Abstract
This paper presents the results of an investigation of the influence of the aluminum-powder concentration on the thermomechanical characteristics of the composites based on the high-density polyethylene. We show that use of a compatibilizer—maleic anhydride–polyethylene graft copolymer—in a mixture with high-density polyethylene has an influence on the regularity of variation of the thermomechanical curves. We study the influence of various cross-linking agents (dicumyl peroxide and sulfur) on the thermomechanical properties of composites. At a particular dicumyl peroxide and sulfur concentration, the composites might reside in three physical states: solid, highly elastic, and viscous flow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The improvement of machinery and technology in the fields of engineering, shipbuilding, military, and aerospace industries is increasing interest in the development of construction high-quality polymer materials on a composite base. The insertion of extenders, plasticizers, light and thermal stabilizers, and compatibilizers; obtainment of polymer mixtures; and chemical cross linking of the polymer matrix make it possible to develop and produce a vast set of composites that are capable, to some extent, to solve a number of problems related with obtainment of the construction material with preset structural and physicomechanical properties [1–4].
The use of polyolefins as a polymer matrix in a mixture with various mineral and metal extenders has been considered as the most problematic because high-density polyethylene (HDPE) is a nonpolar polymer, while extenders and other adjuvants and ingredients, as a rule, are classified as polar components. Mixing of the multipolar components prevented sufficient technological compatibility, which, in the long run, contributed to deterioration or only minor improvement of the composite material properties [5–9].
In this regard, attempts were made to improve the miscibility, compatibility, and final properties of the mixture components by using efficient compatibilizers [10]: their correct choice makes it possible to fundamentally solve not only the problem of mixture-component compatibility, but also closely approach to development of high-quality construction material with the preset properties. This problem is becoming even more urgent in the development of the polyolefin-based metal–polymer composites [10–13]. We believe that, in this case, problems related to the chemical modification of the polymer matrix have become of paramount importance, resulting in the possibility, in the process of thermomechanical studies, to estimate the modifier’s influence on the variation regularity of the phase transitions from one physical condition to another.
Note that, in the literature available, studies of the thermodeformation properties and phase transitions in the polyolefin-based metal–polymer composites are very scarce. Thus, a deep thermomechanical analysis of the metal–polymer systems will make possible a more effective approach to selection of the technological regime of composite treatment by the extrusion and the die-casting methods.
Thus, the present work is aimed at an investigation, by means of the thermomechanical analysis, of the influence of metal temperature and the concentration on the variation regularity of the deformation processes in metal–polymer composites.
As the object of study, we used high-density polyethylene (HDPE) with a breaking stress of 31.3 MPa, a modulus of flexibility of 753 MPa, a relative elongation of 435%, a density of 946 kg/m3, a melt-flow index (MFI) of 5.6 g/10 min, a heat stability of 119°C, a melting point of 131°C, and a degree of crystallinity of 80%.
To modify the HDPE properties, we added 1.0- to 2.0-μm aluminum powder (AP) into its composition and varied its content within 0.5, 1.0, 5.0, 10, 20, and 30 wt %.
PEMA (the HDPE with MA) compatibilizer is a grafted HDPE copolymer with the maleic anhydride (MA). it is aimed at improvement of compatibility of the metals and the minerals carriers with the HDPE. The MA content in the PEMA composition was equal to 4.2 wt %.
We mixed the components on hot rollers at a temperature of 160–170°C by inserting the AP into the HDPE melt for 7–8 min. The cross-linking agent—peroxide dicumylide (PD)—was inserted into a melt of polymer mixtures in an amount of 0.5–2.0 wt %. Then, to test the thermomechanical properties, the tablets were compressed at a temperature of 190–200°C and pressure of 5.0 t.
In the presence of PD, the vulcanization temperature is 140–170°C. The free radicals (that appeared during the peroxide-dicumyl decomposition) detach hydrogen from the polymer macromolecules; the thus-occurring polymer radicals interact with each other with the formation of the C–C bonds. In the presence of a double bond in the polymer chain, the peroxide radical predominantly attaches to it or removes the α-methylene hydrogen. Here, in both cases, the formation of the macroradicals takes place with the subsequent occurrence of transverse connections.
We determined the melt flow index using a CEAST MF50 rheometer (Instron, Italy) at a temperature of 190°C and load of 5 kg. Under these conditions, the MFI of the initial LDPE was 5.8 g/10 min. Derivatographic analysis was carried out on a Paulik, Paulik, and Erdei device.
We studied the thermal conductivity of composites on a DTC-300 device and determined the thermomechanical properties on the Kanavets device [14].
In the process of thermomechanical analysis of the metal–polymer systems on the HDPE and aluminum base, we, first of all, tried to reveal the role of the compatibilizer in the compatibility of the mixture components. In addition, we investigated the influence of the vulcanization agents on variation regularity of the phase transitions from one physical state to another. We used PD and sulfur as vulcanizing agents.
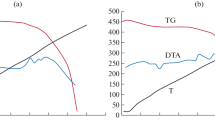
Let us consider the influence of aluminum concentration on the character of the thermomechanical-curve variations for the HDPE + aluminum system (Fig. 1). We see that the thermomechanical curves are characterized by two physical states—solid and viscous-flow. Here, the aluminum concentration does not significantly influence the regularity of the thermomechanical-curve variations. Note that, while the temperature range of the viscous-flow state is somewhat extended if for the initial HDPE, for the filled composites, it does not in fact undergo significant variations and merges into a unified bundle of thermomechanical curves.
Note that, for the initial HDPE, the softening onset temperature is 142°C, yet, for the composites with an aluminum content of 0.5, 1.0, 5.0, 10, 20, and 30 wt %, the softening temperature changes, respectively, in the sequence 142, 140, 138, 138, 136, and 134°C. That is, the insertion of aluminum leads to a decrease of the softening temperature. The data obtained seem to be conflicting, but, in fact, the regular softening-temperature decrease may be caused by the thermal conductivity of the composite in the presence of aluminum. The higher the aluminum content, the greater the composite thermal conductivity, resulting in uniform heating throughout the entire sample volume.
For example, the HDPE thermal conductivity is 0.38 W/(m K); yet, with an increase in aluminum concentration, it changes as follows, W/(m K): Al 0.5 wt %, 0.48; 1.0 wt %, 0.59; 5.0 wt %, 0.84; 10 wt %, 1.96; 20 wt %, 2.23; and 30 wt %, 3.05.
Thus, with the aluminum insertion into the HDPE composition, its thermal conductivity increases by about eight times. On the other hand, in the process of the component mixing and further sample pressing (for the tests), the composites underwent melting and cooling. Therefore, we should not exclude the possibility that the aluminum particles in the HDPE melt might form heterogeneous nucleation centers, which, in the cooling process, transform into heterogeneous crystallization centers with the formation of a fine-spherulite permolecular structure.
To improve the aluminum’s compatibility with HDPE, we inserted a compatibilizer—HDPE with MA (PEMA) graft copolymer. Figure 2 shows the thermomechanical characteristics of the modified HDPE + PEMA + Al composites. Analyzing the thermomechanical curves in this figure, we see that also in this case, like for the HDPE in Fig. 1, the variation regularity of the thermomechanical curve for the HDPE + PEMA-based polymer matrix goes beyond the bundle of thermomechanical curves for the HDPE + PEMA + Al composites. Indeed, when evaluating the thermomechanical curves of the samples under consideration, we found that, depending on the aluminum content increase in the composite within 0.5, 1.0; 5.0; 10; 20 and 30 wt %, the softening temperature of the samples changes, respectively, in the following sequence: for the original HDPE + PEMA, 142°C, and, for the composites, 141, 141, 141. 140. 140, and 139°C.
From a comparative estimate of the composite softening temperature in Figs. 1 and 2, we see that, with the insertion of 30 wt % of Al into the HDPE and the HDPE + PEMA compositions, the difference in this indicator value in relation to the HDPE equals to 8°C and to the HDPE + PEMA mixture – 3°C.
Besides, we investigated the melting point of the composites by the derivatography. We show that this indicator value depends, to a significant extent, on the initial polymer matrix type. For example, insertion of 30 wt % aluminum into the HDPE composition results in the composite melting temperature decrease from 145 to 139°C, while the melting point decreases from 145 to 143°C when using the HDPE + PEMA as a starting polymer matrix. In this case, polarization of the interspherulite space by the compatibilizer provides a favorable effect not only on the compatibility improvement of the mixed components, but also on stabilization of the thermophysical characteristics.
This phenomenon is evidence that insertion of PEMA into the HDPE composition improves the formation of heterogeneous nucleation centers on aluminum-particle surfaces. Another reason to interpret the difference in the composite softening point may be connected with the peculiarities of the spherulite growth during cooling [15–17]. It is well known that, in the process of crystallization and spherulite growth, metal particles and PEMA macrochain fragments containing the MA units are displaced into the interspherulite amorphous space. As a result, in the narrow interspherulite composite space, the concentration of the aluminum particles increases markedly and is predominantly distributed in the PEMA volume. The PEMA polarity provides high compatibility of the metal–polymer systems and more uniform dispersing of the aluminum particles in the polar polymer volume. Therefore, in our further studies, we will engage the composites based on the HDPE + PEMA mixture.
Interchain cross linking of macrochains, making it possible to significantly influence a composite’s thermophysical properties, is an efficient way to improve the properties of polymeric material. Taking it into account that minor attention is paid to this problem in the available literature, in the present work we perform studies demonstrating the advantageous features of the metal–polymer system vulcanization.
Vulcanization as a polymer modification method is very efficient; yet, it requires precise and accurate performance of the mechanochemical reaction. Under insufficient cross linking, we might not detect even a significant difference in the property variation. Nevertheless, the excess concentration of the cross-linking agent may lead to irreversible processes of spatial cross linking when the polymer almost completely loses its ability to be treated by the standard methods such as injection molding and extrusion; that is, it becomes unusable. Therefore, the correct choice of the cross-linking agent type and concentration, as well as of the technological mode of the polymer-matrix chemical modification, are the main factors of mechanochemical synthesis of composite materials with the desired properties.
In this regard, in the present work, we engaged two cross-linking agent types—PD and sulfur. Our task was to identify the limiting concentrations of the cross-linking agents that make it possible to significantly improve the composite thermophysical characteristics with retention of their ability to be treated by injection molding and extrusion methods.
Figure 3 shows the thermomechanical curves of the deformation dependence on the temperature of the composite based on the HDPE + 3.0 wt % PEMA + 5.0 wt % Al. We chose those values because, at higher aluminum concentrations, the composite becomes fragile. Analysis of the thermomechanical curves in this figure shows that the PD concentration has a rather tangible influence on the features of their variation. The original composite was characterized by only one phase transition from the solid state into the viscous fluid; yet, after the vulcanization, an additional, third physical state appears: highly elastic.
The presence of the three physical states is typical for the rubbers. Figure 3 shows that, with an increase in PD concentration from 0.25 to 2.0 wt %, quite noticeable variations in the thermomechanical curves take place. According to the obtained experimental data, the emergence of the three physical states—solid, highly elastic, and viscous-flow—becomes possible when using PD in the amount of 0.25–0.5 wt %. At a PD concentration equal to 1.0–2.0 wt %, the interchain cross-linking density achieves a level such that the polymer immediately transits from a highly elastic to an irreversible glassy state.
Cross-linked polymeric materials of such a type are of no practical value. We show that the increase in PD concentration within 0.25, 0.5, 1.0, and 2.0 wt % entails an increase in the softening temperature of the samples—respectively, to 145, 154, 162, and 170°C. With the insertion of 0.25 and 0.5 wt % PD, the temperature domain of highly elastic deformation changes within the range of 155–165 and 164–180°C, respectively.
Figure 4 shows the influence of sulfur concentration on the regularity of the thermomechanical-curve variations. Analysis of the curves shows that the insertion of sulfur promotes the appearance of all three physical states on the thermomechanical curves. With the increase in sulfur concentration, we see an increase of the temperature range of highly elastic deformation and a decrease of the deformation value being fixed. With sulfur insertion within the range of 1.0, 3.0, 5.0, 7.0, and 10 wt %, the temperature range of highly elastic deformation changes, respectively, as follows, °C: 150–157, 158–169, 164–180, 169–187, and 174–195.
Note that, in contrast to the peroxide cross linking, the sulfuric one allows, in fact, varying the temperature ranges of highly elastic deformation over a wide range with retention of the composite’s ability to be treated. As for the cross-linking efficiency, the sulfur concentration of 5.0 wt % is optimal.
CONCLUSIONS
Thus, we may conclude that, to improve the compatibility of aluminum with HDPE, PEMA (3.0 wt %) should be used as a compatibilizer.
We have showed that, with insertion of aluminum, a slight decrease in the HDPE softening temperature takes place.
Studies of vulcanization of the HDPE-based composites showed that the optimal PD content is 0.5 wt %. At a PD concentration within 0.25–0.5 wt %, cross-linked composites are characterized by three physical states. At higher PD concentration, the polymer composite is completely cross linked and becomes unusable for treatment by injection molding and extrusion methods.
The study of the process of sulfuric vulcanization of HDPE composites showed that, even at high concentrations, samples are characterized by three physical states typical for rubbers. The optimal sulfur concentration as a vulcanizing agent is 5.0 wt %.
REFERENCES
A. A. Tager, Physicochemistry of Polymers, 4th ed. (Nauchnyi Mir, Moscow, 2007) [in Russian].
V. Nelyub and G. Malysheva, “Modern treatment technologies of carbon fibre foe ensuring the high strength carbon fibre reinforced plastic,” MATEC Web Conf. 129, 02001 (2017).
L. F. Kalistratova and V. A. Egorova, “Ordering of the amorphous phase as one of the characteristics of supramolecular structure of amorphous-crystalline polymer,” Inorg. Mater.: Appl. Res. 10, 933–938 (2019).
M. A. Gorodetskii, V. A. Nelyub, G. V. Malysheva, A. Y. Shaulov, and A. A. Berlin, “Technology of forming and the properties of reinforced based on an inorganic binder,” Russ. Metall (Engl. Transl.) 2018 (13), 1195–1198 (2018).
I. D. Simonov-Emel’yanov, “Structuring in dispersive filled polymers and properties of composite materials,” Plast. Massy, Nos. 9–10, 29–36 (2015).
N. T. Kakhramanov, A. D. Ismailzade, N. B. Arzumanova, U. M. Mammadli, and Q. S. Martinova, “Filled composites based on polyolefins and clinoptilolite,” Am. Sci. J. 4 (4), 60–65 (2016).
A. M. Kharaev, R. C. Bazheva, M. B. Begieva, V. A. Nelyub, and A. S. Borodulin, “Polyethersulfones with improved thermohysical properties,” Polym. Sci., Ser. D 12 (1), 24–28 (2019).
S. M. Terekhina, G. V. Malysheva, I. M. Bulanov, and T. V. Tarasova, “Investigation of tribological properties of polymer composite materials based on bismaleimide binder,” Polym. Sci., Ser. D 4 (2), 136–137 (2011).
N. T. Kakhramanov, A. G. Azizov, V. S. Osipchik, U. M. Mamedli, and H. B. Arzumanova, “Nanostructured composites and polymer materials science,” Int. Polym. Sci. Technol. 44 (2), 37–48 (2017).
N. T. Kakhramanov, Kh. V. Allakhverdieva, M. I. Abdullin, and F. A. Mustafayeva, “Influence of aluminum powder concentration on mechanism and kinetic regularities of crystallization of composites based on low density polyethylene,” Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 63 (2), 77–83 (2020).
N. T. Kakhramanov, Z. N. Guseinova, and V. S. Osipchik, “The influence of the technological parameters of injection molding on the physico-mechanical properties of dynamic elastoplastic based on polyolefins,” Polymer Sci., Ser. D 12 (3), 317–321 (2019).
I. P. Petryuk, “Influence of the parameters of a dispersed structure on the interphase layer content in filled polymers,” Plast. Massy, Nos. 5–6, 7–9 (2014).
A. A. Dyakonov, S. N. Danilova, A. P. Vasilyev, A. A. Okhlopkova, S. A. Sleptsova, and A. A. Vasilyeva, “Study of sulfur, diphenylguanidine and 2-mercaptobenzothiazole effect on physical and mechanical properties and structure of ultra-high molecular weight polyethylene,” Perspekt. Mater., No. 1, 43–53 (2020).
Kh. V. Allakhverdieva and N. T. Kakhramanov, “Thermal-mechanical properties of composites and their vulcanizers based on low-density polyethylene and aluminum powder,” Vse Mater., No. 5, 14–19 (2020).
G. V. Kozlov and I. V. Dolbin, “Transfer of mechanical stress from polymer matrix to nanofiller in dispersion-filled nanocomposites,” Inorg. Mater.: Appl. Res. 10, 226–230 (2019).
L. B. Atlukhanova, G. V. Kozlov, and I. V. Dolbin, “The correlation between the nanofiller structure and the properties of polymer nanocomposites: fractal model,” Inorg. Mater.: Appl. Res. 11, 188–191 (2020).
Yu. K. Mashkov, L. F. Kalistratova, and O. V. Kropotin, “Development of methods of formation of effective structure-phase states of polymer composites based on PTFE,” Plast. Massy, Nos. 3–4, 12–14 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Dikhter
Rights and permissions
About this article
Cite this article
Allahverdiyeva, K.V., Kakhramanov, N.T. & Namazly, U.V. Thermomechanical Properties of Composites Based on High-Density Polyethylene and Aluminum. Polym. Sci. Ser. D 14, 598–602 (2021). https://doi.org/10.1134/S1995421222010026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995421222010026