Abstract
We present data on the study of how the concentrations of powdered copper and crosslinking agents influence the properties of composites materials based on low-density polyethylene, compatibilizer, and copper. It was shown that the addition of copper favors an increase in the softening temperature of the composite materials. Two physical states, solid and viscous-flow ones, were detected depending on the test temperature. The optimum concentrations of curing agents, upon addition of which the crosslinked composites based on low-density polyethylene were characterized by three physical states, solid, viscoelastic, and viscous-flow ones, were shown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The use of different types of fillers, plasticizers, compatibilizers, stabilizers, curing agents, and other additives allows one to significantly influence a variation in the structural features and performance properties of polymer composite materials. Polyolefins are polymers widely used in industry, which is due to their valuable set of physicochemical and process characteristics. However, these advantages are not always sufficient to extend the fields of their application [1–3]. This is explained by the fact that, among main drawbacks of nonpolar polyolefins is their poor compatibility with foreign components, which include polar natural minerals or metals. The absence of good process compatibility is usually reflected in agglomeration of filler particles accompanied by certain decrease in the strength indices of composite materials on their basis [4, 5]. For this reason, a greater amount of attention has started to be given to the use of compatibilizers that can improve their miscibility and process compatibility with fillers.
Taking into account that such studies of metal-polymer systems have hardly been performed, it seemed interesting to consider a selective effect of disperse metal particles on the thermomechanical strain–temperature curves by the example of low-density polyethylene (LDPE).
EXPERIMENTAL
The object of research was low-density polyethylene (LDPE) with properties as follows: the fracture stress was 13.1 MPa, the flexural modulus was 196 MPa, the density was 927 kg m–3, the percentage elongation was 720%, the Vicat softening temperature was 95°C, the melting point was 107°C, and the crystallinity was 58%.
The compatibilizer was high-density polyethylene functionalized by maleic anhydride (MA)—PEMA. The degree of grafting of MA as a part of HDPE was 5.6 wt %.
Dicumyl peroxide (DP) is a crosslinking agent appearing as a light yellow powder with a melting point of 40°C, designed to obtain crosslinked structures in polymer compositions.
Sulfur is a chemical element with atomic number 16. It is abundant and multivalent. Elemental sulfur is a bright yellow crystalline solid at room temperature. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. It is used as an agent for vulcanization of polymers.
The metal filler was finely divided copper particles with a particle size of 1.0–2.0 μm.
The particle size of copper was determined on a STA PT1600 Linseiz instrument (Germany).
Mixtures based on LDPE, copper, and PEMA were prepared on hot laboratory roll mills at 160°C by addition of the filler into a polymer melt for 8–10 min. In this case, PEMA was added first to the composition of LDPE and the filler was added second.
In order to modify the properties of LDPE and LDPE + PEMA, their compositions were supplemented by a finely divided copper powder. The amount of copper in LDPE varied within 0.5, 1.0, 5.0, 10, 20, and 30 wt %. The components based on LDPE + copper and LDPE + PEMA + copper were mixed for 8 min on hot roll mills at 150–160°C. The crosslinking agent (DP) was added to the melt of polymer mixtures in the amount of 0.5–2.0 wt %. The concentration of sulfur in the melt of the composite varied in a range of 3.0–10 wt %. After roll milling and preparation of polymer composites, plates were obtained from them by pressing at 150–170°C under a pressure of 50 MPa to test for physicomechanical properties.
The derivatographic analysis was performed on a Paulik–Paulik–Erdey instrument.
The melt flow index (MFI) was determined on a CEAST MF50 melt flow tester (INSTRON, Italy) at 190°C and a load of 5 kg. Under these measurement conditions, the MFI of the starting LDPE was 3.6 g/10 min.
A FOX50 analyzer is designed for the direct determination of the thermal conductivities of polymer materials by the heat flow method. This compact-size analyzer uses thin-film sensors of heat flow for rapid reception of accurate and correct values of thermal conductivity in a wide temperature range. The FOX50 is equipped with a digital meter of sample thickness, a system for temperature control of a sample, and an integrated system for correction of the contact resistance.
The thermomechanical properties were determined on a Kanavts instrument. The strain was measured at gradually varying temperatures (T) under a load of 0.3 kg cm–2 and at a heating rate of 50°C h–1. Upon plotting the thermomechanical curve of the polymer Δ = f(T), it was very important to encompass as much as possible the entire temperature range of polymer existence: glasslike (crystalline), highly elastic, and viscous-flow states. The thermomechanical curves reflect all possible physical, physicochemical, and chemical changes occurring in a sample during variation in the experimental temperature and thereby allow one to acquire reliable data on temperature transitions significant for processing of polymers.
RESULTS AND DISCUSSION
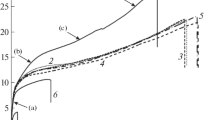
The method of thermomechanical studies provides distinct detection of phase transitions and all possible changes depending on temperature during deformation of composites. The obtained data may allow one to predict to certain degree the temperature conditions for processing of polymer composite materials [6, 7]. The results of studying the thermomechanical curves for the composites based on LDPE and copper are shown in Fig. 1. As this figure shows, the first-order phase transition occurs in a narrow temperature range. For example, the softening temperature of the composites varied in the following order: 107°C (starting LDPE), 110°C (composites containing 0.5 wt % of copper), 114°C (1.0 wt %), 118°C (5.0 wt %), 115°C (10 wt %), 111°C (20 wt %), and 109°C (30 wt %). The comparative analysis of the curves in this figure shows that the concentration of copper has a considerable effect on the first-order phase transition or softening temperature of the composites. The highest softening temperature was observed for the composites based on LDPE + 5.0 wt % of copper. With further increase in the concentration of copper, there was a tendency toward a decrease in the softening temperature of the composites. This is most likely due to the fact that the thermal conductivity of the copper-containing composites naturally increases with an increase in the concentration of copper. This fact favors faster and more uniform distribution of the temperature field throughout the bulk of the sample and, as a consequence, softening of the composite at a relatively low temperature. Indeed, we found that, as the concentration of copper forming a part of LDPE increases, the thermal conductivity of the composites significantly increases: 0.38 (starting LDPE), 0.54 (0.5 wt % of copper), 0.85 (5.0 wt % of copper), 1.92 (10 wt % of copper), 2.22 (20 wt % of copper), and 3.41 W/(m K) (30 wt % of copper). When the strain of the composites reached 2 mm, the maximum temperature range in the region of the viscous-flow state varied from 119 to 130°C.
It is commonly known that polyolefins possess weak adhesion to metals, which is finally expressed only in insignificant improvement in the physicomechanical properties [8, 9]. In this regard, it seemed interesting to show by the example of the LDPE + copper mixture how the compatibilizer (PEMA) influences the thermomechanical properties of the composites under consideration. The results of studying their thermomechanical data are shown in Fig. 2. We experimentally found that the addition of 2 wt % of PEMA to the LDPE + copper composite results in the highest improvement in the properties of the nanocomposites. The function of the compatibilizer is manifested not only in improving the miscibility and process compatibility of the mixture components but also in the capability of influencing the nucleation processes in the melt of the polymer matrix. As a result of hot roll milling of the mixture components and simultaneous exposure to heat and shear stresses, one could not exclude the probability of partial oxidation of the polymer matrix and the emergence of macroradicals capable of recombining with each other to form a relatively small amount of interchain bridges or reacting with maleic anhydride [10–12].
Figure 2 shows that the first-order phase transition occurs in a narrow temperature range. For example, the softening temperature of the LDPE + PEMA + copper composite varied depending on the concentration of copper in the following order: 110°C (0.5 wt %), 114°C (1.0 wt %), 118°C (5.0 wt %), 115°C (10 wt %), 111°C (20 wt %), and 109°C (30 wt %). It follows from the comparative analysis of the curves shown in this figure that the concentration of copper influences the first-order phase transition or softening temperature of the composites. In this case, the highest softening temperature was observed for the composites based on LDPE + PEMA + 5.0 wt % of copper. With further increase in the concentration of copper, this parameter of the composites decreases. As noted above, this fact can be explained by a regular increase in the thermal conductivity of the copper-containing composites with an increase in the concentration of copper.
It is important to note that the thermomechanical curves shown in Fig. 2 change in a slightly different manner. Analyzing these curves, one can note that, in contrast to the LDPE + copper composites, the addition of copper to LDPE + PEMA + copper favors narrowing of the temperature range of the thermomechanical curves in the region of the viscous-flow state. This significant difference in the pattern of changes in the thermomechanical curves is expressed in that the 2-mm strain of the starting LDPE is reached at a lower temperature compared to its composites. It suffices to note that, while the value of this index in the LDPE composites with 0.5, 1.0, 5.0, 10, 20, and 30 wt % of copper varies within 123, 126, 132, 125, 125, and 125°С, respectively, the temperature range in the composites based on LDPE + PEMA + copper becomes narrower and equal to 128–130°C. In this case, narrowing of the temperature range at a strain of 2 mm in the region of the viscous-flow state in the LDPE + PEMA + copper composites suggests the effect of the compatibilizer on strengthening of the adhesion interaction in the polymer–metal system. At the same time, it should be noted that, as compared to LDPE, PEMA is characterized by a relatively low molecular weight, which is evidenced by a high MFI value equal to 19.6 g/10 min.
It is known that most of the foreign particles make certain amendments not only to the formation of nucleation centers in a composite melt but also to the mechanism of their crystallization [9, 10]. While certain fraction of copper particles are involved in the formation of small spherolithic formations of polymer, most of them during the growth of crystalline formations are displaced into the interspherolithic amorphous space consisting of transfer chains. Since the crystallinity in LDPE is equal to 57%, there are grounds to assume that these particles in the semicrystalline polymer will be concentrated predominantly in the interspherolithic space. This space will also involve segments of the PEMA macrochains containing the polar groups of maleic anhydride. Another fraction of PEMA macrochains free of polar groups, most likely, together with LDPE macrochains will be involved in the formation of the crystalline phase of the composites. Copper particles clamped in the narrow interspherolithic space in the supramolecular structure of LDPE will be better compatible and redistributed in the bulk of PEMA. It is likely that, upon increasing the temperature of the composite, a high thermal conductivity of copper will favor first of all a fast heating up of the interspherolithic space.
In order to improve the thermophysical characteristics of the composites, it seemed interesting to consider whether they can be vulcanized using curing agents, such as dicumyl peroxide and sulfur. In this case, it was important to reveal how the concentration of different-type crosslinking agents influences the regularity of change in the thermomechanical curves of the composites based on LDPE + PEMA + 10 wt % of copper. Figure 3 shows the thermomechanical curves for this composite as a function of the DP content. The concentration of DP as a part of the composite varied in a range of 0.25–2.0 wt %. Analyzing the curves in this figure, one can note that, even upon addition of DP in the amount of 0.25 wt %, there emerges a small plateau typical of the highly elastic deformation region. According to the thermomechanical curves shown in Fig. 3, the starting polymer compositions of LDPE + 2.0 wt % of PEMA starts to soften at 109°C, which is two degrees higher than the softening temperature of the starting LDPE. Upon addition of DP in the amount of 0.25, 0.5, 1.0, and 2.0 wt % to the composition LDPE + PEMA + 10 wt % of copper, the softening temperature changes in the following order: 112, 121, 128, and 133°C. The highly elastic deformation plateau for the composite cured with 0.25 wt % of DP varies in a temperature range of 126–135°C, which passes into the viscous-flow state as the temperature increases. Upon vulcanization of this composite with 0.5 wt % of DP, the region of highly elastic deformation varies in a range of 134–152°C, followed by the phase transition into the viscous-flow state. At a strain of 2.0 mm, the highest temperature for the starting composition is 115°C and that for the composites cured with 0.25 and 0.5 wt % of DP is 144 and 166°C, respectively.
Analyzing the thermomechanical curves in Fig. 3, one can establish that, at a DP concentration of 1.0 and 2.0 wt %, the composites during softening immediately pass into a glasslike state characterized by a densely crosslinked structure. Such composites features an irreversible phase state at which they completely lose fluidity and, correspondingly, capability of being processed by extrusion and pressure casting [11, 12].
Figure 4 shows the thermomechanical strain–temperature curves for the composite LDPE + PEMA + 10 wt % of copper cured with sulfur. It can be seen from these curves that, as the concentration of sulfur increases from 3.0 to 10 wt %, the strain–temperature dependence changes in a regular manner. However, in contrast to DP, sulfur does not result in the formation of vulcanizates characterized by a glasslike state. This is interpreted by the fact that, in contrast to DP, sulfur crosslinks macrochains predominantly at the cleavage site of double bonds, the number of which is much lower than that in the considered polymers. Therefore, sulfur vulcanization does not result in the formation of densely crosslinked 3D structure in the composites. In all thermomechanical curves, the vulcanized composites are characterized by three physical states: solid, viscoelastic, and viscous-flow ones. It should be noted that, during sulfur vulcanization, the softening temperature changes less than that during peroxide vulcanization. For example, with an increase in the concentration of sulfur from 3.0 to 10 wt %, the softening temperature increases from 109 to 118°C. Depending on the concentration of sulfur, the region of the highly elastic deformation plateau changes as follows: 118–129°C (3.0 wt %), 127–148°C (5 wt %), 134–162°C (7.0 wt %), and 143–172°C (10 wt %). It follows from the obtained data that, with an increase in the concentration of sulfur, there were an extension of the highly elastic deformation plateau and an increase in the temperature at which the 2-mm strain mark is reached.
Thus, on the basis of the foregoing we can state the following:
1. The addition of copper to the composition of LDPE and LDPE + PEMA results in a certain increase in the softening temperature of the composites.
2. Vulcanization of the LDPE + PEMA + copper composites with 0.25–0.5 wt % of DP favors the formation of a crosslinked 3D structure characterized by three physical states: solid, highly elastic, and viscous-flow ones.
3. Vulcanization of the LDPE + PEMA + copper composites with 3.0–10 wt % of sulfur also favors the formation of a crosslinked structure with three physical states.
REFERENCES
Ashurov, N.R., Dolgov, V.V., Sadykov, Sh.G., and Usmanova, M.M., Nanokompozity. Polimery etilena, napolnennye sloistymi alyumosilikatami (Nanocomposites. Ethylene Polymers Filled with Layered Aluminosilicates), Tashkent: Fan, 2016.
Atlukhanova, L.B., Kozlov, G.V., and Dolbin, I.V., The correlation between the nanofiller structure and the properties of polymer nanocomposites: Fractal model, Inorg. Mater.: Appl. Res., 2020, vol. 11, pp. 188–191. https://doi.org/10.1134/S2075113320010049
Aloev, V.Z., Zhirikova, Z.M., and Tarchokova, M.A., Effectiveness of use of nanofillers of different types in polymeric composites, Izv. Vyssh. Uchebn. Zaved., Khim Khim. Tekhnol., 2020, vol. 63, no. 4, pp. 81–85. https://doi.org/10.6060/ivkkt.20206304.6158
Kalistratova, L.F. and Egorova, V.A., Ordering of the amorphous phase as one of the characteristics of supramolecular structure of amorphous-crystalline polymer, Inorg. Mater.: Appl. Res., 2019, vol. 10, pp. 933–938. https://doi.org/10.1134/S2075113319040208
Evtushenko, Yu.M., Rudakova, T.A., Grigor’ev, Yu.A., and Ozerin, A.N., Low pressure polyethylene modified with organomontmoryllonite, Vse Mater. Entsikl. Sprav., 2018, no. 8, pp. 12–16. https://doi.org/10.31044/1994-6260-2018-0-8-12-16
Kakhramanov, N.T., Ismailzade, A.D., Arzumanova, N.B., Mammadli, U.M., and Martinova, Q.S., Filled composites based on polyolefins and clinoptilolite, Am. Sci. J., 2016, vol. 4, no. 4, pp. 60–65.
Kozlov, G.V. and Dolbin, I.V., Transfer of mechanical stress from polymer matrix to nanofiller in dispersion-filled nanocomposites, Inorg. Mater.: Appl. Res., 2019, vol. 10, pp. 226–230. https://doi.org/10.1134/S2075113319010167
Starokodomskii, D.L., Physicomechanical properties and micro nanostructure of epoxy composites filled with gypsum, chalk and cement, Kompozity Nanostrukt., 2018, vol. 10, no. 1 (37), pp. 39–51.
Kakhramanov, N.T., Guseinova, Z.N., Gadzhieva, R.Sh., Lyalyaeva, R.N., and Khamedova, L.Kh., Thermomechanical properties dynamically vulcanize thermoelastoplastics on the basis of polyolefins and ethylene-propylene-dien rubber, Kompozity Nanostrukt., 2018, vol. 10, no. 3 (39), pp. 123–128.
Allakhverdieva, Kh.V. and Kakhramanov, N.T., Kinetic regulations of crystallization of metal-polymer composites based on copper and low density polyethylene, Kompozity Nanostrukt., 2020, vol. 12, no. 1 (45), pp. 8–13.
D’yakonov, A.A., Danilova, S.N., Vasil’ev, A.P., Okhlopkova, A.A., Sleptsova, S.A., and Vasil’eva, A.A., Study of sulfur, diphenylguanidine and 2-mercaptobenzothiazole effect on physical and mechanical properties and structure of ultra-high molecular weight polyethylene, Perspekt. Mater., 2020, no. 1, pp. 43–53. https://doi.org/10.30791/1028-978X-2020-1-43-53
Mainikova, N.F., Yarmizina, A.Yu., Trofimov, D.V., and Kostromina, N.V., Investigation of the influence of carbon nanofillers on the properties of polypropylene-based composites, Plast. Massy, 2020, nos. 3–4, pp. 23–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Utegenov
Rights and permissions
About this article
Cite this article
Allahverdieva, K.V., Kakhramanov, N.T., Arzumanova, N.B. et al. Thermal Strain Properties of Metal-Polymer Composites Based on Low-Density Polyethylene and Copper. Inorg. Mater. Appl. Res. 13, 116–120 (2022). https://doi.org/10.1134/S2075113322010051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113322010051