Abstract
This paper presents the results of experiments on the oxidative cracking of propane at a pressure of 1 to 2 atm and moderate temperatures (T ≤ 1000 K) in a laboratory-scale reactor. Nitrogen and methane are used as the diluent gases. Kinetic models are analyzed to describe the studied process. The necessity of taking into account heterogeneous reactions on the reactor surface is shown. The introduction of additional stages in the kinetic model, which take into account heterogeneous reactions on the reactor surface, makes it possible to obtain an almost quantitative agreement between the calculations and experimental results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The growing interest in gas-chemical processes for processing natural gas and its individual components is stimulatng the development of more reliable kinetic models to describe these processes occurring in the moderate temperature region (T ≤ 1000 K). There are only a few models that more-or-less reliably describe the oxidation of the closest methane homologs (including propane) at moderate temperatures. Many of the models presented in the literature, which contain a block of propane oxidation reactions, were created for experimental conditions that differ significantly from the area studied in this paper. In order to analyze the applicability of the most popular published models for describing the processes of propane oxidation and cracking at moderate temperatures, experimental studies of propane oxidative cracking (oxycracking) in a plug-flow laboratory reactor and their kinetic modeling based on the most modern published mechanisms were carried out.
EXPERIMENTAL
Experiments on the oxidative cracking of propane were carried out in a laboratory plug-flow type quartz reactor in the temperature range 773–1023 K and pressure 1–2 atm. The initial propane/oxygen ratio was in the range of 1 to 3. The length of the reactor was 350 mm and the inner diameter was 14 mm. The ratio of the area of the inner surface of the reactor to its volume in the working part, taking into account the surface of the pockets for thermocouples, was 5.4 cm–1. The residence time of the gas mixture in the reactor was constant (2.02 ± 0.05 s). The reactor was heated by three independent electric heaters, which made it possible to maintain a constant temperature profile in the high-temperature zone of the reactor, which was 200 mm long. The laboratory setup used is described in more detail in [1–5], which are continued in this study.
The following gases were used in the experiments: oxygen of high purity (99.7%), high purity nitrogen of the first grade (99.999%), helium grade A (99.995%), and pure propane (99.99%). Nitrogen and methane were used as the gas medium. The gas mixtures at the inlet and outlet of the reactor were analyzed using a Kristall 5000 gas chromatograph manufactured by Khromatek (Russia) equipped with three detectors: one flame ionization detector (FID) and two thermal conductivity detectors (TCDs). In TCD 1, the presence of H2 (carrier gas, argon) was determined; in TCD 2, the presence of CO2, O2, N2, and CO (carrier gas, helium); and in the FID, the presence of hydrocarbons (carrier gas, helium).
KINETIC SIMULATION OF PROPANE OXIDATIVE CRACKING
For modeling, several kinetic models were selected that could be considered to describe the propane oxycracking process in the moderate temperature range: UBC Mech 2.0 kinetic mechanism (UBC) [6], methane/propane oxidation mechanism (methane/propane) [7], C1–C3 San Diego Mechanism (San Diego) [8], C1–C5 alkane oxidation mechanism (C1–C5) [9], Butan NUIGALWAY (Butan NUI) [10–14], HEXANE NUIGALWAY (HEXANE NUI) [15], HEPTANE NUIGALWAY (HEPTANE NUI) [16], natural gas to/including C5 (2007/08) HIGH (NG HIGH), natural gas to/including C5 (2007/08) LOW (NG LOW), natural gas (NG) [17], natural gas to/including C5 (2010) (NG3) [5–9], C1–C16 HT + LT + NOx mechanism (Ranzi) [18], and NUIGMech 1.1. (NUIGMech) [19, 20]. It should be noted that we did not use the well-known mechanism GRI-Mech 3.0 [21] for modeling due to the strong differences between the experimental conditions on which its development was based and the conditions considered in this paper. Table 1 gives brief characteristics of the considered mechanisms.
All the mechanisms presented above were developed to describe the processes of light hydrocarbon oxidation and, as a result, they include a propane oxidation block. The mechanisms of the AramcoMech series were not considered, since they are based on other mechanisms that are discussed in detail in this article.
Kinetic modeling was carried out using models of the oxidation of light hydrocarbons in the region of moderate temperatures indicated in Table 1. These models were selected from those presented in the literature according to the criterion of the presence of block C3 in them and the similarity of the conditions of the experiments in which these models were validated with the conditions of this study. It should be noted that all the considered models were developed exclusively for gas-phase processes and they do not take into account the reactions on the reactor surface, the importance of which was shown by us in [26].
According to [27], the oxycracking of light alkanes proceeds by a chain mechanism with degenerate chain branching as a result of the formation of hydrogen peroxide H2O2, formed as a result of the interaction of the peroxide radical \({\text{HO}}_{2}^{\centerdot }\) with an alkane, followed by the decomposition of hydrogen peroxide into OH• hydroxyl radicals. The need to take into account heterogeneous processes in the oxycracking of ethane, and hence other light alkanes, was substantiated in [26]. The same methodology was also given there, including the calculation of accommodation coefficients γi for the interaction of the corresponding molecules with the quartz surface of the reactor. Therefore, the NUIGMech mechanism, which showed the best descriptive ability in the preliminary analysis, was supplemented by three heterogeneous reactions involving radicals \({\text{HO}}_{2}^{\centerdot }\), as well as H2O2 and CO molecules, which ensure the conversion of peroxide radicals and hydrogen peroxide into water and oxygen molecules on the reactor surface, and carbon monoxide into carbon dioxide (Table 2). It was assumed that the H2O, O2, and CO2 products formed as a result of desorption are almost instantaneously returned to the gas phase compared to the characteristic times of the change in the gas-phase concentrations of \({\text{HO}}_{2}^{\centerdot }\) H2O2, and CO.
The corresponding rate constants and accommodation coefficients of particles on the surface to describe the experimental results on propane oxycracking in a quartz reactor were calculated according to the procedure described in [26, 28]. The simulation was carried out in the software environment of the Russian software package CWB 4.3 [29] on the model of an isothermal plug-flow reactor. Table 2 shows the kinetic parameters of the considered heterogeneous processes for the three-parameter form of the Arrhenius equation:
The values of particle accommodation coefficients on the reactor surface, γi, for the three heterogeneous reactions indicated in Table 2 were selected according to the procedure described in [26]: γ(HO2) = 2 × 10–3, γ(H2O2) = 1.1 × 10–4, and γ(СО) = 6.7 × 10–8.
RESULTS AND DISCUSSION
The experimental temperature dependences of changes in the concentration of reagents and main products of propane oxycracking at the outlet of the reactor obtained in this study are shown in Figs. 1–3. The results of modeling based on various published mechanisms indicated in Table 1 are also presented there.
Temperature dependence of the propylene concentration during the oxidative cracking of propane oxidative cracking of propane at P = 1 atm, [C3H8]0 = 5.6%; [O2]0 = 1.9%, diluent gas is nitrogen. The designations are the same as in Fig. 1.
Temperature dependence of propane (a) and oxygen (b) conversion in a methane environment at [C3H8]0 = 5.02%; [O2]0 = 2.48; P = 1 atm; diluent gas, methane. The designations are the same as in Fig. 1.
Figure 1a shows that almost all models give an underestimated value of the temperature of the onset of a rapid change in propane conversion at atmospheric pressure by about 25–50 K. The UBC and San Diego models describe this temperature dependence more accurately than the others; however, when describing the temperature dependence of oxygen conversion, the adequacy of these models is also low (Fig. 1b). The NG HIGH model is noticeably outside the general trend, which once again indicates the need to take into account the i-C4H9O2 radicals and their subsequent low-temperature reactions when modeling such processes.
One of the most important parameters for propane oxycracking is the maximum propylene concentration achievable. Almost all models show that the maximum propylene concentration is reached in the temperature range of 825 to 850 K (Fig. 2). In the case of the HEXANE NUI, HEPTANE NUI, NG LOW, and San Diego models, the temperature at which the concentration maximum is observed corresponds to ~873 K, which is ~50 K lower than the experimental value. The UBC, NG, and NG3 models also show an excessively low propylene concentration. The NG HIGH model again falls outside the general trend; hence, it was decided not to use it in the subsequent calculations.
Propane oxycracking in methane, the main component of natural associated gas, is of great practical interest. Figure 3 shows the results of modeling this process using the models from Table 1. The calculations for all models give a lower reaction start temperature compared to the experimental values, as in [26], which confirms the need to take into account the processes on the reactor surface.
In accordance with the results obtained, the most adequate of the models we considered, NUIGMech, was supplemented by the three heterogeneous stages indicated in Table 2 with the parameters given there. Figure 4 shows a comparison of the experimental results with the results of NUIGMech propane oxycracking modeling with and without heterogeneous stages. The simulation results, taking into account the heterogeneous reactions quite well, actually quantitatively, describe the experimental results, which is a weighty argument in favor of the need to take into account heterogeneous reactions in laboratory-scale reactors.
Temperature dependence of propane (a) and oxygen (b) conversion and the maximum concentration of propylene (b) during propane oxycracking. Symbols, experimental results: ◼, propane; ⚫, oxygen; ▲, propylene; the lines are the results of modeling according to the NUIGMech model without heterogeneous stages: the dashed-dotted curve is propane, the dotted line is oxygen, and the dashed line with two points is propylene; simulation results based on the NUIGMech model supplemented with heterogeneous stages: the dashed curve is propane, the solid curve is oxygen, –⚪– is propylen, [C3H8]0 = 4.59% [O2]0 = 2.49%, P = 1 atm, diluent gas is nitrogen.
Similar results for another value of the initial propane/oxygen ratio are shown in Fig. 5. In this case, too, the results of modeling using the NUIGMech mechanism modified by adding heterogeneous steps are in quantitative agreement with the experimental results.
The same as in Fig. 4, at [C3H8]0 = 4.65% [O2]0 = 5.05%.
Thus, when the NUIGMech gas-phase mechanism is supplemented with reactions describing the most important heterogeneous processes on the reactor surface, the calculated temperature of the onset of a rapid increase in the conversion of reagents increases by ∼50 K, which makes it possible to quantitatively reconcile the calculations with the experiments. Accounting for heterogeneous processes involving other radicals (Н●, OH●, etc.), which play an important role in the gas-phase mechanism of oxycracking and ethane oxycracking [26] did not significantly affect the results, apparently due to the significantly higher rate of gas-phase processes with their participation.
CONCLUSIONS
Analysis of a large group of kinetic models of propane oxidation in the region of moderate temperatures (T ≤ 1000 K) showed that the most modern models, such as NUIGMech, are able to qualitatively describe this process. However, a quantitative description of the process under the conditions of laboratory reactors with a high ratio of the area of the inner surface of the reactor to its volume requires taking into account the heterogeneous processes occurring on the surface of the reactor. Accounting for these processes with the kinetic parameters determined according to the technique proposed in [26, 28] makes it possible to obtain a quantitative, which is accurate up to the experimental error, agreement between the simulation results and experimental results.
REFERENCES
V. S. Arutyunov, R. N. Magomedov, A. Yu. Proshina, and L. N. Strekova, Chem. Eng. J. 238, 9 (2014). https://doi.org/10.1016/j.cej.2013.10.009
V. S. Arutyunov, A. S. Dmitruk, and A. V. Nikitin, Russ. Chem. Bull. 65, 2405 (2016). https://doi.org/10.1007/s11172-016-1597-3
R. N. Magomedov, A. Yu. Proshina, and V. S. Arutyunov, Kinet. Catal. 54, 383 (2013). https://doi.org/10.1134/S0023158413040113
R. N. Magomedov, A. Yu. Proshina, B. V. Peshnev, and V. S. Arutyunov, Kinet. Catal. 54, 394 (2013). https://doi.org/10.1134/S0023158413040125
A. S. Dmitruk, A. V. Nikitin, L. N. Strekova, and V. S. Arutyunov, in Combustion and Explosion, Ed. by S. M. Frolov (Torus Press, Moscow, 2016), Iss. 9, No. 3, p. 21.
J. Huang and W. K. Bushe, Combust. Flame 144, 74 (2006). https://doi.org/10.1016/j.combustflame.2005.06.013
E. L. Petersen, D. M. Kalitan, S. Simmons, et al., Proc. Combust. Inst. 31, 447 (2007). https://doi.org/10.1016/j.proci.2006.08.034
J. C. Prince, C. Treviño, and F. A. Williams, Combust. Flame 175, 27 (2017). https://doi.org/10.1016/j.combustflame.2016.06.033
D. Healy, D. M. Kalitan, C. J. Aul, et al., Energy Fuels 24, 1521 (2010). https://doi.org/10.1021/ef9011005
D. Healy, M. M. Kopp, N. L. Polley, et al., Energy Fuels 24, 1617 (2010). https://doi.org/10.1021/ef901292j
N. Donato, C. Aul, E. Petersen, et al., J. Eng. Gas Turbine Power 132, 051502 (2010). https://doi.org/10.1115/1.3204654
D. Healy, N. S. Donato, C. J. Aul, et al., Combust. Flame 157, 1526 (2010). https://doi.org/10.1016/j.combustflame.2010.01.016
D. Healy, N. S. Donato, C. J. Aul, et al., Combust. Flame 157, 1540 (2010). https://doi.org/10.1016/j.combustflame.2010.01.011
D. Healy, D. M. Kalitan, C. J. Aul, et al., Energy Fuels 24, 1521 (2010). https://doi.org/10.1021/ef9011005
K. Zhang, C. Banyon, C. Togbé, et al., Combust. Flame 162, 4194 (2015). https://doi.org/10.1016/j.combustflame.2015.08.001
K. Zhang, C. Banyon, J. Bugler, et al., Combust. Flame 172, 116 (2016). https://doi.org/10.1016/j.combustflame.2016.06.028
G. Bourque, D. Healy, H. J. Curran, et al., Proc. ASME Turbo Expo. 3, 1051 (2008). https://doi.org/10.1115/GT2008-51344
C1-C16 HT + LT + NOx mechanism, The CRECK Modeling Group, Politecnico di Milano. http://creckmodeling.chem.polimi.it/menu-kinetics/menu-kinetics-detailed-mechanisms/107-category-kinetic-mechanisms/406-mechanisms-1911-tot-ht-lt-nox/. Accessed 2020.
NUIGMech 1.1. https://c3.nuigalway.ie/combustionchemistrycentre/mechanismdownloads/
S. Martinez, M. Baigmohammadi, V. Patel, et al., Combust. Flame 228, 401 (2021). https://doi.org/10.1016/j.combustflame.2021.02.009
Gri-Mech 3.0. http://combustion.berkeley.edu/gri_ mech/version30/text30.html/
W. K. Metcalfe, S. M. Burke, S. S. Ahmed, and H. J. Curran, J. Chem. Kinet. 45, 638 (2013). https://doi.org/10.1002/kin.20802
Y. Li, C. W. Zhou, K. P. Somers, et al., Proc. Combust. Inst. 36, 403 (2016). https://doi.org/10.1016/j.proci.2016.05.052
C.-W. Zhou, Y. Li, E. O’Connor, et al., Combust. Flame 167, 353 (2016). https://doi.org/10.1016/j.combustflame.2016.01.021
C.-W. Zhou, Y. Li, U. Burke, et al., Combust. Flame 197, 423 (2018). https://doi.org/10.1016/j.combustflame.2018.08.006
M. G. Bryukov, A. S. Palankoeva, A. A. Belyaev, and V. S. Arutyunov, Kinet. Catal. 62, 703 (2021). https://doi.org/10.1134/S0023158421060021
J. A. Miller and S. J. Klippenstein, Int. J. Chem. Kinet. 33, 654 (2001). https://doi.org/10.1002/kin.1063
A. S. Palankoeva, Ya. S. Zimin, M. G. Bryukov, A. A. Belyaev, and V. S. Arutyunov, Gorenie Vzryv 14 (4), 42 (2021). https://doi.org/10.30826/CE
Chemical Workbench 4.3. Kintech Laboratory. http://www.kintechlab.com/products/chemical-workbench/. Accessed 2021.
Funding
The study was financially supported by the Russian Foundation for Basic Research and the Science Committee of the Republic of Armenia as part of scientific project no. 20-53-05001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palankoeva, A.S., Belyaev, A.A. & Arutyunov, V.S. Oxidative Cracking of Propane in a Plug-Flow Laboratory Reactor. Russ. J. Phys. Chem. B 16, 399–406 (2022). https://doi.org/10.1134/S1990793122030204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122030204


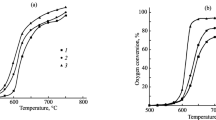
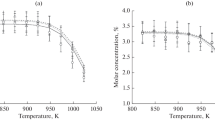


 , experimental values;
, experimental values;  , San Diego;
, San Diego;  , methane/propane;
, methane/propane;  , UBC;
, UBC;  , Ranzi;
, Ranzi;  , Butan NUI;
, Butan NUI;  , Heptane NUI;
, Heptane NUI;  , Hexane NUI;
, Hexane NUI;  , NG;
, NG;  , NG High;
, NG High;  , NG Low;
, NG Low;  , NG3.
, NG3.


