Abstract
The thermal pyrolysis of ethane and propane at a pressure of 1–3 atm was experimentally studied in a through-flow reactor. Kinetic modeling of the process under these conditions showed good agreement with experimental data in terms of both the conversion of alkanes and the yield of pyrolysis products, which allowed us to expand the modeling range of pressure. The results obtained show that the pressure change in the range of 1–15 atm has no noticeable effect on the pyrolysis of light alkanes. On this basis, we concluded that the previously established effect of pressure on the oxidative cracking of ethane was associated with its effect on the oxidative stages of the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, the role of natural gas as a raw material for the production of various gas chemical products is growing. Accordingly, the number of studies devoted to this alternative to the use of gas as a fuel is increasing [1]. However, the vast majority of these works relate to the studies of catalytic processes. At the same time, a high temperature, which is necessary for the catalytic conversion of methane, almost always requires taking into account parallel gas-phase reactions; moreover, these reactions are of great independent interest, and they open up prospects for the development of fundamentally new technologies [2].
The oxidative cracking of light alkanes directly in an atmosphere of methane, the main component of natural gas, can be such a promising process. The possibility of its implementation was shown by Arutyunov et al. [3]. The oxidative conversion of methane homologues directly in the atmosphere of methane can proceed due to a large difference in the reactivity of methane and its homologues without using complex and energy-intensive technologies for their separation from natural gas.
Previously, a number of experimental studies on the oxycracking of ethane and propane were carried out in the region of moderate temperatures and pressures to 5 atm [4]. Nikitin et al. [5] hypothesized that the noted effect of pressure on the oxycracking of light alkanes was associated with its role in oxidative processes, while its effect on thermal cracking processes was insignificant or absent. In this work, we carried out an experimental study of the pyrolysis of light С2–С3 alkanes and the kinetic modeling of this process in order to verify this assumption, which is extremely important for the practical implementation of technologies based on oxycracking.
It should be noted that, despite the existence of published kinetic models that adequately describe the oxycracking processes of light alkanes, none of them takes into account reactions on the reactor surface. Bryukov et al. [6] found that these reactions play a significant role in oxidative processes in laboratory-scale reactors in a temperature range typical for the cracking and oxycracking of light alkanes. Bryukov et al. [6] supplemented a kinetic model [7] with a block of heterogeneous reactions in order to accurately describe the process of the oxidative cracking of ethane in a pressure range of 1–2 atm at moderate temperatures. However, according to Bryukov et al. [6], the role of heterogeneous reactions in oxycracking is mainly associated with the interaction of oxygen-containing molecules and radicals with the surface, and it can be expected that their effect in thermal pyrolysis will be much lower.
The possibility of a quantitative description of the partial oxidation (oxidative pyrolysis) of ethane in a laboratory reactor [6] allowed us to draw a conclusion on the effect of pressure on the processes of oxidative and thermal pyrolysis of light alkanes based on an experimental study of the thermal pyrolysis of ethane and propane and its comparison with the results of kinetic modeling.
EXPERIMENTAL
Experiments on the pyrolysis of ethane and propane were carried out using a flow-type laboratory setup with a cylindrical quartz reactor in a temperature range of 773–1023 K at pressures of 1–3 atm. The reactor length and inner diameter were 350 and 14 mm, respectively. The ratio of the inner surface area of the reactor to the reactor volume S/V in the working part was 5.4 cm–1 with consideration for the surface area of thermocouple pockets. The reactor was heated by three independent electric heaters, which made it possible to maintain a constant temperature profile in a 200 mm long high-temperature zone of the reactor. The laboratory setup was described in detail elsewhere [3, 5, 8–10], and this study is a continuation of previous works.
The following gases were used in the experiments: high-purity nitrogen of the first grade (99.999%), helium of grade A (99.995%), pure ethane (99.99%), and pure propane (99.99%). Nitrogen served as a carrier gas. Gas mixtures at the reactor inlet and outlet were analyzed using a Kristall 5000 gas chromatograph (Chromatec, Russia).
Kinetic modeling was carried out using the NUIGMech1.1 model [7] for the oxidation of light hydrocarbons in a moderate temperature range, which was successfully used for describing complex processes of this type, including the process of ethane oxycracking in this temperature range [6]. The simulation was carried out based on the Russian software package CWB 4.3 [11] using the model of an isothermal plug-flow reactor.
RESULTS AND DISCUSSION
Figure 1 shows the dependence of the molar concentration of ethane at the outlet of the reactor on the pyrolysis temperature. It can be seen that a decrease in the concentration of ethane began at about 925 K, which is close to the temperature of the onset of conversion observed in its oxidative cracking. In this case, the change in pressure in a range of 1–3 atm did not significantly affect the process of pyrolysis. A twofold increase in the initial concentration of ethane (Figs. 1a, 1b) also had almost no effect on the temperature dependence of pyrolysis. The results of kinetic simulation shown in Fig. 1 are in good agreement with the experimental data.
Dependence of the molar concentration of ethane on the temperature of its pyrolysis at pressures of 1, 2, and 3 atm: (a) [C2H6]0 = 3.3 mol % and the rest was nitrogen, t = 2 s; (b) [C2H6]0 = 6.1–6.4 mol % and the rest was nitrogen, t = 2 s. Points refer to experimental results for ethane: p = (◻) 1, (Δ) 2, and (○) 3 atm. Lines show the results of modeling: solid line, p = 1 atm; dashed line, p = 2 atm; and dotted line, p = 3 atm.
Similar experiments on the pyrolysis of propane were carried out. In Figs. 2a and 2b, it can be seen that the temperature of the onset of a rapid decrease in the propane concentration was slightly lower, ~900 K, which is in good agreement with the results obtained by Nikitin et al. [5]. As in the case of ethane, pressure does not significantly affect the rate of propane pyrolysis. However, note that the used kinetic model describes propane pyrolysis less accurately than ethane pyrolysis.
Dependence of the molar concentration of propane on the temperature of its pyrolysis at pressures of 1, 2, and 3 atm: (a) [C3H8]0 = 2.3–2.4 mol % and the rest was nitrogen, t = 2 s; (b) [C3H8]0 = 5.0–5.7 mol % and the rest was nitrogen, t = 2 s. Points refer to experimental results for ethane: p = (◻) 1, (△) 2, and (○) 3 atm. Lines show the results of modeling: solid line, p = 1 atm; dashed line, p = 2 atm; and dotted line, p = 3 atm.
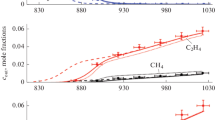
Figures 3 and 4 show the temperature dependences of the formation of the main products of ethane and propane pyrolysis. It can be seen that a change in the pressure in a range of 1–3 atm did not have a noticeable effect on the yields of unsaturated hydrocarbons and hydrogen in this process. The calculations described well the yield of products in the pyrolysis of ethane, and the concentrations of ethylene and hydrogen formed almost coincided with each other both in experiments and in calculations. Of course, there was no such coincidence between the concentrations of propylene and hydrogen in the pyrolysis of propane because the resulting propylene was also partially subjected to further pyrolysis with the appearance of ethylene and other products. However, the calculations adequately described the yield of hydrogen in the pyrolysis of propane but the calculated yield of propylene was clearly overestimated; apparently, this can be due to the shortcomings of the model, which should be refined.
Temperature dependences of the formation of the main products of ethane pyrolysis—ethylene and hydrogen: (a) [C2H6]0 = 2.78 mol % and the rest was nitrogen, р = 1 and 2 atm; (b) [C2H6]0 = 6.4 mol % and the rest was nitrogen, р = 1, 2, and 3 atm, t = 2 s. Points refer to experimental results: (◻) Н2, р = 1 atm; (Δ) H2, p = 2 atm; (◇) Н2, р = 3 atm; (○) С2Н4, р = 1 atm; (∇) С2Н4, р = 2 atm; and ( ) С2Н4, p = 3 atm. Lines show the results of modeling: solid line, H2, p = 1 atm; dashed line, Н2, р = 2 atm; dash-dotted line with two points, H2, р = 3 atm; dotted line, С2Н4, р = 1 atm; dash-dotted line, С2Н4, p = 2 atm; and dashed line with short strokes, С2Н4, р = 3 atm.
) С2Н4, p = 3 atm. Lines show the results of modeling: solid line, H2, p = 1 atm; dashed line, Н2, р = 2 atm; dash-dotted line with two points, H2, р = 3 atm; dotted line, С2Н4, р = 1 atm; dash-dotted line, С2Н4, p = 2 atm; and dashed line with short strokes, С2Н4, р = 3 atm.
Temperature dependences of the formation of the main products of propane pyrolysis—propylene and hydrogen—at pressures of 1, 2, and 3 atm: (a) [C3H8]0 = 5.38 mol % and the rest was nitrogen, t = 2 s; (b) [C3H8]0 = 2.3–2.4 mol % and the rest was nitrogen, t = 2 s. Points refer to experimental results: (◻) Н2, р = 1 atm; (Δ) H2, p = 2 atm; (◇) Н2, р = 3 atm; (○) С3Н6, р = 1 atm; (∇) С3Н6, р = 2 atm; and ( ) С3Н6, p = 3 atm. Lines show the results of modeling: solid line, H2, p = 1 atm; dashed line, Н2, р = 2 atm; dash-dotted line with two points, H2, р = 3 atm; dotted line, С3Н6, р = 1 atm; dash-dotted line, С3Н6, p = 2 atm; and dashed line with short strokes, С3Н6, р = 3 atm.
) С3Н6, p = 3 atm. Lines show the results of modeling: solid line, H2, p = 1 atm; dashed line, Н2, р = 2 atm; dash-dotted line with two points, H2, р = 3 atm; dotted line, С3Н6, р = 1 atm; dash-dotted line, С3Н6, p = 2 atm; and dashed line with short strokes, С3Н6, р = 3 atm.
Good agreement between the experimental results and kinetic calculations of the thermal pyrolysis of light alkanes allowed us to expand the range of test pressures beyond the limits of the experimental capabilities of the equipment used. In order to more reliably establish the effect of pressure on the pyrolysis of light alkanes, a kinetic calculation was carried out at much higher pressures of 10 and 15 atm (Figs. 5 and 6).
Calculated temperature dependence of ethane conversion during its thermal pyrolysis at pressures of 1, 10, and 15 atm: [C2H6]0 = 3.29 mol % and the rest was nitrogen, t = 2 s; (◻) experimental results for C2H6 at р = 1 atm. Lines show the results of modeling: solid line, р = 1 atm; dashed line, р = 10 atm; and dotted line, р = 15 atm.
Calculated temperature dependence of hydrogen formation during thermal pyrolysis of ethane at pressures of 1, 10, and 15 atm: [C2H6]0 = 3.29 mol % and the rest was nitrogen, t = 2 s; (◻) experimental results for H2 at р = 1 atm. Lines show the results of modeling: solid line, р = 1 atm; dashed line, р = 10 atm; and dotted line, р = 15 atm.
As can be seen in Figs. 5 and 6, an increase in the pressure to 15 atm had almost no effect on thermal pyrolysis. Similar results were obtained with an approximately twofold increase in the initial ethane concentration and for propane pyrolysis. A slight difference in the curves calculated for different pressures was apparently due to the fact that there was a slight redistribution of some secondary routes in the reaction scheme. Thus, the experimental data and the results of kinetic modeling allowed us to draw a completely unambiguous conclusion that pressure does not play a significant role in the process of ethane and propane pyrolysis. Therefore, the observed effect of pressure on the oxycracking of light alkanes should be attributed to oxidative rather than thermal stages of the process, as was expected by Nikitin et al. [5].
CONCLUSIONS
In this work, a combined experimental and theoretical study of the thermal pyrolysis of ethane and propane under the conditions of a flow reactor confirmed the possibility of a quantitative description of the conversion of light alkanes based on modern kinetic models. The results obtained show an insignificant role of heterogeneous reactions on the reactor surface in the pyrolysis of ethane and propane. An important practical conclusion is that a change in pressure, at least within a range of 1–15 atm, does not have a noticeable effect on the process of pyrolysis. Thus, the practically important conclusion [5] that the effect of pressure on the oxidative cracking of ethane is associated with its action on the oxidative stages of the process is confirmed.
REFERENCES
Arutyunov, V.S., Okislitel’naya konversiya prirodnogo gaza (Oxidative Conversion of Natural Gas), M.: KRASAND, 2011.
Arutyunov, V.S., Golubeva, I.A., Eliseev, O.L., and Zhagfarov, F.G., Tekhnologiya pererabotki uglevodorodnykh gazov: uchebnik dlya vuzov (Technology of Processing of Hydrocarbon Gases: Textbook for Universities), Moscow: Yurait, 2020.
Arutyunov, V.S., Magomedov, R.N., Proshina, A.Yu., and Strekova, L.N., Chem. Eng. J., 2014, vol. 238, p. 9.
Arutyunov, V.S., Savchenko, V.I., Sedov, I.V., Nikitin, A.V., Magomedov, R.N., and Proshina, A.Yu., Russ. Chem. Rev., 2017, vol. 86, no. 1, p. 47.
Nikitin, A.V., Arutyunov, V.S., and Dmitruk, A.S., Russ. Chem. Bull., 2016, no. 10, p. 2405.
Bryukov, M.G., Palankoeva, A.S., Belyaev, A.A., and Arutyunov, V.S., Kinet. Catal., 2021, vol. 62, no. 6, p. 703.
NUIGMech1.1. National University of Ireland Galway. 2020. http://c3.nuigalway.ie/combustionchemistrycentre/mechanismdownloads/.
Magomedov, R.N., Proshina, A.Yu., and Arutyunov, V.S., Kinet. Catal., 2013, vol. 54, no. 4, p. 383.
Magomedov, R.N., Proshina, A.Yu., Peshnev, B.V., and Arutyunov, V.S., Kinet. Catal., 2013, vol. 54, no. 4, p. 394.
Dmitruk, A.S., Nikitin, A.V., Strekova, L.N., and Arutyunov, V.S., Gorenie i Vzryv, 2016, vol. 9, no. 3, p. 21.
Chemical Workbench 4.3. Kintech Laboratory. 2021. http://www.kintechlab.com/products/chemical-workbench/.
Funding
This study was supported by the Russian Foundation for Basic Research and the Science Committee of the Republic of Armenia within the framework of scientific project no. 20-53-05001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Palankoeva, A.S., Zimin, Y.S., Zakharov, A.A. et al. Pyrolysis of Ethane and Propane in a Temperature Range of 773–1023 K. Kinet Catal 63, 243–247 (2022). https://doi.org/10.1134/S0023158422030077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422030077