Abstract
This review considers the development history of hydrogen energy generation in the USSR and Russia and its current state. The main domestic achievements in the field of production, storage, and transportation of hydrogen as well as its application to power engineering and other fields are summarized. The paper mainly focuses on various aspects to the application of nanomaterials and nanotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
TABLE OF CONTENTS
Introduction
1. Main application areas of hydrogen
2. Development of hydrogen energy in the USSR and Russia
Conclusions
INTRODUCTION
Maintaining high rates of development in the modern power and transport industries can lead humanity to a large-scale environmental crisis. Current trends in energy production involve replacing traditional fuels with more environmentally friendly and renewable ones. One promising energy carrier is hydrogen The transition to the hydrogen energy and economy is one of the most promising ways to preserve the sustainable ecosystem of planet Earth suitable for life.
In the 1970s, the world oil crisis gave a powerful impetus to the progress in research and development aimed at finding alternative fuels, primarily for transport engines. One environmentally friendly fuel that can be used as a propellant due to its unique physical and chemical properties is hydrogen [1, 2].
The possibilities for the industrial use of hydrogen have been known for a long time, and interest in them has periodically increased: in the 1970s, in connection with the oil crises, and in the 1990s and 2000s, due to growing problems with climate change resulting in tightening climate policy. This spurred relevant research and development (with a notable emphasis on transport) but large-scale practical implementation outside certain industrial sectors has not yet followed. Now, it seems that the situation may change, as more and more countries, in connection with the transition to green energy, are beginning to support hydrogen at the state level [3]. For example, in Europe, the US, Japan, China, and others, large-scale programs at the state level have been launched [4, 5].
Russia, possessing significant potential in the field of hydrogen energy production, has not yet joined this group, although some very successful projects have been implemented. However, the situation is currently changing dramatically. Russian President V.V. Putin has called for concentrating resources on the development of renewable energy sources (RES), hydrogen sources, and other clean sources that “will preserve nature for future generations for many millennia” [6]. The RF Ministry of Energy, on behalf of the government, has drawn up the Draft Energy Strategy of the Russian Federation for the period up to 2035 [7] and is preparing a program of work in the field of hydrogen energy production in the Russian Federation [8] with the involvement of leading researchers and specialists [9]. Major Russian and foreign companies are showing interest in cooperation and investment in this area. In January 2020, the implementation of the comprehensive program Atomic Science, Engineering, and Technology, developed by the State Atomic Energy Corporation Rosatom, aimed, inter alia, at the development of hydrogen energy, was launched in Russia. Its estimated funding will be 88.5 billion rubles by 2025.
Since June 2013, PJSC RusHydro has been cooperating with Kawasaki Heavy Industries and the government of the Magadan oblast on the implementation of a project for the industrial production of liquefied hydrogen in the Far East. In September 2019, as reported by Rosatom, Rusatom Overseas and Japan’s Natural Resources and Energy Agency signed a cooperation agreement for 2020–2021, which, in particular, concerns a pilot project for the export of hydrogen from Russia to Japan. The project includes potential hydrogen production by electrolysis for the Japanese market [10].
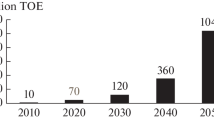
It should be noted that according to the estimates of the international Hydrogen Council, by 2050, hydrogen energy will account for 18% of all global energy needs [11].
1 MAIN APPLICATION AREAS OF HYDROGEN
It is difficult to imagine modern industry without the use of industrial gases at various stages of production. Today hydrogen is one of the three most in-demand gases in the industry, second only to oxygen and nitrogen.
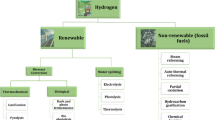
The application scope of hydrogen in Russia is quite wide: in the food industry (hydrogenation of fats), in the chemical industry (production of ammonia, hydrogen chloride, and methanol, hydrogenation of oils), in petrochemistry (for converting low-grade fuels into high-quality ones and for removing sulfur), in metallurgical production (creation of a protective-reducing atmosphere during high-temperature operations, for example, in the production of stainless steel, welding and cutting, production of molybdenum and tungsten, refining of chromium, production of hard alloys based on tungsten and molybdenum, and sintering of special powders), in the glass industry (creating a protective atmosphere in the float glass production), in power engineering (cooling of turbine generators and nuclear reactors), in the space industry (rocket fuel), etc. [12].
Petrochemical and chemical enterprises use mainly hydrogen obtained from steam reforming (especially if a direct access to natural gas is available). The semiconductor, glass, food industry, metallurgy, and power industries use electrolytic hydrogen benefiting from the simplicity and reliability of cells for the electrolysis of water, the high purity of the generated hydrogen and oxygen, the possibility of obtaining these gases under high pressure directly in the electrolysis cells, and a high degree of automation.
Currently, the world consumes about 75 million tons of hydrogen, which is then used in industry (mainly in oil refining and ammonia production). So far, more than three quarters of this volume is produced from natural gas, which requires more than 205 billion m3 of gas [13]. In 2013, ammonia production (55%) prevailed in the structure of hydrogen consumption, followed by oil refining (22%) and methanol production (13%). By 2020, consumption growth in these areas was projected to 2.8, 2.2, and 0.8 million tons, respectively [14].
In Russia, the main area of hydrogen consumption is the output of chemical products, primarily ammonia and methanol. The leaders in the consumption of hydrogen are the enterprises producing ammonia NH3. Currently, 28 enterprises in Russia use 2.46 million tons of hydrogen per year. Hydrogen consumption in synthesis of methanol (CH3OH) in 2013 amounted to 0.6 million tons [15]. The consumption of hydrogen in other segments of the chemical industry does not exceed 90 000 tons, 3% of the total consumption volume [5].
It should be noted that in the oil refining industry, the need for hydrogen in oil refineries also increases (hydrodesulfurization, hydrocracking of distillates, hydrotreating, etc.).
An important field of hydrogen application in metallurgy is the production of raw materials by direct reduction of iron ore. Now this process consumes about 330 000 tons of hydrogen per year. Significant volumes of hydrogen are consumed in the technological processes of rolling production (during heat treatment of cold-rolled steel). Here, the annual hydrogen consumption is about 15 000 tons [15].
In the power industry, hydrogen is still used mainly for cooling powerful turbine generators due to its high thermal conductivity and diffusion coefficient, as well as non-toxicity. According to statistics, in the power industry thermal and nuclear power plants annually consume about 4000–5000 tons of hydrogen [15].
In the food industry, hydrogen is used for the hydrogenation of oils and fats to obtain solid fats (margarine). The annual volume of hydrogen consumption by oil and fat plants is estimated to be at the level of 1500 tons [15].
Other consumers of hydrogen include the glass industry, ore-dressing plants, plants producing nuclear fuel, enterprises of the electronic and electrical industries, transport and gas companies, and producers of pharmaceuticals [15].
In this review, we would primarily like to consider the production and use of hydrogen for the purposes of energy generation.
2 DEVELOPMENT OF HYDROGEN ENERGY IN THE USSR AND RUSSIA
The total volume of hydrogen production in the world is currently estimated by various sources at 55–65 million tons, compared to 18 million tons in 1970 and 40 million tons per year in 1980 [16]. More than 90% of hydrogen is produced at the place of its consumption (so-called captive product), and less than 10% is supplied by specialized companies operating in the industrial gas market (Air Liquide, Linde, Praxair Inc., etc.) [17].
Russia’s share is approximately 8% [14]. The bulk of hydrogen (up to 96%) is usually produced by steam catalytic conversion of natural gas, as a result of which technical hydrogen is obtained with a purity of 96–97 vol %. This hydrogen is suitable for hydrotreating and hydrocracking [18].
However, the conversion of hydrocarbons causes air pollution. Massive emissions of CO2 into the atmosphere and their subsequent adverse impact on the environment call for alternative solutions.
As already noted, the energy crisis of the early 1970s, which in many respects had political and economic causes, gave a significant impetus to the research and development in the field of hydrogen energy production. At the same time, work in the field of hydrogen (atomic–hydrogen) energy in the USSR was started at the NRC (National Research Center) Kurchatov Institute (at that time, the Kurchatov Institute of Atomic Energy) on the initiative of Academician V.A. Legasov. For their successful development, a specialized subdivision was set up in the IAE, the Institute of Hydrogen Energy and Plasma Technologies (IHEPT) headed by Academician V.D. Rusanov, which made a great contribution to the development of this direction, not only in Russia but also abroad [19]. IAE specialists addressed a wide range of issues related to developing plasma-chemical and electrochemical methods for hydrogen production, hydrogen safety of nuclear power plants, etc. [20, 21].
The results of fundamental research carried out under scientific guidance of V.D. Rusanov successfully passed large-scale approbation at the industrial level. In particular, the centrifugal effect in a rotating plasma was the basis for creating the technology for plasma processing of hydrogen sulfide-containing gases and the basis for pilot production at the Orenburg gas processing plant. After the discovery of the phenomenon of plasma catalysis, development of plasma technologies for the conversion of hydrocarbon raw materials and fuels as well as various methods for hydrogen production were an important contribution to the progress of hydrogen energy technologies [22, 23].
The possibility of using nuclear energy in electrolysis, thermochemical and thermoelectrochemical processes has been intensively studied. Dollezhal Scientific Research and Design Institute of Energy Technologies (NIKIET) and IAE showed that decommissioned radioactive aggregates could be used for cheap water splitting. The preparation of solid hydrogen by throttling a hydrogen-helium mixture into a vacuum was also considered [24].
The above-mentioned plasma-chemical technologies aroused great interest in Russia and abroad. In Russia, such pioneers in this area of hydrogen energy can also be mentioned as the Keldysh Research Center, St. Petersburg Polytechnic University, RAS Institute of Electrophysics, and others [25].
The main advantage of the plasma method for the conversion of hydrocarbons, in addition to their high specific productivity, is the practical independence of the converter operation from the type of hydrocarbon (from methane to fuel oil, including oxygen-containing organic matter, waste, and various types of biofuels). Figure 1 shows a plasma converter developed by NIK NEP in cooperation with NRC Kurchatov Institute with a productivity of 7 m3 of synthesis gas per hour from gasoline. The disadvantage of plasma-chemical technologies is the additional power consumption (0.10–0.15 kW h/m3 H2) [26].
Fuel processor of the company NIK NEP [26].
It is extremely attractive to produce synthetic gas and hydrogen from organic household and industrial waste, as this makes it possible simultaneously to address the urgent issue of recycling rubbish. Highly efficient and environmentally friendly plasma and plasma-melt waste processing technologies developed at the Kurchatov Scientific Research Center make it possible not only to dispose of almost any type of waste without preliminary sorting but also to simultaneously produce up to 3500 kW h of electricity per ton of organic component through the use of synthetic gas (hydrogen) [19].
Nuclear technology provides virtually unlimited resources of cheap energy for the production of hydrogen, especially at night. Using the electricity generated at a nuclear power plant, it is possible, by electrolysis, to produce hydrogen and oxygen from water [27]. If electrolysis is carried out at high temperatures, then the thermal energy obtained from the nuclear reactor can replace part of the electricity, and the practical efficiency of the process will increase significantly. Such technologies, as well as technologies for converting natural gas using waste heat from reactors, were based on the developments of the SRC Kurchatov Institute. In thermochemical (and thermoelectrochemical) cycles of water decomposition using the thermal and electrical energy of reactors, hydrogen can be obtained at an efficiency of up to 50% [28].
Since the 1970s, Russia has had complete necessary scientific and technical substantiation and experimental confirmation for the projects of high-temperature helium reactors (HTHR) of nuclear power technology plants for the chemical industry and ferrous metallurgy [29, 30]. The HTHR projects were based on the development of hydrogen nuclear rocket engines [31]. It is reactors of this type that are of greatest interest for the production of hydrogen using waste heat and excess electricity at night, and interest in them has now increased.
One of the most innovative in this area was the international project for the development of a high-temperature modular helium reactor with a gas turbine (GT-MHR), which was designed jointly by Russian institutes (OJSC Afrikantov Experimental Mechanical Engineering Design Bureau (OKBM), SRC Kurchatov Institute, OJSC VNIINM, OJSC GI VNIPIET, FSUE NII NPO Luch) and the American company GA, managed and financed by the RF Ministry of Atomic Energy and the US Department of Energy. Framatom and Fuji Electric also collaborated with the project [31].
Hydrogen is of great interest for nuclear power plants as an energy accumulator and for load smoothing. The oxygen obtained in this case could become the basis for the production of ozone, for example, for the purification of industrial effluents in St. Petersburg [31].
Of particular interest are the R&D of hydrogen-oxygen steam generators, which were developed in the USSR in the late 1970s and early 1980s. Such systems make it possible to increase the temperature of steam for turbines and their efficiency [32].
In this case, in the off-peak hours, hydrogen and oxygen are produced by electrolysis of water, sent to the storage facility, and used during peak hours to generate additional power by burning hydrogen in oxygen and by additional superheating and increasing the steam flow before the turbine [33]. At the Joint Institute for High Temperatures of the Russian Academy of Sciences (JIHT RAS) and Keldysh Research Center, experimental samples of high-pressure hydrogen–oxygen steam generators of megawatt power class were created [34–39].
However, the practical implementation of this technology was slowed, largely due to accidents at nuclear power plants.
The first industrial production of liquid hydrogen in the USSR began in 1965 by the FKP SRC of the Rocket and Space Industry (FKP SRC RKP) as part of the KSVI-106 hydrogen test complex, which is currently the only functioning large-scale production of liquid hydrogen in Russia [40].
By 1980, in the USSR, the production of liquid hydrogen functioned in Chirchik, Zagorsk, and Dneprodzerzhinsk. Moreover, in Chirchik and Zagorsk there were installations for the production of hydrogen by electrolysis of water.
The introduction of hydrogen fuel, for example, in aviation was held back by the need for a more confident solution to the issue of operating hydrogen systems and their ground handling, as well as reducing the cost of hydrogen.
However, on the initiative of S.P. Korolev, large-scale effort was undertaken in the USSR 50 years ago to create equipment and technologies for using effective hydrogen-oxygen rocket fuel in rocket and space technology [41]. This necessarily required designing vehicles for the transportation of liquid hydrogen [42, 43].
One of the main ways of transporting liquid hydrogen is in rail tank cars. The first unit for the transportation of liquid hydrogen was the TRZhV-20 tank truck, created in 1966 at the Uralkriomash enterprise in Nizhny Tagil [44].
Since then, the tank has been modernized several times in order to improve the safety of liquid hydrogen transportation. The currently produced model of the tank has a number of advantages over its predecessors: the transported mass of hydrogen is increased and the losses during transportation are reduced [14].
The current demand for liquid hydrogen in Russia is still quite limited, although it is planned to create the latest launch vehicles and upper stages of space complexes, using liquid hydrogen as fuel.
It should be noted that the situation with hydrogen production in Russia is developing somewhat differently from that in foreign countries. First, 40% of hydrogen in the world is produced by coal gasification while in Russia CTL technology (production of liquid fuel from coal) is not used now. Second, the market for commercial hydrogen is well developed in world practice, while in Russia this direction is just emerging. Third, the main role in the structure of world hydrogen consumption is played by oil refining, and in Russia, it is through the output of chemical products, primarily ammonia and methanol. The main hydrogen producer in Russia in 2014–2016 was Uralelectromed’ OJSC. In 2016, the share of this manufacturer was 56% of the physical output. The second largest producer in 2016 was KMEZ CJSC with 23% of the physical output, followed by MMC (mining and metallurgical company) Noril’sk Nickel’ PJSC with 21% [45].
In Russia, developments are already underway to create an integrated infrastructure for the production and supply of hydrogen. It should be noted that an active part here is taken by the Linde Gas Rus company [14].
To create a carbon-free and environmentally friendly economy, it is considered feasible to use water electrolysis to produce hydrogen, as well as to develop solutions for distributed generation on demand and on site [46–48].
However, the hydrogen produced from natural gas, which Russia is rich in, is several times cheaper than electrolytic hydrogen. Therefore, the main method for producing hydrogen in Russia at present is catalytic conversion of natural gas with water vapor. A significant contribution to the development of technologies for the conversion of natural gas and other types of fossil fuels has been made by the Boreskov Institute of Catalysis (Siberian Branch of the Russian Academy of Sciences) [49, 50].
According to the calculations performed by the analysts of the DISCOVERY Research Group, the volume of the hydrogen market in Russia in 2016 exceeded 93 000 tons, a value equivalent to 6.81 billion US dollars. The growth rate of the market volume amounted to 4.9% of the market physical output and 7.6% of its total worth [45].
Unfortunately, the foreign economic activity with hydrogen of the Russian Federation is characterized by insignificant export–import operations. There are only small supplies of bottled gas that do not significantly affect the domestic market. The volume of hydrogen imports to Russia in 2016 amounted to 1.97 tons [45].
The volume of hydrogen exports from Russia in 2016 amounted to 645 kg [45]. The main buyers of domestic production are the CIS countries (primarily Kazakhstan). However, let us return to the history of the hydrogen energy development in Russia (USSR) and its current state.
Since the 1920s and up to the early 1940s, significant and extensive investigations into the reaction of hydrogen combustion in oxygen and air in various conditions were carried out by Russian scientists belonging to the school of N.N. Semenov [51]. The discovery of branched chain reactions in 1928 opened up broad perspectives for the control of chemical processes important for hydrogen power engineering [52].
In the Soviet Union, the study of hydrogen as a motor fuel began in 1935 at Moscow Lomonosov Mechanical Engineering Institute (MMMI) (now Bauman Moscow State Technical University). Professor V.I. Soroko-Novitskii and his colleagues published the research report “On the possible use of an engine running on hydrogen.” In this work, the effect of hydrogen additions to gasoline on the ZIS-5 engine was investigated [53]. There are also well-known works on the use of hydrogen as a fuel, carried out by F.B. Perelman [54].
The real practical application of hydrogen as a motor fuel began in 1941 in besieged Leningrad during the Great Patriotic War. Technician-lieutenant B.I. Shelishch proposed to apply hydrogen that was already used up in aerostats as a motor fuel for the engines of the GAZ-AA car [55, 56]. In total, in 1941–1942, about 200 trucks were converted to hydrogen. Bringing down aerostats which had partially lost their lift required a lot of effort. This operation was carried out using a mechanical winch installed on a GAZ-AA vehicle. The internal combustion engine (ICE) rotated the winch to lower the aerostats. In besieged Leningrad, several hundred air defense posts were equipped with GAZ-AA vehicles running on hydrogen [57].
Figure 2 shows a hydrogen-filled aerostat in the background, being brought down to the ground, from which hydrogen is pumped into a gas holder located in the foreground [54]. The used-up hydrogen from a cloth gas tank was supplied to the intake manifold of a GAZ-AA engine through a process plug. Bypassing the carburetor, gas entered the working cylinders. The dosage of hydrogen and air was provided by a throttle valve or an accelerator pedal.
GAZ-AA car running on hydrogen [58].
With regard to using hydrogen as a fuel for internal combustion engines, we should recall the work of the Central Scientific Research Automotive and Automotive Institute (NAMI), the Institute of Mechanical Engineering Problems of the Academy of Sciences of the Ukrainian SSR (IPMASh Academy of Sciences of the Ukrainian SSR), NPO KVANT, the Sector of Mechanics of Inhomogeneous Media of the USSR Academy of Sciences (SMNS AS USSR), higher technical college-plant at ZIL, etc.
Along with the scientific aspects of theoretical study of internal combustion engines running on hydrogen and its additives to gasoline, much attention was paid to investigations into the safety of using hydrogen as a fuel for vehicles, as well as ways of storing it on board an automobile [54, 55].
The pilot operation of Volga petrol–hydrogen vehicles was begun in Kharkov in 1980 and showed that transferring part of the city’s motor transport to petrol-hydrogen mixtures was a promising project. In 1986, the USSR Ministry of Automotive Industry made decision on the production and subsequent operation in the Soviet cities of an experimental batch of urban minibuses RAF (200 units) (Fig. 3) running on petrol-hydrogen mixtures. However, this decision was not implemented due to political and economic unrest [51].
In 2006, the National Association for Hydrogen Energy (NAVE) presented a Gazelle vehicle with an internal combustion engine running on hydrogen-benzene fuel compositions. The car was developed with the participation of the Moscow Power Engineering Institute (MPEI (TU)), Avtokombinat No. 41 (Moscow), and Audit-Premier LLC.
In 2007, NAVE, in cooperation with JSC AVEX, MPEI (TU), and JSC Avtokombinat No. 41, presented another version of the hydrogen car (Fig. 4). The payload of the car is 2000 kW. The power of the electric drive is 65–70 kW, the power of the internal combustion engine is 10 kW. The drive range of the vehicle is 200 km [60].
(Color online) NAHE (National Association for Hydrochloric Energy) car at the exhibition Innovative Achievements held within the framework of the forum, 2006 [59].
In the early 1980s, Kuznetsov Design Office developed aircraft engines intended for Tupolev passenger aircraft. These hydrogen-fueled engines have passed bench and flight tests. Tupolev Design Bureau on the basis of the Tu-154 serial passenger aircraft created the experimental hydrogen aircraft Tu-155 (Fig. 5). On April 15, 1988, the Tu-155 aircraft made its first flight. It underwent an extensive set of tests, during which 14 world records were set. It carried out about 100 long-term flights on liquid hydrogen.
(Color online) TU-155 aircraft with hydrogen engines and the crew of the Tu-155 aircraft [61].
The work on the Energiya-Buran complex (Fig. 6) took about 10 years. The NPO (R&D production center) Molniya became the lead product developer of the Buran orbital spacecraft. An RD-0120 liquid-propellant rocket engine operating on liquid hydrogen and liquid oxygen was installed as an engine on the second stage of the Energiya launch vehicle. The space flight of Buran took place on November 15, 1988. The automatic flight of Buran was entered into the Guinness Book of Records and is still unsurpassed [62].
(Color online) Space shuttle Energiya-Buran [63].
Currently, one way to ensure a phased transition to hydrogen energy in the transport industry is the production of synthesis gas and/or hythane (a mixture of hydrogen and carbon monoxide or methane). Moreover, in the case of synthesis gas, its production is possible directly on board a vehicle using part of the main (gaseous or liquid) hydrocarbon fuel. The use of syngas and hythane can have a positive effect on the parameters of internal combustion engines. Plasma-chemical fuel conversion was discussed earlier, but at the Siberian State University of Railways, together with the Boreskov Institute of Catalysis SB RAS, experimental studies were carried out on the use of additives of hydrogen-containing synthesis gas on diesel internal combustion engines [64]. One of the options is an on-board synthesis gas generator, consisting of a catalytic reactor for air conversion of hydrocarbon fuel into synthesis gas using a catalyst based on a metal mesh carrier with an active component based on nickel nanoparticles [65]. This institute also carried out a large number of developments on converting various types of fuel using highly efficient nanostructured catalysts [49, 50].
Operational tests were carried out in 2008 and 2009 in real conditions: the GAZ-2310 Sobol and Barguzin vehicles, equipped with on-board synthesis gas generators, took part in the Blue Corridor rally organized by Gazprom along the St. Petersburg–Moscow and Moscow–Sochi routes. Tests have shown that additives of synthesis gas to the main fuel significantly reduce the emissions of CO, CH, and NOx and provide an overall reduction in fuel consumption.
Studies [66] have shown that the air conversion of natural gas is the simplest and cheapest way to obtain synthesis gas on board a vehicle. However, the use of synthesis gas requires compliance with a standard proportional composition of the fuel mixture since exceeding the permissible level of hydrogen content in the combustion chamber of the internal combustion engine causes the permissible combustion temperature limits to be exceeded and leads to a change in the nature of the vibration load [64, 67].
In the 1970s, several scientific research organizations in the USSR began work on the use of hydrogen as a fuel, not only for internal combustion engines but also for electrochemical generators (ECG) based on fuel cells (FC) [68].
In the USSR, the first publications on alkaline fuel cells (AFC) appeared in 1941, and the first practically significant developments were carried out in the 1960s at the Ural Electrochemical Plant (UEKhK) and at Korolev Rocket and Space Corporation Energiya (RSC Energiya) for spaceships.
In 1969–1975, UEKhK developed the ECG Volna-20 for RSC Energiya (Fig. 7), with an AFC battery with a circulating electrolyte, and set up their pilot production [69].
(Color online) Hydrogen–oxygen electrochemical generator Volna-20 [70].
In the late 1980s, UEKhK developed an AFC for the Buran spacecraft, the electrochemical generator Foton (Fig. 8), with a nominal power of 10 kW with the possibility of increasing power, combining several modules. Generator efficiency was 60%, and the guaranteed service life was 2000 h [71], which, however, was not reached during the Buran flight.
Alongside the development of AFC for spacecraft. starting from the first half of the 1970s, Soviet engineers and researchers worked on creating a submarine with power plants on AFC (CDB Lazurit, NPO Kvant, SKB Kotlostroeniya, CDB MT Rubin, and later they were joined by the AFC developers for spacecraft).
For the submarine Katran of project 613E, a power plant with an AFC with a capacity of 280 kW was created. The reagents, liquid oxygen and hydrogen, were stored in cryogenic containers outside the main hull of the submarine.
In 1988, the Katran submarine successfully passed tests and, for the first time in the world, confirmed the fundamental possibility of creating and effectively using FC for this type of equipment. Unfortunately, work on the creation of power plants for submarines in the USSR was suspended, in contrast to similar developments abroad [71].
In 2001 and 2003, the Ural Electrochemical Plant, RSC Energiya, and AvtoVAZ demonstrated the Lada ANTEL-1 and Lada ANTEL-2 vehicles with their electric motor and a power supply unit based on the Foton ECG at Moscow motor shows, as shown in Fig. 9. In the first system, oxygen served as an oxidizer, and in the second, it was air purified from CO2. On one filling of hydrogen stored in cylinders, these cars could travel 300 km [72]. Certainly, the cost of such a system (taking into account its spacecraft origin) was excessively high.
(Color online) VAZ vehicles on fuel cells ANFCL-1 (a) and ANFCL-2 (b) [73].
In the 1970s and 1980s, NPO Kvant, together with the Riga bus plant RAF, created the world’s first experimental hydrogen minibus Kvant-RAF with a combined power plant based on a hydrogen-air FC with a capacity of 2 kW and a nickel-zinc storage battery (5 kW h), which was presented at the Moscow International Exhibition Elektro-82 and was experimentally commissioned and operated [51].
Since 1966, RSC Energiya has been developing phosphoric acid FCs for the Soviet lunar program. From 1987 to 2005, Energiya produced about 100 fuel cells, which worked in total about 80 000 h [74]. However, further developments in this area were abandoned.
In 1999, modules of two nickel-hydrogen storage batteries were created for the Yamal spacecraft (Fig. 10), i.e. hydrogen was used not only for FC but also for their analogs [72].
The first prototype of an electrochemical generator based on solid oxide fuel cells (SOFC) with a capacity of 1 kW was created in 1989 at the Institute of High-Temperature Electrochemistry of the Ural Branch of the Russian Academy of Sciences (IVET). It was the most powerful SOFC in the European territory, but during the crisis of 1990s, this work slowed down and was only resumed in 2008 in cooperation with Rosatom’s subsidiary, the TVEL fuel company. Gazprom, in particular, is interested in SOFC developments, since autonomous energy sources based on natural gas are promising for power supply systems for gas pipelines, including cathodic protection stations. At the sites of OJSC Gazprom transgaz Yekaterinburg, a 1.5 kW power plant has successfully operated for more than a year [75]. This institute has successfully continued research work in the field of SOFC [76, 77].
Since the end of the 1980s, work on the development of SOFC-based power systems has also been successfully carried out at Zababakhin All-Russian Scientific Research Institute for Technical Physics (RFNC VNIITF, Snezhinsk). Developments were carried out on all structural designs of SOFC (tubular, planar, block), and the attained capacity was 1–2.5 kW.
In 2005, VNIITF also manufactured an SOFC battery module for cathodic protection systems for gas pipelines. The module was based on a tubular SOFC with a supporting electrolyte. The operating temperature of the SOFC is 950°С. The fuel utilization ratio reached 86% [78]. Since 2007, within the framework of the Federal Target Program, significant successes in this area have been achieved by FSUE TsNII SET with the participation of RFNC VNIITF.
In 1994, OJSC Special Design Bureau for Boiler Building (SKBK) developed a project for a combined power generating facility electric power plant (PGF) with an electrochemical generator based on SOFC and an end steam and gas turbine cycle.
TVEL Corporation (ChMZ OJSC (Chepetsk Mechanical Plant OJSC) has a pilot site for the production of zirconium dioxide, powders for plasma spraying and products from solid electrolytes.
The comprehensive development program of the TVEL Corporation until 2020 provided for a significant increase in production and expansion of the product range.
In recent years, significant progress has also been achieved at the Institute of Solid State Physics, Russian Academy of Sciences (Chernogolovka), where the development of planar SOFCs is being successfully carried out [79].
In Russia, research and development of polymer membrane fuel cells (PEMFC) is carried out at IPCPhT RAS, SRC Kurchatov Institute, RFNC-VNNIIEF, TsNII SET, as well as in several other research organizations.
Development and pilot production of the polymer electrolyte membrane (PEM) were carried out in the mid-1970s in Russia at Plastpolymer OJSC [82]. However, at the moment the production of PEM (and membranes based on it) has been discontinued.
In recent years, research in hydrogen energy in Russia have been focused mainly on the development of new materials, nanostructured catalysts, membrane-electrode assemblies, and related technologies. In particular, at the SRC Kurchatov Institute (Moscow), MPEI (TU) (Moscow), Institute of Problems of Chemical Physics of the Russian Academy of Sciences (Chernogolovka), Ioffe Physico-Technical Institute RAS (St. Petersburg), Institute of Catalysis SB RAS (Novosibirsk), Frumkin Institute of Physical Chemistry and Electrochemistry RAS (Moscow), and others, a number of fundamental and applied research projects have been successfully completed [83].
In 2015, at the branch TsNII SET of FSUE Krylov State Scientific Center, a prototype of PEMFC-based power plant was developed and manufactured, operating on converted diesel fuel and equipped with a system for recycling waste oxidation products and intended for the new-generation marine engineering facilities [84]. Several experimental hybrid solar/wind/hydrogen power systems for decentralized power supply have been developed at SRC Kurchatov Institute [85]. Also worth mentioning is the unmanned aerial vehicle (Fig. 13) [86], developed in 2013 at SRC Kurchatov Institute, together with the United Aircraft Corporation (UAC). Subsequently, great advances in this area were achieved at the Institute for Problems of Chemical Physics, Russian Academy of Sciences (IPCP) [87]. Currently, AT Energy [88] and BMPower [89] are successfully producing PEMFCs for unmanned aerial vehicles. Original nanostructured electrocatalysts on nanocarbon carriers have been developed [90–101].
(Color online) First Russian drone equipped with PEMFC, 2013 [86].
For FC, hydrogen production by the electrolysis of water, which ensures high purity of hydrogen, is certainly optimal. In Russia, alkaline water electrolysis cells have been successfully produced by the Uralkhimmash enterprise since the 1950s; electrolysis cells with asbestos diaphragms are shown in Fig. 11, with a hydrogen capacity from 4 to 250 nm3/h (SEU-4, 10, 20, and 40, with a hydrogen capacity of 4, 10, 20, and 40 nm3 H2/h, respectively) operate at pressures up to 1.0 MPa, and the electrolysis unit FV-500 with hydrogen capacity up to 500 nm3/h operating at atmospheric pressure [2].
Unfortunately, the production of domestic alkaline water electrolysis cells has actually been discontinued since the company could not withstand competition with foreign developers.
However, research and development of water electrolysis systems with PEM have been carried out in Russia for more than 20 years by FSBI SRC Kurchatov Institute, FSUE Krasnaya Zvezda, and other organizations. Electrolysis cells have been created with levels of PEM productivity from a few milliliters to several cubic meters of hydrogen per hour (Fig. 12) for various purposes and pressures up to 150 bar [2]. Much attention has been paid to the development of nanostructured catalysts and nanostructured membrane-electrode units (MEU) based on platinum and iridium [46, 102–110].
The creation of hydrogen storage and transportation systems is of key importance, and in many respects it is a limiting factor in the development of hydrogen energy production. Storage on board vehicles is crucial for the development of hydrogen technologies in transportation. In the 1980s, prototypes of storage systems (metal hydride, gas cylinder, and cryogenic systems) were developed in the USSR. We should also recall that hydrogen storage systems were developed at Podgorny Institute of Mechanical Engineering Problems using low-temperature and high-temperature hydrides of intermetallic alloys based on FeTiVa and Mg2Ni [48], with light composite super-balloons with a mass hydrogen content of about 8–10% at pressures of 300–500 atm [51]. In the 2000s, at the Moscow Aviation Institute, in cooperation with RSC Energiya and OJSC Splav, at the request of the Ministry of Emergency Situations, cylinders with a capacity of 10 L and a mass of 2.5 kg designed for a pressure of 30 MPa were manufactured and tested [111].
In 2001, LLC NPO Poisk (St. Petersburg), by order of the companies CJSC LEM and AO Avtovaz developed metal-plastic motor transport cylinders with a capacity of 65 L, a working pressure of 22 MPa, and a mass of 35 kg for compressed natural gas (methane) and hydrogen intended for passenger cars. In 2003, by order of RSC Energiya and JSC Avtovaz, work began on the development of a cylinder for storing compressed hydrogen onboard vehicles of the ANFCL family with a working pressure of 39.2 MPa [112].
However, it is metal hydrides for transport and stationary applications that have especially motivated intensive research and development. Work in the USSR on this discovery of the phenomenon reversible absorption of hydrogen by alloys and intermetallic compounds was discovered [113–115] in the end of 1960s. Three leading organizations collaborated within the consortium for the creation of metal hydride storage systems: IAE, NAMI, and Moscow State University [54]. In 1979, application nos. 26 3140 and 263 141 were registered in the State Register of Inventions of the USSR with the priority of invention on June 22, 1978, which were among the first inventions in this area in the USSR and in the world [54].
The storage of hydrogen in hydrides has a number of advantages over storage under pressure or in a liquefied form, namely, the energy consumption is reduced, transportation is simplified, and, most importantly, storage safety is increased. Hydrides provide a high bulk density that is comparable to liquid hydrogen but do not require low temperature maintenance. In some cases, high mass density can also be achieved [116].
Currently, in Russia at the IPCP (Institute for Problems of Chemical Physics) RAS, a variety of hydrogen-storage materials have been developed (Fig. 14) and various types of reusable metal hydride batteries have been created on their basis [114, 117–120]. The JIHT (Joint Institute for High Technologies) RAS is also developing hydride materials But special attention is paid to the creation of power plants based on them [118, 121–124].
(Color online) Materials for metal hydride storage of hydrogen developed at IPCP RAS [120].
The possibilities of creating cryogenic storage systems for liquid hydrogen on board a vehicle were also investigated. An experimental RAF vehicle with a cryogenic hydrogen storage system was tested at the NAMI test site in the 1980s, and based on the results of this work, an experimental cryogenic tank for storing liquid hydrogen on board the vehicle was developed at NPO Cryogenmash. However, after 1985, this work was gradually curtailed [51].
In the USSR, OJSC Cryogenmash, OJSC Geliymash, OJSC Uralkriomash, the institutes of the Russian Academy of Sciences, the Scientific Research and Design Institute of the Nitrogen Industry and Organic Synthesis Products (OJSC GIAP), and others created an infrastructure to supply the country with liquid hydrogen, primarily for rocket and space programs. Liquefaction units with a capacity of 180 kg/h of parahydrogen were developed, manufactured and put into operation (NIIkhimmash in Sergiev Posad, PA Electrokhimprom in Chirchik) [125]. We should also mention the Cryogenic Center at Baikonur, which provided the entire cycle of operations with the Energiya–Buran system [126].
Nanostructured porous materials can also be used to store hydrogen in molecular form. The main materials capable of providing effective physical sorption of hydrogen are carbon nanostructures, BN nanostructures, metal-organic framework systems (MOF), and porous organic polymers. Sorbents should have a large specific surface area, have an ordered pore system, and be light enough but stable. This problem has also received much attention in domestic developments [116].
Also noteworthy is also the original concept of aluminum–hydrogen energy, where hydrogen is produced by the reaction of aluminum with water [127, 128]. In this case, aluminum acts as an intermediate energy carrier in stationary, transport, and portable applications. The direct electrochemical oxidation of aluminum and its nanostructural modifications [129] in air–aluminum fuel cells is also promising. However, aluminum–hydrogen technologies are also very attractive, since there is a possibility of generating additional heat energy [130].
Since 2006, JIHT RAS has successfully developed prototypes of compact power supplies for portable electronics based on the free-breathing hydrogen–air fuel cells of an aluminum-water microgenerator of hydrogen [131, 132].
The technologies under development for aluminum–hydrogen energy can find their place in the future hydrogen economy of the future as an efficient and safe way of transporting the source of hydrogen and stored energy [130].
Of particular interest are the developments of hydrogen storage systems in glass/quartz capillaries carried out at SRC Kurchatov Institute, which make it possible to achieve a mass fraction of the stored hydrogen of more than 10% [133, 134], but these developments have not yet been brought to practical implementation.
In recent years, SRC Kurchatov Institute has been actively developing the use of the hydrogen cycle (implemented in the electrolysis cell water–hydrogen storage system–fuel cell) for storing energy generated by RES-based power plants, including in the Far North and the Arctic zones. Effective technical solutions have been developed for the use of electrochemical hydrogen systems for energy storage with an efficiency of cyclic conversion of more than 40%. It has been shown that the storage of energy in the form of hydrogen is environmentally safe and significantly surpasses traditional storage batteries in terms of capacitive performance, which is especially important for the prolonged absence of energy from renewable energy sources, for example, under polar night conditions and in calm weather. It has been confirmed that the use of hydrogen electrochemical systems (water electrolysis cells and FC) with an appropriate hydrogen storage system makes it possible to store a significant amount of energy and smooth out daily, monthly, and seasonal irregularities in the energy supply from RES [135].
An important advantage of a hydrogen energy storage system is its ability to increase the energy intensity of hydrogen storage by attaching additional gas cylinders or using larger gas tanks. Other undoubted advantages of hydrogen storage in relation to traditional electrochemical batteries are ease of disposal and the absence of toxic components. Important advantages of hydrogen storage systems are the absence of self-discharge, self-balancing, modular architecture, process reversibility, and a large number of charge/discharge cycles. It is noteworthy that with an increase in storage capacity, the specific cost of the system decreases, since only additional volume for storing hydrogen is required to store additional energy. The advantage of power plants with hydrogen storage in terms of weight and size compared to traditional batteries is especially significant when creating remote systems in hard-to-reach areas, where equipment is delivered, for example, by helicopters [136].
The SRC Kurchatov Institute has developed, tested and implemented a line of power plants based on renewable energy sources and hydrogen energy generation and storage systems. A feature of the created power plants is their northern climatic design, which ensures efficient energy supply to various consumers in the polar night, low temperatures and other factors inherent in the northern regions of countries such as Russia, Norway, Finland, Denmark, Ireland, USA, and Canada. A fundamentally important advantage of the created power plants is abandoning use of diesel generators as auxiliary energy sources and their replacement with environmentally friendly ECGs based on FC [137]. The capacity range of the developed autonomous power plants and their modular scalable architecture allow the power supply needs of both small remote systems, such as meteorological stations, cellular communication stations, sea and river beacons, and other navigation systems to be met, as well as more energy-intensive consumers, such as local residential and industrial facilities, charging stations for electric vehicles, etc. The combined use of fundamentally different primary energy sources, such as sun and wind, as well as an electrochemical system for storing and generating electricity, can significantly increase the stability of power supply to autonomous off-grid consumers [138].
It should be emphasized that developing the regulatory framework for hydrogen power engineering (GOSTs, codes, and standards) was practically neglected in the USSR but now this issue has attracted the necessary attention [139].
Thus, NAHE (National Association for Hydrochloric Energy) believes that establishing a national technical policy in the field of hydrogen technologies and FC based on a modern national regulatory and technical base harmonized with international ISO and IEC standards, as well as the organization of international cooperation within the framework of the International Association for Hydrogen Energy (IAHE), necessary for the development of hydrogen technologies that provide favorable conditions for the formation of a hydrogen economy, the popularization of hydrogen energy, and the organization of hydrogen education, are the main priorities for the coming years [140].
The IAHE also included scientists from the USSR, whose work was coordinated by the Commission of the USSR Academy of Sciences on Hydrogen Energy and the IAE. The head and leader of the hydrogen movement in the USSR was Academician V.A. Legasov. Under his leadership, since 1978, Atomizdat has published the collections Atomic-Hydrogen Energy and Technology and held All-Union seminars on atomic-hydrogen energy based on the IAE.
The first all-Union school on this subject, under the supervision of A.M. Styrikovich, took place in 1979 at the Donetsk Polytechnic Institute (now Donetsk National Technical University). In 2004, the rector of MIREA Russian Technological University, Academician A.S. Sigov, and Professor A.A. Evdokimov (since 2007 the head of the Department of Chemistry) were actively promoting hydrogen education. They have a special role in the development of universal hydrogen education in Russia [141].
First, they supported additional specialization in hydrogen energy at the Department of Informatization of Journalism at MIREA as part of hydrogen education, and then the organization of a student hydrogen club as one of the forms of non-academic education. The MIREA Hydrogen Club, founded by students in 2004, participates in the organization and holding of the international symposium Hydrogen Energy of the Future and Platinum Group Metals in the CIS Countries, publishes the illustrated newspaper Hydrogen Gas Turbine, the magazine Hydrogen All-Education, etc. [142].
CONCLUSIONS
Hydrogen energy generation, as a scientific and technical direction covering the problems of obtaining, storing, transporting, and using hydrogen, began in the USSR in the 1970s and successfully developed over the following decades, in line with world trends. At the same time, several unique domestic developments appeared that have no analogues in the world. In modern Russia, hydrogen energy technologies are becoming extremely popular in connection with the renaissance of atomic–hydrogen energy (which is based on the production of hydrogen using the energy of nuclear power plants) and the rapid development of renewable energy (requiring highly efficient energy storage systems). There has been a steady trend towards the transition from hydrocarbon energy to environmentally friendly hydrogen energy, within which hydrogen, a universal energy carrier, is becoming an integral part of the development of modern society. The use of nanomaterials and nanotechnologies is an innovative component of hydrogen energy technologies and opens up prospects for large-scale implementation.
REFERENCES
S. I. Kozlov and V. N. Fateev, Hydrogen Energy: Current State, Problems, Prospects (Gazprom VNIIGAZ, Moscow, 2009) [in Russian].
S. A. Grigor’ev, V. I. Porembskii, V. N. Fateev, et al., Transp. Al’tern. Topl., No. 3 (2008).
Analytical Center for the RF Government, “Hydrogen economy: New hopes for success” (2019). http://ac.gov.ru/files/publication/a/22861.pdf.
Vestn. Atomproma, No. 9 (2019). http://atomvestnik.ru/wp-content/uploads/2019/12/internet_9_isp.pdf.
N. Grib, Neftegaz. Vertikal’, No. 19, 6 (2019). http://www.ngv.ru/upload/iblock/224/224b8a5647503ebe18e4180a43431d41.pdf.
V. V. Putin, Report on Investment Forum “Russia Calls!”, Nov. 20, 2019. https://tass.ru/ekonomika/7161985.
Draft Energy Strategy of the Russian Federation for the Period up to 2035. https://minenergo.gov.ru/node/1920.
P. Sorokin, Report on Meeting of the Working Group on the Development of Hydrogen Energy in the Russian Federation. https://minenergo.gov.ru/node/16922.
Analytical Center for the RF Government, Energet. Byull., No. 73 (2019). http://ac.gov.ru/files/publication/a/22861.pdf.
Energy Holding RusHydro. http://www.rushydro.ru/press/news/104245.html.
A. Nekrasov, Hydrogen Prospects. https://plus.rbc.ru/news/5dfc2e607a8aa9fb3e34dbf3.
V. Fateev and S. Grigoriev, “H2 technologies in Russia,” in Hydrogen in an International Context: Vulnerabilities of Hydrogen Energy in Emerging Markets (River Publ., 2017), p. 171.
A. Egorov, Korp. Zh. Gazprom, No. 9, 42 (2019). http://www.gazprom.ru/f/posts/91/915005/gazprom-magazine-2019-9.pdf.
CREON Energy. https://neftegaz.ru/analisis/oil_gas/328834-po-protorennoy-dorozhke/.
Air Tekhnik, Applications of Hydrogen. https://airtechnik.ru/blog/sfery-primenenija-vodoroda/.
E. E. Shpil’rain, S. P. Malyshenko, and G. G. Kuleshov, Introduction to Hydrogen Power Engineering (Energoatomizdat, Moscow, 1984) [in Russian].
T. Mitrova, Yu. Mel’nikov, and D. Chugunov, A Hydrogen Economy—the Path to Low-Carbon Development (Tsentr Energet. Mosk. Shkoly Upravl. Skolkovo, Moscow, 2019) [in Russian]. https://energy.skolkovo.ru/downloads/documents/SEneC/Research/SKOLKOVO_EneC_Hydrogen-economy_Rus.pdf.
N. L. Solodova, E. I. Cherkasova, I. I. Salakhov, and V. P. Tutubalina, Probl. Energet. 19 (11–12), 39 (2017).
M. V. Koval’chuk, “Hydrogen energy as a component of the fuel and energy complex of Russia,” Fed. Sprav. Topl.-Energet. Kompleks Ross., No. 10, 369 (2011). http://federalbook.ru/files/TEK/Soderzhanie/Tom%2010/V/Kovalchuk.pdf.
Special Issue, To the 70th Anniversary of the Kurchatov Institute (2013). http://www.kuriermedia.ru/data/objects/2225/80_Kurchatov_institut.indd.pdf.
V. A. Stukalov, S. A. Subbotin, and T. D. Shchepetina, “Hydrogen energy and technogenic hydrogen cycle as a basis for consolidated development of fuel producing and nuclear energy industries,” in Proceedings of the Joint Symposium on Energy Links between Russia and Eastern Asia: Development Strategies in the XXI Century, Aug. 30–Sept. 2, 2010, Irkutsk, Russia (Kurchatov Inst., Moscow). http://isem.irk.ru/symp2010/papers/RUS/S3-09r.pdf.
V. D. Rusanov, A. A. Fridman, and G. V. Sholin, Sov. Phys. Usp. 24, 447 (1981). https://doi.org/10.1070/PU1981v024n06ABEH004884
V. K. Zhivotov, Innovatsii, No. 11 (98), 112 (2006).
Hydrogen Again. http://www.buran.ru/htm/12-3.htm.
K. P. Latyshenko and S. A. Garelina, Izv. MGTU, No. 3 (17), 63 (2013).
M. F. Krotov, S. V. Korobtsev, and V. N. Fateev, Energ.: Ekon., Tekh., Ekol., No. 12, 26 (2011).
A. I. Miller, Nucl. Eng. Int. 50 (612), 16 (2005).
N. N. Ponomarev-Stepnoi, A. Ya. Stolyarevskii, and V. P. Pakhomov, Atomic-Hydrogen Energy: Systemic Aspects and Key Problems (Energoatomizdat, Moscow, 2008) [in Russian].
A. P. Aleksandrov and N. N. Ponomarev-Stepnoi, in 20 Years of Nuclear Power (Atomizdat, Moscow, 1974), p. 205 [in Russian].
V. A. Legasov, N. N. Ponomarev-Stepnoi, A. N. Protsenko, et al., Vopr. At. Nauki Tekh., Ser. At.-Vodor. Energet., No. 1, 5 (1976).
N. N. Ponomarev-Stepnoi and A. Ya. Stolyarevskii, Energiya, No. 1, 3 (2004).
The main problems of hydrogen energy, Report of the Commission of the USSR Academy of Sciences on Hydrogen Energy (IVTAN, Moscow, 1978).
S. P. Malyshenko, O. V. Nazarova, and Yu. A. Sarumov, At.-Vodor. Energet. Tekhnol., No. 7, 106 (1986).
S. P. Malyshenko, Ross. Khim. Zh. 41, 112 (1997).
S. P. Malyshenko, V. I. Prigozhin, and V. S. Rachuk, Sovrem. Mashinostr., Nos. 2–3 (8–9), 54 (2009).
S. P. Malyshenko, Research and Development of JIHT RAS in the Field of Hydrogen Energy Technologies JIHT RAS. Results and Prospects (OIVT RAN, Moscow, 2010), No. 1 [in Russian].
I. N. Bebelin, A. G. Volkov, A. N. Gryaznov, and S. P. Malyshenko, Therm. Eng. 44, 657 (1997).
S. P. Malyshenko, O. V. Nazarova, and Yu. A. Sarumov, Teploenergetika, No. 10, 43 (1986).
S. P. Malyshenko, Al’tern. Energet. Ekol. 95 (3), 10 (2011).
Hydrogen Production of United Rocket and Space Corporation. http://www.nic-rkp.ru/default.asp?page=productions_hydrogen_production.
Korolev Rocket and Space Corporation Energia, 1946–1996 (Menonsovpoligraf, Moscow, 1996) [in Russian].
Uralkriomash—Vagonki Small Land (SV-96, Yekaterinburg) [in Russian].
A. M. Arkharov and I. D. Kunis, Cryogenic Filling Systems of Launching Rocket and Space Complexes, Ed. by I. V. Barmin (MGTU im. Baumana, Moscow, 2006) [in Russian].
O. Ya. Cheremnykh, Inzh. Zh.: Nauka Innov. 3 (63) (2017). https://doi.org/10.18698/2308-6033-2017-10-1684
Discovery Research Group. https://marketing.rbc.ru/articles/9910/
S. A. Grigoriev, V. N. Fateev, D. G. Bessarabov, and P. Millet, Int. J. Hydrogen Energy (2020, in press). https://doi.org/10.1016/j.ijhydene.2020.03.109
I. Dincer, Int. J. Hydrogen Energy 37, 1954 (2012). https://doi.org/10.1016/j.ijhydene.2011.03.173
M. F. Orhan, I. Dincer, M. A. Rosen, and M. Kanoglu, Renewable Sustainable Energy Rev. 16, 6059 (2012).
S. D. Badmaev and P. V. Snytnikov, Int. J. Hydrogen Energy 33, 3026 (2008).
A. V. Samoilov, V. A. Kirillov, A. B. Shigarov, A. S. Brayko, D. I. Potemkin, T. B. Shoinkhorova, P. V. Snytnikov, S. I. Uskov, A. A. Pechenkin, V. D. Belyaev, and V. A. Sobyanin, Catal. Ind. 10, 321 (2018).
E. Khrustalev, Energet. Prom-st’ Ross., Nos. 15–16, 107 (2008). http://www.eprussia.ru/epr/107/8367.htm.
N. N. Semenov, Russ. Chem. Rev. 36, 1 (1967). https://doi.org/10.1070/RC1967v036n01ABEH001579
V. I. Soroko-Novitskii and A. Kurenin, On Using the Possibility of Engine Operation on Hydrogen, Research Report (Lomonosov Mosc. Mech. Eng. Inst., Moscow, 1935).
A. Yu. Ramenskii, The History of the Development of Hydrogen Cars in Russia. http://www.cleandex.ru/articles/2015/11/06/the_use_of_hydrogen_as_a_fuel_for_cars.
A. Yu. Ramenskii, Using Hydrogen as Fuel. https://abs-magazine.ru/article/primenenie-vodoroda-v%C2%A0kachestve-topliva.
A. Yu. Ramenskiy, P. B. Shelichsh, and S. I. Nefedkin, Int. Sci. J. Altern. Energy Ecol. 11 (43), 63 (2006).
A. Bernshtein, Tekh.-Molodezhi, No. 11, 37 (1984).
Hydrogen Lieutenant: The History of the Formation of Hydrogen Technologies in Russia. http://h2center.ru/index.phpıd=8&option=com_content&task=view.
10th Peterburg International Economy Forum 2006. http://h2org.ru/index.php?option=com_content&task=view&id=135&Itemid=114.
Hydrogen Vehicle with Combined Power Plant. http://h2org.ru/index.php?option=com_content&task=view&id=85&Itemid=118.
Unique Aircraft Tu-155 with Hydrogen Engine. https://uacrussia.livejournal.com/15784.html.
Buran: The First and Only. https://rostec.ru/news/buran-pervyy-i-edinstvennyy/.
Energiya—Buran. http://www.minspace.ru/Education/edu9phys/buran.html.
S. P. Glushkov, V. I. Kochergin, and V. V. Krasnikov, Vestn. AGTU, Ser. Morsk. Tekh. Tekhnol., No. 1, 24 (2018).
O. F. Brizitskii, V. Ya. Terent’ev, V. A. Kirillov, et al., Transp. Al’tern. Topl., No. 6 (6), 25 (2008).
N. G. Pevnev, V. A. Kirillov, O. F. Brizitskii, and V. A. Burtsev, Transp. Al’tern. Topl., No. 3 (15), 40 (2010).
N. G. Pevnev and V. V. Ponamarchuk, Vestn. SibADI, No. 2 (48), 75 (2016).
I. Iordache, K. Bouzek, M. Paidar, et al., Int. J. Hydrogen Energy 44, 19036 (2019).
D. G. Kondrat’ev, V. I. Matrenin, A. T. Ovchinnikov, et al., Energ.: Ekon., Tekh., Ekol., No. 4, 12 (2009).
A. Y. Ramenskiy, Altern. Energy Ecol., No. 20, 13 (2015).
S. I. Kozlov and V. N. Fateev, Transp. Al’tern. Topl., No. 3 (51), 41 (2016).
O. I. Manaev, Energobezopasn. Energosberezh., No. 2 (23), 34 (2008).
A. S. Stikhin, FGUP UEKhK, Possibilities and Application of Nanotechnology in the Development and Organization of Production of Power Plants on Hydrogen Fuel. http://www.myshared.ru/slide/58566/.
Fuel Cell. https://ru.wikipedia.org/wiki/Топливный_элемент.
Institute of High-Temperature Electrochemistry, Ural Branch of the Russian Academy of Sciences. http://www.ihte.uran.ru/?p=7741.
L. P. Putilov, A. K. Demin, V. I. Tsidilkovski, and P. Tsiakaras, Appl. Energy 242, 1448 (2019).
D. Medvedev, J. Lyagaeva, E. Gorbova, et al., Prog. Mater. Sci. 75, 38 (2016).
I. D. Romanov, E. A. Chernyshov, and E. A. Romanova, Int. J. Appl. Fundam. Res., No. 10, 38 (2015).
I. S. Erilin, D. A. Agarkov, I. N. Burmistrov, et al., Mater. Lett. 266, 127439 (2020).
Electrolysis Module of Uralkhimmash, Type SEI. http://vodorod-energy.ru/wp-content/uploads/2017/03/9-1.jpg.
Electrolysis Module of Uralkhimmash, Type FH. http://chem.prompages.ru/images/gallary/71_.jpg.
S. I. Kozlov and V. N. Fateev, Transp. Al’tern. Topl., No. 2 (38), 7 (2014).
V. A. Grinberg, V. V. Emets, A. D. Modestov, et al., Prot. Met. Phys. Chem. Surf. 55, 277 (2019).
Krylov State Scientific Center. http://krylov-centre.ru/press/news/199/.
S. A. Grigor’ev, A. S. Grigor’ev, N. V. Kuleshov, et al., Therm. Eng., No. 62 (2), 81 (2015). https://doi.org/10.1134/s0040601515020032
I. E. Baranov, A. A. Kalinnikov, S. V. Korobtsev, et al., Energ.: Ekon., Tekh., Ekol., No. 4, 31 (2014).
I. Cheberko, Izvestiya (2016). https://iz.ru/news/611043.
AT Energy. http://atenergy.pro/products/energoobespechenie-bpla.html.
BMPower. http://bmpower.ru/fuelcel/energoobespechenie-bpla.html.
V. E. Guterman, S. V. Belenov, A. A. Alekseenko, et al., Electrocatalys 9, 550 (2018).
A. A. Kalinnikov, I. V. Pushkareva, V. I. Porembskii, et al., Chem. Probl. 17, 535 (2019). https://doi.org/10.32737/2221-8688-2019-4-535-545
I. E. Baranov, V. I. Porembskii, E. K. Lyutikova, et al., Chem. Probl. 17, 489 (2019). https://doi.org/10.32737/2221-8688-2019-4-489-499
D. D. Spasov, N. A. Ivanova, A. S. Pushkarev, et al., Catalysts 9, 803 (2019). https://doi.org/10.3390/catal9100803
I. E. Baranov, S. A. Grigoriev, D. Ylitalo, et al., Int. J. Hydrogen Energy 31, 203 (2006).
S. Grigoriev, V. Fateev, A. Pushkarev, et al., Materials 11, 1405 (2018). https://doi.org/10.3390/ma11081405
K. Novikova, A. Kuriganova, I. Leontyev, et al., Electrocatalysis 9, 22 (2018). https://doi.org/10.1007/s12678-017-0416-4
A. A. Alekseenko, E. A. Moguchikh, O. I. Safronenko, and V. E. Guterman, Int. J. Hydrogen Energy 43 (51), 22885 (2018). https://doi.org/10.1016/j.ijhydene.2018.10.139
E. A. Astafev, A. E. Ukshe, R. A. Manzhos, et al., Int. J. Electrochem. Sci., 1742 (2017). https://doi.org/10.20964/2017.03.56
A. S. Pushkarev, I. V. Pushkareva, S. A. Grigoriev, et al., Int. J. Hydrogen Energy 40, 14492 (2015).
A. A. Fedotov, S. A. Grigoriev, E. K. Lyutikova, et al., Int. J. Hydrogen Energy 38, 426 (2013).
K. Novikova, A. Kuriganova, I. Leontyev, et al., Electrocatalys 9, 22 (2018).
A. S. Pushkarev, M. A. Solovyev, S. A. Grigoriev, et al., Int. J. Hydrogen Energy (2020, in press). https://doi.org/10.1016/j.ijhydene.2020.02.098
A. S. Pushkarev, I. V. Pushkareva, S. P. du Preez, et al., Chem. Probl. 17, 9 (2019). https://doi.org/10.32737/2221-8688-2019-1-9-15
I. V. Pushkareva, A. S. Pushkarev, S. A. Grigoriev, et al., Int. J. Hydrogen Energy (2019, in press). https://doi.org/10.1016/j.ijhydene.2019.11.011
S. V. Akel’kina, A. S. Pushkarev, S. A. Grigoriev, I. V. Pushkareva, and V. N. Fateev, Russ. J. Electrochem. 54, 251 (2018). https://doi.org/10.1134/S1023193518030023
O. A. Varzatskii, D. A. Oranskiy, S. V. Vakarov, et al., Int. J. Hydrogen Energy 42, 27894 (2017). https://doi.org/10.1016/j.ijhydene.2017.05.092
S. A. Grigoriev, A. S. Pushkarev, I. V. Pushkareva, et al., Int. J. Hydrogen Energy 42, 27845 (2017). https://doi.org/10.1016/j.ijhydene.2017.05.048
S. A. Grigor’ev, M. M. Khaliullin, N. V. Kuleshov, and V. N. Fateev, Russ. J. Electrochem. 37, 819 (2001).
V. N. Kuleshov, N. V. Kuleshov, S. A. Grigoriev, et al., Int. J. Hydrogen Energy 41, 36 (2016).
P. Millet, S. A. Grigoriev, and V. I. Porembskiy, Int. J. Energy Res. 37, 449 (2013).
E. N. Aleksin, Persp. Nauki, No. 6 (08), 63 (2010).
NPO Poisk. http://poisk-ltd.com/about/aboutk_39.html.
V. V. Burnasheva and K. N. Semenenko, Russ. Chem. Rev. 52, 299 (1983). https://doi.org/10.1070/RC1983v052n04ABEH002818
B. P. Tarasov, M. V. Lototskii, and V. A. Yartys’, Ross. Khim. Zh. 50 (6), 34 (2006).
K. N. Semenenko and V. N. Verbetskii, Ross. Khim. Zh. 37 (2), 70 (1993).
Chemical Probl., No. 4, 453 (2018). https://cyberleninka.ru/article/n/problemy-akkumulirovaniya-i-hraneniya-vodoroda.
B. P. Tarasov and M. V. Lototskii, Ross. Khim. Zh. 50 (6), 5 (2006).
B. P. Tarasov, Int. J. Hydrogen Energy 36, 1196 (2011).
B. P. Tarasov, A. A. Volodin, P. V. Fursikov, et al., Al’tern. Energet. Ekol., No. 22, 30 (2014).
B. P. Tarasov, Hydrogen Storage for Energy Accumulation. http://www.hse.ru/data/2018/06/10/ 1149860616/%D0%A2%D0%B0%D1%80%D0%B%D1 %81%D0%BE%D0%B2%20%D0%91.%D0%92..pdf.
V. Borzenko, D. Dunikov, and S. Malyshenko, High Temp. 49, 249 (2011).
D. Dunikov and D. Blinov, Int. J. Hydrogen Energy 45, 9914 (2020).
A. G. Khayrullina, D. O. Blinov, and V. I. Borzenko, Energy 183, 1244 (2019).
B. P. Tarasov, M. V. Lototskii, and V. A. Yartys’, Russ. J. Gen. Chem. 77, 694 (2007).
A. M. Domashenko and Yu. V. Gorbatskii, “State, problems and prospects for the development of liquid hydrogen infrastructure in Russia,” in Proceedings of the International Forum on Hydrogen Technologies for Energy Production, Moscow, Feb. 6–10, 2016, p. 29.
O. K. Alekseeva, S. I. Kozlov, R. O. Samsonov, and V. N. Fateev, Transp. Al’tern. Topl., No. 4 (10), 68 (2009).
A. E. Sheindlin and A. Z. Zhuk, Herald Russ. Acad. Sci. 80, 143 (2010).
A. E. Sheindlin and A. Z. Zhuk, Ross. Khim. Zh., No. 6, 105 (2006). https://cyberleninka.ru/article/n/kontseptsiya-alyumovodorodnoy-energetiki-1.
A. Z. Zhuk, B. V. Kleimenov, E. I. Shkol’nikov, et al., Aluminum-Hydrogen Energy (OIVT RAN, Moscow, 2007) [in Russian].
E. I. Shkol’nikov, Ekol. Zhizn’, No. 7, 57 (2010). https://elementy.ru/nauchno-populyarnaya_biblioteka/431264/Chto_takoe_alyumoenergetika.
E. I. Shkol’nikov, S. A. Yanushko, S. A. Tarasova, et al., Elektrokhim. Energet. 8 (2), 86 (2008).
E. I. Shkol’nikov, M. S. Vlaskin, A. S. Ilyukhin, and A. B. Tarasenko, Elektrokhim. Energet. 7 (4), 175 (2007). https://cyberleninka.ru/article/n/osobennosti-raboty-svobodno-dyshaschego-toplivnogo-elementa-s-tverdym-polimernym-elektrolitom-v-usloviyah-ogranichennogo-obema.
N. K. Zhevago and V. I. Glebov, Energy Convers. Manag. 48, 1554 (2007). https://doi.org/10.1016/j.enconman.2006.11.017
N. K. Zhevago, E. I. Denisov, and V. I. Glebov, Int. J. Hydrogen Energy 35, 169 (2010).
S. A. Grigor’ev, A. S. Grigor’ev, N. V. Kuleshov, et al., Therm. Eng. 62, 81 (2015).
A. S. Grigoriev, V. V. Skorlygin, S. A. Grigoriev, et al., Int. J. Electrochem. Sci. 13, 1822 (2018).
A. S. Grigoriev, V. V. Skorlygin, S. A. Grigoriev, et al., Russ. Electr. Eng. 90, 505 (2019).
G. Doucet, C. Etievant, C. Puyenchet, et al., Int. J. Hydrogen Energy 34, 4983 (2009).
A. Yu. Ramenskiy, S. A. Grigoriev, E. A. Ramenskaya, and A. S. Grigoriev, Int. J. Hydrogen Energy 42, 21250 (2017).
National Hydrogen Energy Association. http://h2org.ru/.
Yu. A. Kotlyar and V. V. Shinkarenko, Hydrogen Universal Education in Russia. To the History of the Issue. Documents. Materials. Comment (ASMI, Moscow, 2008) [in Russian].
A. S. Sigov, V. V. Shinkarenko, and A. A. Evdokimov, Al’tern. Energet. Ekol., No. 6, 94 (2006).
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under an agreement on the provision of grants from the federal budget in the form of subsidies in accordance with paragraph 4 of Article 78.1 of the Budget Code of the Russian Federation no. 075-15-2019-1847 dated December 4, 2019 (unique identifier of the RFMEFI60419X0243).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Pertsovskaya
Rights and permissions
About this article
Cite this article
Fateev, V.N., Grigoriev, S.A. & Seregina, E.A. Hydrogen Energy in Russia and the USSR. Nanotechnol Russia 15, 256–272 (2020). https://doi.org/10.1134/S1995078020030040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078020030040