation with parameters of moisture were observed in 1997–2010 with a closer relationship to the cohort of mortality trees (Fig. 8). During the period of mass stand mortality (after 2010), the correlation of IP with climatic variables was insignificant.
DISCUSSION
Mass mortality of the fir in Stolby Nature Reserve, when 2/3 of the stands died in a short time interval, was a phenomenon not previously observed either in the reserve or in the range of the fir. Mortality of stands was preceded by a decrease in the radial growth index (GI) of fir trees, which began in the mid-1980s. The depression of the GI was associated with an increase in air temperature at the beginning of the growing season, which caused water stress due to a decrease in the moisture content of the root layer (RLM) and an increase in atmospheric aridity (SPEI). Earlier, when the GI increased (since the 1960s), the increase in air temperature at the beginning of the growing season stimulated an increase in the GI. Thus, the “inflection point” of the growth curve (mid-1980s) is breakpoint with the achievement of the temperature threshold, after which the stimulation of the increase by the temperature of the air was replaced by its oppression through the water stress induced by the increasing temperature. It should be noted that a similar trajectory of the GI (an increase with subsequent depression) was noted in other parts of the fir area, as well as in pine (Pinus sibirica) and larch (Larix sibirica) grown under conditions of moisture limitation, whereas, in areas of sufficient moisture, the depression of the GI was missing or poorly expressed (Kharuk et al., 2018b).
The dependence of IP on the parameters of moisture supply (RLM and SPEI) and, consequently, water stress, reached the maximum in the first decade of the 21st century (Figs. 7, 8b). During this period, the appearance of P. proximus, an aggressive xylophage not previously observed in the area of Siberian fir, was noted. This bark beetle was first discovered in the reserve in 2008 (Knorre et al., 2015). Ground surveys showed that mortality and dried fir trees were inhabited or used out by this insect; its attacks were also recorded on most living trees. It is known that water stress reduces the synthesis of protective substances: phenols and terpenes (Kolb et al., 2016; Sangüesa-Barreda et al., 2015). Along with this, the deficiency of moisture leads to an increase in the nutritional value of plant tissues for dendrophages due to an increase in the concentration of soluble carbohydrates (Liu et al., 2011). The reproduction of pest insects is also facilitated by an increase in the vegetation period (Bark Beetles…, 2015), which increased in the reserve since the 1970s, by ~8 days. The synergism of effects of water stress and insects caused the mortality of stands, which, by 2017, covered 75% of the fir range. The greatest damage occurred in just 3 years (2014–2016). A sharp change in the status of plants (from living to dead) is typical when exposed to pest insects. A similar phenomenon was observed in the Sierra Nevada Mountains (United States), where the area of dead conifer stands, previously weakened by drought, increased by 40–50% during 2015–2016 (Pile et al., 2018).
The growth index of a cohort of dried trees is more sensitive to climate variables than that in a cohort of living trees (Fig. 8). This “predisposition” to mortality is caused by microconditions of growth and the localization of trees on relief elements with the greatest risk of water stress. In the initial phase of mortality, the death of tree stands is confined mainly to the relatively steep (10°) northeastern slopes located in the rain shadow, since precipitation is accompanied mainly by the transfer of air masses from the southwest (Figs. 4b, 4c). The dependence of the mortality area on the tree stands from height above sea level was not detected due to low relief areas (<800 m above sea level). As was shown earlier (Kharuk et al., 2018), the mortality of dark conifers in the Altai–Sayan region, including the Stolby reserve, was observed mainly in low mountains (<1000 m), while in the upper altitude belt there is an increase in radial increment and a closeness of dark conifer stands. It should be noted that there is no direct connection between GI and precipitation, which is due to a lack of significant trends in precipitation (Fig. 2c) and increased evapotranspiration with increasing air temperature (displayed by the drought index SPEI).
The negative impact of air temperature in May on the increment of fir is noteworthy. An abnormal increase in air temperature in early spring activates photosynthesis and enhances evapotranspiration, which occurs against the background of a lack of moisture recharge from the soil and desiccation of needles; observations show that, in years with abnormally high spring temperatures, fir needles have a redness (mainly annual, having relatively less dense cuticles) in large areas.
During the mortality of trees (Fig. 5), there is a close relationship between the GI and the mortality of fir trees (r = –0.79). This confirms the possibility of using growth parameters as predictors of woody plant mortality (Cailleret et al., 2017). There is a lack of connection between the GI and climatic variables in the phase of mass dying of tree stands (after 2010), due to the dominant impact of the polygraph beetle on the vital condition of woody plants.
Unlike fir, Siberian pine stands were not mortality, although some of the trees showed signs of weakening (gum emission) and traces of insect attacks, mainly by the six-pointed bark beetle (Ips sexdentatus Boern). Differences in the resistance between Pinus sibirica and Abies sibirica, equally hygrophilous species, can be attributed to differences in the depth of the root layer, as well as in the leaf area index (LAI). The depth of the Siberian pine root layer (to 2–3 m on light soils) significantly exceeds that of fir, the roots of which are localized mainly in the layer to 0.5 m. Exact values of LAI of Abies sibirica and Pinus sibirica, the most important parameter of the plant water balance, are absent in the literature, whereas in similar members of the woody flora of North America, Pseudotsuga menzieslii Mirb., Pinus strobus L., value of LSI are 9.3 and 4.5, respectively (Thomas et al., 2000; Guiterman et al., 2012). These values can be considered indicative for fir and Siberian pine.
Along with differences in the LAI and root-layer depth, it is important to note the exceptional aggressiveness of P. proximus in comparison with the pests of P. sibirica. For the period since the discovery of P. proximus in the early 2000s, the mortality of fir has spread over a territory of over 400 000 ha. In other parts of the boreal zone, in the forests of the United States, the synergy of aridity of climate and the attacks of xylophage Dendroctonus ponderosae are related, as in P. proximus, to the subfamily Scolytinae, which has caused the catastrophic (~25 million hectares) mortality of conifers (Millar and Stephenson, 2015).
The renewal of fir under a canopy of lost tree stands is mainly trustworthy without visible signs of damage that, under favorable climatic conditions, can allow the fir to be restored. The preservation of renewal is probably due to the buffer role of the upper canopy; Morozov (1926) pointed to a relatively higher air humidity under the tree canopy. However, the most likely climate scenarios predict an increase in aridity of the climate in the southern taiga subzone, which will lead to the disappearance of fir from the southern part of its range. In this regard, the proximity of the GI trajectories of living and dry trees (Fig. 5), indicating the probability of death and this cohort of trees in the conditions of the projected increase in aridity and frequency of droughts, is noteworthy (IPCC…, 2014).
CONCLUSIONS
(1) The mass death of fir tree stands was preceded by a decrease in the radial growth index (GI) of fir with an increase in its dependence on the moisture content of the root layer and the drought index SPEI (atmospheric drought).
(2) Fir mortality of fir began in the 2000s, when firs weakened by water stress were attacked by Polygraphus proximus. In the initial phase of mortality, dead stands were localized mainly on the relief elements with the highest probability of water stress (steep slopes located in the rain shadow). By 2017, the mortality had spread to the entire territory of the fir forests, which led to the death of ~75% of the stands. The surviving trees grew under conditions of higher humidity of the root zone in comparison with the dead ones.
(3) During the mortality period, there was a close relationship between the GI and the mortality of fir trees. The similarity of the trajectories of dead and survived trees indicates the probability of death of “surviving” trees under the conditions of a predicted increase in the climate aridity.
(4) The decrease in the GI and mortality of Abies sibirica in the Stolby Nature Reserve is part of the phenomenon of mass mortality of fir stands in the southern taiga subzone in the 21st century. The beginning of the process was provoked by an increase in aridity of the climate and the frequency of droughts. Weakening of fir forests by water stress induced an outbreak of mass reproduction of P. proximus both in the fir forests on the territory of Stolby reserve and in the southern part of the fir area as a whole.
REFERENCES
Akulov, E.N. and Mandel’shtam, M.Yu., New finds of the bark beetles (Coleoptera: Curculionidae: Scolytinae) in the south of Krasnoyarsk krai and Khakassia Republic, Materialy Vserossiiskoi konferentsii “Ekologicheskie i ekonomicheskie posledstviya invazii dendrofil’nykh nasekomykh,” Krasnoyarsk, 25–27 sentyabrya 2012 g. (Proc. All-Russ. Conf. “Ecological and Economic Consequences of Invasion of Dendrophilic Insects,” Krasnoyarsk, September 25–27, 2012), Krasnoyarsk, 2012, pp. 123–128.
Anderegg, L.D.L, Anderegg, W.R.L., and Berry, J.A., Tree physiology review: not all droughts are created equal: translating meteorological drought into woody plant mortality, Tree Physiol., 2013, vol. 33, no. 7, pp. 701–712.
Bark Beetles: Biology and Ecology of Native and Invasive Species, Vega, F.E. and Hofstetter, R.W., Eds., Amsterdam: Elsevier, 2015.
Cailleret, M., et al., A global synthesis of radial growth patterns preceding tree mortality, Global Change Biol., 2017, vol. 23, pp. 1675–1690. https://doi.org/10.1111/gcb.13535
Chilakhsaeva, E.A., First find of Polygraphus proximus Blandford, 1894 (Coleoptera, Scolytidae) in Moscow oblast, Byull. Mosk. O-va. Ispyt. Prir., Otd. Biol., 2008, vol. 113, no. 6, pp. 39–42.
Gu, Y., Brown, J.F., Verdin, J.P., and Wardlow, B., A five-year analysis of MODIS NDVI and NDWI for grassland drought assessment over the Central Great Plains of the United States, Geophys. Res. Lett., 2007, vol. 34, no. 6, p. L06407
Guiterman, C., Seymour, R.S., and Weiskittel, A.R., Long-term thinning effects on the leaf area of Pinus strobes L. as estimated from litterfall and individual-tree allometric models, For. Sci., 2012, vol. 58, no. 1, pp. 85–93. https://doi.org/10.5849/forsci.10-002
Holmes, R.L., Computer-assisted quality control in tree-ring dating and measurement, Tree-Ring Bull., 1983, vol. 44, pp. 69–75.
IPCC, Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Pachauri, R.K. and Meyer, L.A., Eds., Geneva: Intergov. Panel Climate Change, 2014.
Kharuk, V.I., Im, S.T., Oskorbin, P.A., Petrov, I.A., and Ranson, K.J., Siberian pine decline and mortality in southern Siberian mountains, For. Ecol. Manage., 2013, vol. 310, pp. 312–320.
Kharuk, V.I., Im, S.T., Petrov, I.A., and Yagunov, M.N., Decline of dark coniferous stands in Baikal region, Contemp. Probl. Ecol., 2016a, vol. 9, no. 5, pp. 617–625.
Kharuk, V.I., Im, S.T., and Dvinskaya, M.L., Decline of spruce (Picea abies) in forests of Belarus, Russ. J. Ecol., 2016b, vol. 47, no. 3, pp. 241–248.
Kharuk, V.I., Im, S.T., Petrov, I.A., Dvinskaya, M.L., Fedotova, E.V., and Ranson, K.J., Fir decline and mortality in the Southern Siberian mountains, Reg. Environ. Change, 2017, vol. 17, no. 3, pp. 803–812. https://doi.org/10.1007/s10113-016-1073-5
Kharuk, V. I., Im, S.T., and Yagunov, M.N., Migration of the northern boundary of the Siberian silkmoth habitat, Contemp. Probl. Ecol., 2018a, vol. 11, no. 1, pp. 26–34.
Kharuk, V.I., Petrov, I.A., Dvinskaya, M.L., Im, S.T., and Shushpanov, A.S., Comparative reaction of larch (Larix sibirica Ledeb.) radial increment on climate change in the forest steppe and highlands of Southern Siberia, Contemp. Probl. Ecol., 2018b, vol. 11, no. 4, pp. 388–395.
Kharuk, V.I., Im, S.T., and Petrov, I.A., Warming hiatus and evergreen conifers in Altay-Sayan region, Siberia, J. Mt. Sci., 2018c, vol. 15, no. 12, pp. 2579–2589. https://doi.org/10.1007/s11629-018-5071-610.1007/s11629-016-4286-7
Kharuk, V.I., Ranson, K.J., Petrov, I.A., Dvinskaya, M.L., Im, S.T., and Golyukov, A.S., Larch (Larix dahurica Turcz) growth response to climate change in the Siberian permafrost zone, Reg. Environ. Change, 2018d, vol. 19, no. 1, pp. 233–243. https://doi.org/10.1007/s10113-018-1401-z
Knorre, A.A., Raznobarskii, V.G., Vagnoryus, P.A., Shaidurov, R.L., Lesnikov, E.M., Astapenko, S.A., and Akulov, E.N., Endangered fir stands in Stolby Nature Reserve due to invasion of bark beetle (Polygraphus proximus Blandf.), Tr. Gos. Zapoved. Stolby, 2015, no. 20, pp. 211–222.
Kolb, T.E., Fettig, C.J., Ayres, M.P., Bentz, B.J., Hicke, J.A., Mathiasen, R., Stewart, J.E., and Weed, A.S., Observed and anticipated impacts of drought on forests insects and diseases in the United States, For. Ecol. Manage., 2016, vol. 380, pp. 321–324. https://doi.org/10.1016/j.foreco.2016.04.051
Krivets, S.A., Kerchev, I.A., Bisirova, E.M., Pet’ko, V.M., and Baranchikov, Yu.N., Distribution of the bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) in Siberia, Izv. S.-Peterb. Lesotekh. Akad., 2015, 211, pp. 190–211.
Krivolutskaya, G.O., Ecological-geographic characteristics of diversity of the bark beetles (Coleoptera, Scolytidae) in Northern Asia, Entomol. Obozr., 1983, vol. 62, no. 2, pp. 287–301.
Liu, C., Liu, Y., Guo, K., Fan, D., Li, G., Zheng, Y., Yu, L., and Yang, R., Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China, Environ. Exp. Bot., 2011, vol. 71, pp. 174–183.
Logan, J. A., Regniere, J., and Powell, J.A., Assessing the impacts of global warming on forest pest dynamics, Front. Ecol. Environ., 2003, vol. 1, pp. 130–137.
Martínez-Vilalta, J., Lloret, F., and Breshears, D.D., Drought-induced forest decline: causes, scope and implications, Biol. Lett., 2012, vol. 8, no. 5, pp. 689–691.
Millar, C.I. and Stephenson, N.L., Temperate forest health in an era of emerging megadisturbance, Science, 2015, vol. 349, no. 6250, pp. 823–826. https://doi.org/10.1126/science.aaa9933
Morozov, G.F., Uchenie o lese (The Concept about Forest), Leningrad: Gosizdat, 1926.
Pile, L.S., Marc, D.M., Rojas, R., and Roe, O., Characterizing tree mortality after extreme drought and insect outbreaks in the southern Sierra Nevada, Proc. 19th Biennial Southern Silvicultural Research Conf., Asheville, NC: U.S. Dep. Agric. For. Service, Southern Res. Stn., 2018, pp. 89–96.
Proekt organizatsii i vedeniya lsenogo khozyaistva (A Draft of Organization and Management of Forestry), Krasnoyarsk: Gos. Prirod. Zapoved. Stolby, 2007.
Raffa, K.F., Aukema, B.H., Bentz, B.J., et al., Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions, Bioscience, 2008, vol. 58, pp. 501–517.
Riaño, D., Chuvieco, E., Salas, J., and Aguado, I., Assessment of different topographic corrections in Landsat-TM data for mapping vegetation types, IEEE Trans. Geosci. Remote Sens., 2003, vol. 41, pp. 1056–1061
Rinn, F., TSAP V. 3.6 Reference Manual: Computer Program for Tree-Rings Analysis and Presentation, Heidelberg: Frank Rinn, 1996.
Sangüesa-Barreda, G., Linares, J.C., and Camarero, J.J., Reduced growth sensitivity to climate in bark-beetle infested Aleppo pines: connecting climatic and biotic drivers of forest dieback, For. Ecol. Manage., 2015, vol. 357, pp. 126–137.
Sazonov, A.A., Kukhta, V.N., Blintsov, A.I., et al., Mass drying of spruce forests in Belarus and its prevention, Lesn. Okhotn. Khoz., 2013, no. 7, pp. 10–15.
Speer, J.H., Fundamentals of Tree Ring Research, Tucson: Univ. of Arizona Press. 2010.
Thomas, S.C. and Winner, W.E., Leaf area index of an old-growth Douglas-fir forest estimated from direct structural measurements in the canopy, Can. J. For. Res., 2000, vol. 30, pp. 1922–1930.
Tropina, E.F., Soil studies in Stolby Nature Reserve, Tr. Gos. Zapoved. Stolby, 2015, no. 20, pp. 69–98.
Vicente-Serrano, S.M., Begueria, S., and López-Moreno, J.I., A multi-scalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index, J. Clim., 2010, vol. 23, pp. 1696–1718.
Yousefpour, R., Hanewinkel, M., and Le Moguédec, G., Evaluating the suitability of management strategies of pure Norway spruce forests in the black forest area of Southwest Germany for adaptation to or mitigation of climate change, Environ. Manage., 2010, vol. 45, no. 2, p. 387.
Funding
This work was supported by the Russian Foundation for Basic Research, project nos. 18-45-240003 and 18-05-00432. Dendrochronological and dendroclimatic analysis was performed with support from the Russian Science Foundation, project no. 17-74-10113.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement of the welfare of animals. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by S. Kuzmin
Rights and permissions
About this article
Cite this article
Kharuk, V.I., Shushpanov, A.S., Petrov, I.A. et al. Fir (Abies sibirica Ledeb.) Mortality in Mountain Forests of the Eastern Sayan Ridge, Siberia. Contemp. Probl. Ecol. 12, 299–309 (2019). https://doi.org/10.1134/S199542551904005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199542551904005X