Abstract—
Diadenosine polyphosphates (DAP) are now considered as a new class of endogenous regulatory cardiotropic compounds. In previous studies DAP were demonstrated to affect cardiac electrical activity and contractility in various animal species including rats. DAP decreased the action potential duration and reduced the contractility of the rat myocardium. At the same time, DAP did not affect repolarizing potassium currents (IK1, IKACh, Ito1, IKur), which normally participate in repolarization after the action potentials (AP), and had a little effect on L-type calcium current in isolated rat cardiomyocytes. However, in addition to these ionic currents, AP duration can be regulated via chloride currents. In this study the presence of a transient inward calcium-dependent chloride current Ito2 has been shown in rat ventricular myocardium and an influence of DAP on this current has been demonstrated for the first time. Ionic currents were recorded in isolated rat ventricular cardiomyocytes using whole-cell patch clamp method. Action potentials were recorded in isolated preparations of rat right ventricle with sharp glass microelectrodes. In the absence of Na+ and K+ and in the presence of potassium current blockers 4-aminopyridine (5 × 10–3 M) and tetraethylammonium (1.5 × 10–2 M) transient outward current was present in ventricular myocytes. This current was sensitive to non-selective chloride channel blocker 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS, 10–5 M), L-type calcium current blocker nifedipine (10–5 M), and a selective blocker of calcium-dependent chloride channels 6-(1,1-dimethyl ethyl ethyl)-2-[(2-furanyl carbonyl)amino]-4,5,6,7-tetrahydrobenzo[b]thiophen-3 carbonic acid (CaCCinh-A01, 10–5 M). In the presence of diadenosine tetraphosphate (Ap4A, 10–4 M) in the external solution the peak amplitude of the current increased by 44 ± 11%. Diadenosine pentaphosphate (Ap5A) and NAD+ failed to produce any significant effects on the current density. In isolated preparations of rat ventricular myocardium DIDS (10–5 M) and CaCCinh-A01 (10–5 M) blocked the Ap4A-induced acceleration of repolarization. Thus, the effects of Ap4A on cardiac electrical activity in rats are at least partially mediated by its influence on the amplitude of repolarizing chloride current Ito2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Diadenosine polyphosphates (DAP) are purinergic compounds, considered now as a new class of endogenous signaling molecules. DAP molecules consist of two adenosine bases linked by several (2–7) phosphoric acid residues [1, 2]. DAP are synthetized by specific enzymatic systems in different tissues including peripheral and central nervous system, where they function as neurotransmitters [3]. In mammals, activated platelets are the most important source of DAP, which indicates cardiovascular system as a direct target for DAP [4]. Presynaptic nerve terminals can also secrete NAD+ – a purinergic compound, structurally similar to DAP and causing certain cardiotropic effects [5, 6]. According to the latest data, DAP and NAD+ can be released as cotransmitters together with catecholamines, regulating heart function [7].
The effects of DAP in cardiovascular system depend on the animal’s species, age, type of organ or tissue and the structure of the molecule used. Thus, depending on the number of phosphoric acid residues, DAP can cause vasodilatation or vasoconstriction of resistive arteries [8]. In rat myocardium, both DAP and NAD+ act via different subtypes of purinergic receptors at different stages of ontogenesis, which determines the difference in effects in animals of different age [9, 10].
In our previous studies we have shown that DAP and NAD+ reduce action potentials (AP) duration in rat atrial and ventricular myocardium [6, 11]. Such changes in the bioelectrical activity are usually mediated by modulation of transmembrane ionic currents. Nevertheless, the experiments using patch clamp method did not reveal any pronounced effects of DAP or NAD+ neither on repolarizing potassium currents, nor on L-type calcium current in isolated rat cardiomyocytes [12, 13]. In this regard, we hypothesized that DAP and NAD+ effects in rat myocardium could be mediated by their influence on ionic current of another nature – namely, on repolarizing chloride current.
At the present time, several repolarizing chloride currents are known, some of them can be found in mammalian cardiomyocytes. The pattern of cardiac bioelectrical activity can be modulated by protein kinase A activated chloride current (ICl, PKA), by stretch-activated chloride current (ICl, SWELL) and by calcium-dependent chloride current (ICl,Ca, also known as Ito2) [14, 15]. Under normal conditions, in the absence of adrenergic stimulation transient calcium-dependent current Ito2 makes the greatest contribution to bioelectrical activity of the myocardium [16]. Calcium-dependent chloride current, previously described in isolated working cardiomyocytes from rabbits [17] and mice [18], is defined as outward transient current resistant to tetraethylammonium and 4-aminopyridine (potassium currents blockers), but sensitive to calcium L-type current blocker nifedipine [17]. Nevertheless, Ito2 was not described in rat myocardium previously.
In the present study we have for the first time described transient current Ito2 in rat ventricular myocardium and investigated its regulation by purinergic compounds P1,P 4-di(adenosine-5')tetraphosphate (Ap4A), P1,P 4-di(adenosine-5')pentaphosphate (Ap5A) and NAD+ in ventricular myocytes isolated from rat.
MATERIALS AND METHODS
Ventricular myocytes were isolated from outbred male rats weighing 150–200 g as was described earlier [19]. Prior to the experiments the animals were anesthetized with intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). In addition to the anesthetics, rats were injected with heparine (1000 ME/kg) to prevent blood coagulation. The animals were decapitated, the heart was quickly excised, mounted onto Langendorff apparatus and retrogradely perfused with the following isolation solution (mM): NaCl 116; KCl 4; NH2PO4 1.7; NaHCO3 25; MgCl2 0.5; taurine 20; sodium pyruvate 5; glucose 11; bovine serum albumin 1 mg/kg; pH 7.4 maintained by aeration with carbogen (95% O2, 5% CO2) at 37°C. After 7 min, the perfusion was switched to isolation solution containing 0.5 mg/mL collagenase II (Worthington, USA), 0.1 mg/mL protease XIV (Sigma Aldrich, USA), and 10–5 M CaCl2. After 25–30 min the perfusion was stopped, the ventricles were dissected and gently triturated for mechanical isolation of cardiomyocytes in Kraft–Brühe solution (mM): monopotassium glutamate, 50; KCl, 30; KH2PO4, 30; MgSO4 · 7H2O, 3; taurine, 20; EGTA, 0.5; HEPES, 20; glucose, 10; pH 7.2 (KOH). This solution was used to store the cells for 6–7 h at room temperature. The experiments were performed in accordance with actual requirements of the local legislation on the proper handling of laboratory animals.
Ionic currents were recorded in the whole-cell configuration of the patch-clamp method using amplifier EPC-800 (HEKA Elektronik, Germany). Cardiomyocytes were placed in RC-26 experimental chamber (Warner Instruments, UK) and were constantly superfused with modified Tyrode’s solution (mM): NMDG-Cl 126; CsCl 5.4; MgCl2 1; CaCl2 2; HEPES 10; glicose 10; pH 7.4 (CsOH) at room temperature (24 ± 1°C), containing also potassium currents blockers 4-aminopyrydine (4-AP, 5 mM) and tetraethylammonium (TEA, 15 mM) [20]. The patch pipettes were made of borosilicate glass without filament (Sutter Instrument, USA) using PIP-6 puller (HEKA Elektronik, Germany) and filled with the following pipette solution (mM): Cs-aspartate, 110; CsCl, 20; MgCl2, 1; EGTA, 0.1; Mg-ATP, 5; Na-GTP, 0.03; HEPES, 10; pH 7.4 (CsOH). The resistance of pipettes tips was 2–3 MOhm. Thus, the extracellular concentration of Cl– was 137.4 mM and intracellular concentration was 20 mM, which is close to physiological concentrations of Cl– [21]. Before the registration of ionic currents, cell capacity, pipette capacity, and access resistance were compensated. The data were recorded and processed using WinWCP 4.8.6 (University of Strathclyde, UK) and Clampfit 10.3 (Molecular Devices, USA) software. The amplitude of transient current Ito2 was defined as the peak amplitude of the current blocked by DIDS (10–5 M) and CaCCinh-A01 (10–5 M). Current amplitude was normalized to cell capacitance (pA/pF).
Action potentials (AP) were recorded in preparations of rat right ventricle using sharp glass microelectrodes as described earlier [22]. Right after the excision, the heart was rinsed with physiological solution of the following composition (mM): NaCl 130; KCl 5.6; NaH2PO4 0.6; MgCl2 1.1; CaCl2 1.8; NaHCO3 20; glucose 11; pH 7.4 maintained by aeration with carbogen. Then the preparation of the right ventricle was isolated and pinned to the bottom of experimental chamber, endocardial side up, and superfused with Tyrode solution at 10 mL/min (temperature 37 ± 0.5°C). Transmembrane potentials were recorded after 2 h of adaptation. The experiments were performed using sharp glass microelectrodes (tip resistance 20–50 MOhm) made of borosilicate glass capillaries (Sutter Instrument, USA) using P-30 puller (Sutter Instrument, USA) and filled with KCl solution (3 M). The potentials were recorded using 1600 amplifier (A-M Systems, USA) and PowerGraph Professional 3.0 software (L-Card, Russia). During the processing of the recorded data, AP duration at 50% and 90% levels of repolarization (APD50 and APD90, respectively) were evaluated.
In the present study we used the following chemicals: P1,P 4-di(adenosine-5′)tetraphosphate ammonium salt (Ap4A, Sigma, USA); P1,P 4-di(adenosine-5′)pentaphosphate ammonium salt (Ap4A, Sigma, USA); NAD+ (AppliChem, Germany); nonselective blocker of chloride permeability 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS, Sigma, USA); 4-aminopyridine (4-AP, Santa Cruz, Biotechnology, USA); selective blocker of calcium-dependent chloride channels 6-(1,1-dimethylethyl)-2-[(2-furanylcarbonyl)amino]-4,5,6,7-tetrahydro-benzo[b]thiophene-3-carboxylic acid (CaCCinh-A01, Tocris Bioscience, UK); nonselective chloride channels blocker niflumic acid (Sigma, USA); tetraethylammonium chloride (TEA, MERCK, Germany).
All results are presented as mean ± standard error of the mean (SE) for n experiments. Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, USA). The normality of sampling distribution was evaluated using Pearson test. The statistical significance of the effects of DAP, NAD+ and chloride currents blockers were revealed using one-way repeated measures analysis of variance (one-way ANOVA), followed by Bonferroni test. The differences between groups were considered statistically significant at p ≤ 0.05.
RESULTS AND DISCUSSION
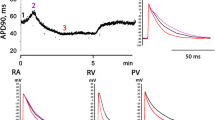
In rat ventricular cardiomyocytes at potentials more positive than –40 mV one can register transient outward current Ito, characterized by big amplitude and defining a fast repolarization phase of AP. However, after the replacement of Na+ ions with NMDG and K+ ions with Cs+ and in the presence of TEA (15 mM) and 4-AP (5 mM), the transient outward current induced by cell repolarization does not disappear completely. Since in the described experimental conditions only calcium and chloride currents remain unblocked, we can suppose the presence of transient repolarizing chloride current Ito2 in rat ventricular myocardium (Fig. 1a).
Transient chloride current Ito2 in rat ventricular cardiomyocytes. (a) Representative recordings of net outward transient current in control conditions and its 4-AP-resistant component, referring to as Ito2 current. (b) Representative recordings of Ito2 current in control conditions and in the presence of non-selective chloride channels blocker DIDS (10–5 M). (c) Representative recordings of Ito2 current in control conditions and in the presence of L-type calcium current blocker nifedipine (10–5 M). (d) Representative recordings of Ito2 current in control conditions and in the presence of selective calcium-dependent chloride channels blocker CaCCinh-A01 (10–5 M). The appropriate stimulation protocols are provided in the panels.
According to the results of the previous studies, chloride current Ito2 is calcium-dependent: its amplitude depends on the free Ca2+ concentration in the cytoplasm. Thus, Ito2 current was induced using step protocol with first 20-ms depolarizing step at –10 mV for calcium L-type current activation. It allowed us to achieve relatively high Ca2+ level in the cytoplasm and, therefore, to activate Ito2 current at the second step of the protocol [18]. We have demonstrated that 4-AP-resistant component of the Ito current can be completely blocked by nonselective blocker of chloride channels and transporters DIDS (10–5 M), calcium L‑type blocker nifedipine (10–5 M), and selective blocker of calcium-dependent chloride channels CaCCinh-A01 (10–5 M), while niflumic acid (10–5 M) was ineffective. It proves the chloride nature and calcium-dependence of the studied current and makes it possible to identify it as Ito2 current (Figs. 1b, 1c, 1d).
Figure 2 demonstrates the current–voltage relationship of the Ito2 current recorded in isolated rat ventricular myocytes. Though the amplitude of the current in rat ventricular myocardium was quite low in comparison to that in murine ventricular myocardium, the shape of the current–voltage characteristics completely fits the curve obtained in earlier experiments on isolated murine cardiomyocytes [18]. At membrane potentials more positive than –40 mV the current had an outward direction; its amplitude increased with membrane depolarization. At holding potentials more negative than –50 mV the current had an inward direction, but its amplitude was much lower. However, due to low Ca2+ concentration in cytoplasm, the Ito2 current may hardly exert a significant influence on cardiac electrical activity at these membrane potentials.
Current–voltage characteristics of the transient chloride current Ito2 in rat ventricular myocytes. (a) Representative recordings of Ito2 current at different holding potentials. (b) Current–voltage curve of the Ito2 current. Amplitude of the transient current Ito2 was determined as a peak amplitude of the current obtained after subtraction of the current recorded in the presence of the blocker (DIDS, 10–5 M; CaCCinh-A01, 10–5 M).
To study how DAP and NAD+ influence chloride current Ito2 in isolated rat ventricular cardiomyocytes, we added Ap4A, Ap5A and NAD+ to the extracellular solution (10–4 M) and registered the current at 40 mV. According to the published data, at this holding potential Ito2 has maximum amplitude, while ICaL current is almost absent. We evaluated the magnitude of the current by its peak amplitude after subtraction of the current recorded in the presence of blocker. The amplitude of the current was normalized to cell capacity. The application of Ap4A caused a statistically significant increase in the current amplitude by 44 ± 11% (p = 0.0065, n = 7). The effect of Ap4A was reversible. Ap5A (n = 6) and NAD+ (n = 6) did not induce any statistically significant changes in Ito2 magnitude or kinetics (Figs. 3a, 3b). Thus, among all the studied P2Y receptor agonists, only Ap4A exerted a pronounced effect on Ito2.
The influence of DAP and NAD+ on transient chloride current Ito2 in rat ventricular myocytes. (a) Representative recordings of Ito2 current in control conditions and in the presence of Ap4A (10–4 M). (b) Ito2 amplitudes in control conditions and in the presence of Ap4A (10–4 M, n = 7), Ap5A (10–4 M, n = 6) or NAD+ (10–4 M, n = 6); **p < 0.01, ****p < 0.0001, one-way ANOVA. (c) Action potentials in isolated preparation of rat right ventricle in control and in the presence of DIDS (10–4 M, n = 6) or DIDS (10–4 M) together with Ap4A (10–5 M, n = 6). (d) Action potentials in isolated preparation of rat right ventricle in control and in the presence of CaCCinh-A01 (10–5 M, n = 7) or CaCCinh-A01 (10–5 M) together with Ap4A (10–5 M, n = 7).
To estimate the contribution of Ap4A-induced enhancement of Ito2 to Ap4A-induced changes in the electrical activity of myocardium we performed a series of experiments using standard technique of intracellular AP recording with sharp glass microelectrodes (Figs. 3c, 3d). In the course of the experiment, an isolated preparation of rat right ventricle was superfused for at least 5 min with physiological solution containing DIDS (10–5 M) or CaCCinh-A01 (10–5 M) to achieve a complete blockade of calcium-dependent chloride channels. After that the preparation was supplied with physiological solution containing the blocker together with Ap4A (10–5 M). Previous studies showed that 5-min perfusion of a myocardium preparation with DAP causes pronounced and reversible changes in its electrical activity [11]. In the present study, in the presence of DIDS (n = 6) or CaCCinh-A01 (n = 7) Ap4A did not induce any statistically significant changes in the AP duration in isolated preparation of rat right ventricle. Therefore, we can conclude that the effects of Ap4A in rat ventricular myocardium are at least partially mediated by the Ap4A influence on the amplitude of chloride current Ito2.
We have previously shown that DAP and NAD+ are not only able to decrease AP duration in isolated preparations of rat myocardium; these compounds also suppress the contractility of isolated rat heart and calcium waves in isolated rat cardiomyocytes [6, 11, 23]. In rat ventricular cardiomyocytes Ap4A did not have a pronounced effect on the L-type calcium current, the amplitude of which due to the features of electromechanical coupling mechanism defines the force of the myocardium contractions. Considering the demonstrated enhancement of calcium-dependent chloride current Ito2 in the presence of Ap4A, it can be assumed that since this current contributes to early repolarization of cardiomyocytes, its enhancement can lead to a decrease in the AP duration at the levels of depolarization 25% and 50% and, consequently, to a decrease in plateau phase duration due to a decreased Ca2+ influx [24].
The main contenders to be molecular correlates of calcium-dependent chloride current are anoctamines (Ano, also known as TMEM16A channels), bestrophines and the CLCA family [25, 26].
It is unlikely that the discovered current is mediated by proteins of CLCA family, since these channels differ from the classic calcium-dependent chloride current in their biophysical properties (thus, CLCA channels can be activated by cell depolarization without an increase in the intracellular Ca2+ concentration and have linear current–voltage relationship) and expression patterns [27].
Ano family includes 10 different proteins, nevertheless, only Ano1 and Ano2 can mediate calcium-dependent chloride current in physiological conditions; the functions of the other members of Ano family are unclear [28, 29]. Ano1 is expressed in murine myocardium and mediate calcium-dependent chloride current in ventricular cardiomyocytes isolated from mice [20]. The data on similar studies performed on rat myocardium are not available for the present moment. Ano2 is expressed in olfactory epithelium, but no data are available on its expression in mammalian myocardium [28]. Bestrophines are expressed in human, canine and murine myocardium and form functional channels in cardiac cells [30–32]. Anoctamines and bestrophines differ in their sensitivity to non-selective chloride channels blockers. Thus, the concentration of half-maximum inhibition (IC50) of Ano1 channels by niflumic acid is about 7.5 × 10–6 M, when for bestrophin 1 channels (Best1) IC50 is higher, more than 10–4 M. On the contrary, bestrophines are more effectively inhibited by DIDS (IC50 about 4 × 10–6 M) in comparison to anoctamines (IC50 about 5.5 × 10–4 M) [25]. Thus, considering the sensitivity of the studied current to DIDS and niflumic acid, it can be assumed that its molecular correlate is rather channels of the bestrophines family, than anoctamines.
The possibility that the described current is transported by Cs+ cations instead of Cl– ions through non-selective cation channels of the TRP family, is excluded due to the sensitivity of the current to selective blocker CaCCinh-A01.
Taking into account the receptor mechanism, mediating DAP effects in mammalian myocardium, it was previously shown that purinergic P2Y receptors, but not P1 and P2X receptors contribute to that [33]. Among P2Y purinergic receptors the highest level of expression in mammalian myocardium was demonstrated for P2Y1, P2Y2, P2Y6 and P2Y11 receptors coupled with Gq-proteins [34]. It is known that Gq-protein-coupled P2Y receptors are able both to initiate protein kinase C signaling cascade and to activate nitric oxide- (NO-) dependent signaling pathway [35, 36]. In the previous study performed by our research group it was shown that the blockers of NO-dependent signaling pathway did not affect the effects of Ap4A and Ap5A in isolated rat heart, while protein kinase C blocker and non-selective blocker of phosphodiesterases prevented those effects [23]. It allows us to conclude that the effects of DAP in rat myocardium are mediated by the Gq-protein-coupled P2Y receptors and the following activation of signaling pathway including protein kinase C and certain phosphodiesterases.
Noteworthy, that in experiments on cells from other mammalian organs (bronchial smooth muscle cells, renal tubular epithelial cells) the activation of P2Y receptors (including P2Y2 receptors found in rat myocardium) leads to an increase in calcium-dependent chloride current mediated by channels of Ano family [37, 38]. However, in the described cases the increase in the amplitude of calcium-dependent chloride current occurs due to the activation of its channels by an increase in the intracellular Ca2+. On the contrary, in rat ventricular myocardium the activation of P2Y receptors by the DAP application was accompanied by negative inotropic effect due to a decrease of calcium transients and, consequently, a decrease in the intracellular Ca2+ level [23]. Thus, the purinergic activation of calcium-dependent chloride current in rat cardiomyocytes is mediated by a different signaling mechanism.
The obtained results suggest the contribution of the Ito2 current to the bioelectrical activity of rat ventricular myocardium in normal conditions and evidence for the possibility of its regulation by purinergic agonists. These data allow us to complement the existing model of AP generation and regulation in rat working myocardium. Nevertheless, further studies employing molecular biology techniques are required to clarify the molecular correlates of the Ito2 current in rat heart and to find possible ways of the Ito2 regulation.
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation (project no. 14-15-00268).
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare no conflicts of interests.
Statement on the welfare of animals. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the USA National Institutes of Health (NIH Publication no. 85–23, revised 1996) and the Declaration on humane treatment of animals. The experimental protocol was approved by the Bioethics Committee of Moscow Lomonosov State University.
REFERENCES
Baxi M.D., Vishwanatha J.K. 1995. Diadenosine polyphosphates: Their biological and pharmacological significance. J. Pharmacol. Toxicol. Methods. 33 (3), 121–128.
Hoyle C.H., Hilderman R.H., Pintor J.J., Schlüter H., King B.F. 2001. Diadenosine polyphosphates as extracellular signal molecules. Drug Dev. Res. 52 (1–2), 260–273.
Mutafova-Yambolieva V.N., Durnin L. 2014. The purinergic neurotransmitter revisited: A single substance or multiple players? Pharmacology & Therapeutics. 144 (2), 162–191.
Flores N.A., Stavrou B.M., Sheridan D.J. 1999. The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc. Res. 42, 15–26.
Smyth L.M., Yamboliev I.A., Mutafova-Yambolieva V.N. 2009. N-type and P/Q-type calcium channels regulate differentially the release of noradrenaline, ATP and β‑NAD in blood vessels. Neuropharmacology. 56 (2), 368–378.
Pustovit K.B., Kuzmin V.S., Sukhova G.S. 2014. The influence of extracellular NAD+ on contractile and bioelectrical activity of rat heart. Ros. Fiziol. Zhurnal im. Sechenova (Rus.). 100 (4), 445–457.
Pakhomov N.V., Pustovit K.B., Abramochkin D.V., Kuzmin V.S. 2017. The role of diadenosine pentaphosphate and nicotinamide adenine dinucleotide (NAD+) as potential nucleotide comediators in the adrenergic regulation of cardiac function. Neurochem. J. 11 (1), 63–71.
Steinmetz M., Schlatter E., Boudier H.A.J.S., Rahn K.H., De Mey J.G.R. 2000. Diadenosine polyphosphates cause contraction and relaxation in isolated rat resistance arteries. J. Pharmacol. Exp. Ther. 294 (3), 1175–1181.
Pustovit K.B., Ivanova A.D., Kuz’min V.S. 2018. Extracellular NAD+ suppresses adrenergic effects in the atrial myocardium of rats during the early postnatal ontogeny. Bull. Eksp. Biol. Med. (Rus.). 165, 4–8.
Pustovit K.B., Potekhina V.M., Pakhomov, N.V., Kuzmin, V.S. 2018. The effects of extracellular diadenosinetetraphosphate on the bioelectrical activity of atrial and ventricular rat myocardium at early stages of postnatal ontogeny. Vestnik Moskovskogo Univ. Seria 16, Biologia (Rus.). 73 (1), 52–59.
Pustovit K.B., Kuzmin V.S., Abramochkin D.V. 2016. Diadenosine tetra- and pentaphosphates affect contractility and bioelectrical activity in the rat heart via P2 purinergic receptors. Naunyn Schmiedebergs Arch. Pharmacol. 389 (3), 303–313.
Abramochkin D.V., Pustovit K.B., Filatova T.S. 2015. Effects of diadenosine polyphosphates on inward rectifier potassium currents in rat cardiomyocytes.Vestnik Moskovskogo Univ. Seria 16, Biologia (Rus.). 70 (4), 153–157.
Kuzmin V.S., Pustovit K.B. 2016. Effekty i mekhanizmy deistvia diadenozinovykh polifosfatov i ikh proizvodnykh v serdtse mlekopitayushchikh (Effects and mechanisms of action of diadenosine polyphosphates and their derivatives in mammalian heart). Moscow: Universitetskaya kniga.
Kuzmin V.S., Rosenstrauch L.V. 2010. The ionic mechanisms of action of class III antiarrhytmic drugs. Kardiologia (Rus.). 7, 49–61.
Bokeria O.L., Akhobekov A.A. 2014. Ionic channels and their role in the development of cardiac arrhythmias. Annaly Aritmologii (Rus.). 11 (3), 176–184.
Hiraoka M., Kawano S., Hirano Y., Furukawa T. 1998. Role of cardiac chloride currents in changes in action potential characteristics and arrhythmias. Cardiovasc. Res. 40, 23–33.
Zygmunt A.C., Gibbons W. 1992. Properties of the calcium-activated chloride current in heart. J. Gen. Physiol. 99 (3), 391–414.
Xu Y., Dong P.H., Zhang Z., Ahmed G.U., Chiamvimonvat N. 2002. Presence of a calcium-activated chloride current in mouse ventricular myocytes. Am. J. Physiol. Heart. Circ. Physiol. 283, H302–H314.
Isenberg G., Klockner U. 1982. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium”. Pflugers Arch. 395 (1), 6–18.
Ye Z., Wu M.M., Wang C.Y., Li Y.C., Gong Y.F., Zhang J., Wang Q.S., Song B.L., Yu K., Hartzell H.C., Duan D.D., Zhao D., Zhang Z.R. 2015. Characterization of cardiac anoctamin1 Ca2+-activated chloride channels and functional role in ischemia-induced arrhythmias. J. Cell Physiol. 230 (2), 337–346.
Pogorelov A., Pogorelova V., Pogorelova M. 2010. Does an electroneutral K+/Cl− antiport occur in cardiomyocyte during acute ischemia? Biophysics. 55 (5), 771–774.
Abramochkin D.V., Borodinova A.A., Nikolsky E.E., Rosenstrauch L.V. 2012. Modulation of non-quantum acetylcholine secretion by nitric oxide in the myocardium of rat right atrium. Biol. Membrany (Rus.). 29 (5), 317–323. (English version: Nitric oxide modulates intensity of non-quantal acetylcholine release in myocardium of the right atrium of rat. Biochemistry (Moscow) Suppl. Series A. 6 (4) 288–293).
Pakhomov N., Pustovit K., Potekhina V., Filatova T., Kuzmin V., Abramochkin D. 2018. Negative inotropic effects of diadenosine tetraphosphate are mediated by protein kinase C and phosphodiesterases stimulation in the rat heart. Eur. J. Pharmacol. 820, 97–105.
Oudit G.Y., Kassiri Z., Sah R., Ramirez R.J., Zobel C., Backx P.H. 2001. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J. Mol. Cell Cardiol. 33 (5), 851–872.
Liu Y., Zhang H., Huang D., Qi J., Xu J., Gao H., Du X., Gamper N., Zhang H. 2015. Characterization of the effects of Cl– channel modulators on TMEM16A and bestrophin-1 Ca2+ activated Cl– channels. Pflugers Arch. 467 (7), 1417–1430.
Cho H., Oh U. 2013. Anoctamin 1 mediates thermal pain as a heat sensor. Curr. Neuropharmacol. 11 (6), 641–651.
Eggermont J. 2004. Calcium-activated chloride channels: (Un)known, (un)loved? Proc. Am. Thorac. Soc. 1 (1), 22–27.
Tian Y., Schreiber R., Kunzelmann K. 2012. Anoctamins are a family of Ca2+-activated Cl– channels. J. Cell Sci. 125 (Pt 21), 4991–4998.
Pedemonte N., Galietta L.J. 2014. Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94 (2), 419–459.
Horvath, B., Vaczi K., Hegyi B., Gonczi M., Dienes B., Kistamas K., Banyasz T., Magyar J., Baczko I., Varro A., Seprenyi G., Csernoch L., Nanasi P.P., Szentandrassy N. 2016. Sarcolemmal Ca2+ entry through L-type Ca2+ channels controls the profile of Ca2+-activated Cl– current in canine ventricular myocytes. J. Mol. Cell Cardiol. 97, 125–139.
O’Driscoll K.E., Hatton W.J., Burkin H.R., Leblanc N., Britton F.C. 2008. Expression, localization, and functional properties of Bestrophin 3 channel isolated from mouse heart. Am. J. Physiol. Cell Physiol. 295 (6), C1610–C1624.
O’Driscoll K.E., Leblanc N., Hatton W.J., Britton F.C. 2009. Functional properties of murine bestrophin 1 channel. Biochem. Biophys. Res. Commun. 384 (4), 476–481.
Pustovit K.B., Abramochkin D.V. 2016. Effects of nicotinamide adenine dinucleotide (NAD+) and diadenosine tetraphosphate (Ap4A) on electrical activity of working and pacemaker atrial myocardium in guinea pigs. Bull. Exp. Biol. Med. 160 (6), 733–736.
Erlinge D., Burnstock G. 2008. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 4 (1), 1–20.
Buvinic S., Briones R. Huidobro-Toro J.P. 2002. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br. J. Pharmacol. 136 (6), 847–856.
Erb L., Weisman G.A. 2012. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1 (6), 789–803.
Mitchell C., Syed N.I.H., Gurney A.M., Kennedy C. 2012. A Ca2+-dependent chloride current and Ca2+ influx via Cav1.2 ion channels play major roles in P2Y receptor-mediated pulmonary vasoconstriction. Br. J. Pharmacol. 166 (4), 1503–1512.
Rajagopal, M., Kathpalia P.P., Thomas S.V., Pao A.C. 2011. Activation of P2Y1 and P2Y2 receptors induces chloride secretion via calcium-activated chloride channels in kidney inner medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 301 (3), F544–F553.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Filatova
Abbreviations: AP, action potential; DAP, diadenosine polyphosphates; TEA, tetraethylammonium; 4-AP, 4-aminopyridine.
Rights and permissions
About this article
Cite this article
Filatova, T.S., Abramochkin, D.V. Purinergic Regulation of Transient Calcium-Dependent Chloride Current Ito2 in Rat Ventricular Myocardium. Biochem. Moscow Suppl. Ser. A 13, 147–154 (2019). https://doi.org/10.1134/S1990747818060041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747818060041