Abstract
Diadenosine polyphosphates (Ap(n)As) are endogenously produced molecules which have been identified in various tissues of mammalian organism, including myocardium. Ap(n)As contribute to the blood clotting and are also widely accepted as regulators of blood vascular tone. Physiological role of Ap(n)As in cardiac muscle has not been completely elucidated. The present study aimed to investigate the effects of diadenosine tetra- (Ap4A) and penta- (Ap5A) polyphosphates on contractile function and action potential (AP) waveform in rat supraventricular and ventricular myocardium. We have also demonstrated the effects of A4pA and Ap5A in myocardial sleeves of pulmonary veins (PVs), which play a crucial role in genesis of atrial fibrillation. APs were recorded with glass microelectrodes in multicellular myocardial preparations. Contractile activity was measured in isolated Langendorff-perfused rat hearts. Both Ap4A and Ap5A significantly reduced contractility of isolated Langendorff-perfused heart and produced significant reduction of AP duration in left and right auricle, interatrial septum, and especially in right ventricular wall myocardium. Ap(n)As also shortened APs in rat pulmonary veins and therefore may be considered as potential proarrhythmic factors. Cardiotropic effects of Ap4A and Ap5A were strongly antagonized by selective blockers of P2 purine receptors suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), while P1 blocker DPCPX was not effective. We conclude that Ap(n)As may be considered as new class of endogenous cardioinhibitory compounds. P2 purine receptors play the central role in mediation of Ap4A and Ap5A inhibitory effects on electrical and contractile activity in different regions of the rat heart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diadenosine polyphosphates (Ap(n)As) belong to the wide group of dinucleotide compounds, which consist of two adenosine molecules connected with two (Ap2A) or up to six (Ap6A) inorganic phosphate residues. Ap(n)As have been recently considered as endogenous signaling compounds which are present in a number of mammalian tissues and participate in the regulation of various physiological functions (Delicado et al. 2006; Flores et al. 1999; Stavrou et al. 2001a, b; Vollmayer et al. 2003).
Ap(n)As contribute to blood clotting since they are stored in platelets in a high concentration and may be released by degranulation following the platelets activation (Lüthje and Oqilvie 1983; Lüthje et al. 1987; Schlüter et al. 1994). Ap(n)As have been also widely accepted as a regulator of vascular tone (Steinmetz et al. 2002; 2003; 2005; Sumiyoshi et al. 1997). However, pattern of Ap(n)As effects in vascular smooth muscle has not been completely resolved, because similar Ap(n)As produced vasoconstriction in some types of veins and arteries (Conant et al. 2008) and vasodilatation in the others (Steinmetz et al. 2000). Sympathetic and parasympathetic nerve terminals also contain Ap(n)As capable of modulating the neurotransmitter effects (Miras-Portugal et al. 1998). Recently, Ap(n)As have been suggested as neurotransmitters both in peripheral and central nervous system (Mutafova-Yambolieva and Durnin 2014). Also, Ap(n)As are considered as “alarmones,” molecules involved in signal transduction during tissue stress, like ischemia or heat shock (Stavrou et al. 2001a, b; Stavrou 2003). Ap(n)As are structurally related to purine nucleotides, and effects of extracellular Ap(n)As are likely to be mediated via different subtypes of purine receptors (Brandts et al. 2003; Conant et al. 2000; Laubinger et al. 2003; Nahum et al. 2006).
Intracellular localization of Ap2A-Ap6A was demonstrated in the myocardium of various species, including human (Jankowski et al. 2003; Luo et al. 2004). Ap4A and Ap5A were found in myocardial-specific granules in a high concentration (Luo et al. 1999). Moreover, release of granules containing Ap(n)As after stimulation of cells by cholinomimetic carbachol has been shown in the brain (Pereira et al. 2000). As mentioned above, Ap(n)As are vasoactive compounds that may be released from platelets during ischemia-reperfusion, affect coronary circulation, and alter cardiac performance in direct or oblique manner (García-Villalón et al. 2009).
Nevertheless, the molecular mechanisms of Ap(n)As cardiotropic effects are poorly investigated. Several studies were dedicated to the effects of different diadenosine polyphosphates in multicellular preparations of guinea pig, rabbit, canine, and human myocardium (Stavrou 2003). The authors reported extremely contradictory data concerning changes in cardiac contractility and electrical activity induced by Ap(n)As. The receptor mechanism of described effects is also controversial, although P1 and P2 purinergic receptors appear to be the best candidates for Ap(n)As target. Moreover, effects of Ap(n)As have never been compared in different regions of the heart, while it is well known that effects of classical neurotransmitters, acetylcholine, and noradrenaline, greatly vary depending on the type of myocardium.
In the rat cardiac muscle, modulation of contractile or electrical activity by Ap(n)As has been scarcely studied. To our knowledge, only one report of Ap4A effects in rat concerning ventricle contractility is available (Vahlensieck et al. 1999). Also, no comparison between effects of Ap4A and Ap5A has been provided by earlier investigators.
Thereby, the present study aimed to investigate the effects of Ap4A and Ap5A on a contractile function and action potentials of the rat heart. Here, we provide the first comparison of Ap(n)As-induced action potential (AP) waveform alteration in different types of supraventricular and ventricular myocardium. The present study demonstrates for the first time the effects of A4pA and Ap5A in pulmonary veins (PVs) myocardium that plays crucial role in genesis of atrial fibrillation.
Materials and methods
Animals
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The experimental protocol was approved by Bioethics Committee of Moscow State University. Male Wistar rats weighing 250–300 g were used in the study (n = 104, 8 weeks old). Rats were held in the animal house for 4 weeks under a 12-h:12-h light:dark photoperiod in standard T4 cages prior to the experiment and fed ad libitum.
Heart preparation
Rats were anesthetized with intraperitoneal injection of 80 mg/kg ketamine and 10 mg/kg xylazine. Heparin (1000 U/kg) was added to the anesthetics solution to prevent blood coagulation in the coronary vessels of the excised heart. The chest was opened, and the heart was rapidly excised and placed into a bath with cold (+4 °C) Krebs-Henseleit solution (KH) that contained (in mM): NaCl 118.0, KCl 4.7, NaHCO3 25.0, MgSO4 1.2, CaCl2 2.5, KH2PO4 1.2, and glucose 5.5, bubbled with carbogen (95 % O2, 5 % CO2), with pH 7.4 ± 0.1.
Measurements of mechanical activity in the isolated Langendorff-perfused heart
The aorta was cannulated and the heart was placed into the constant pressure (80 mmHg) Langendorff perfusion system. Normoxic perfusion with KH solution was carried out at 37 °C in spontaneously beating or paced hearts. In the latter case, heart rate (4 Hz) was governed by platinum electrodes placed on the surface of the right atria after the dissection of sinoatrial node region.
A latex balloon connected to the pressure transducer by a plastic catheter was inserted into the left ventricle (LV) through mitral valve. End-diastolic pressure was set to 5 mmHg by filling the balloon with water. Left ventricle pressure was recorded continuously using bridge amplifier and data acquisition system (L-Card, Russia) with PowerGraph software (Disoft, Russia). LV developed pressure (LVDP) was calculated as the difference between the systolic and end-diastolic pressure (LVEDP) values, received from LV pressure curve. Also, maximal rates of contraction and relaxation (dP/dtmax, dP/dtmin respectively) were calculated. Next, dP/dtmax and dP/dtmin were divided by LVDP to receive the normalized values (dP/dtmax/LVDP, dP/dtmin/LVDP). Heart rate was measured and rate-pressure production was calculated to estimate heart performance in spontaneously beating preparations.
Experimental protocol for isolated heart
Hearts were allowed to equilibrate for 10 min. Then three concentrations (0.1, 1, 10 μM) of diadenosine tetra- (Ap4A, first series of experiments) or pentaphosphate (Ap5A, second series) were consequently administrated for 5 min each, with 15 min of washout after each concentration. Total duration of whole experiments never exceeded 60 min. Special time-control experiments (n = 3) were performed to ensure that heart activity does not change significantly in the interval from 10th to 60th minute of perfusion.
Isolation of cardiac multicellular preparations
The heart was excised and rinsed as described in Heart preparation section. Fragments of the left auricle (LA), right auricle (RA), interatrial septum (IAS), and right ventricular wall (Ventr) were isolated and pinned endocardial side up to the bottom of the experimental chamber (3 ml) supplied with physiological solution at 10 ml min−1 (37.5 °C). The composition of the solution was as follows (mM): NaCl 118.0, KCl 2.7, NaH2PO4 2.2, MgCl2 1.2, CaCl2 1.2, NaHCO3 25.0, and glucose 11.0, bubbled with carbogen, with pH 7.4 ± 0.1. Since all the types of preparations lacked intrinsic pacemaker activity, they were paced throughout the experiment with a pair of silver Teflon-coated electrodes (pacing rate—3 Hz, pulse duration—2 ms, pulse amplitude—2 times threshold).
Isolation of multicellular pulmonary veins preparations
Rats were anesthetized as described in Heart preparation section. The chest was opened; the heart with lung lobes was rapidly excised and rinsed with physiological solution. To allow outflow of the solution, the outer edges of the lung lobes were trimmed. The left atrium was incised at the atrioventricular border and cannulated. Blood from the left atria and pulmonary veins was flushed out by injection of physiological solution. Following that fascia and pulmonary arteries were removed and preparation of isolated supraventricular region including LA, pulmonary veins (PVs) and lung lobes were pinned in a preparation bowl. Finally, tubular PV preparations were isolated from one or two lung lobes. Isolated PVs were cut along the axis and pinned in experimental chamber with inner side up. During the following experiments, preparations were perfused and paced as described above.
Microelectrode AP recording
After 1 h of equilibration, transmembrane potentials were recorded from LA, RA, IAS, Ventr, and PVs with glass microelectrodes (30–45 MΩ) filled with 3 M KCl connected to a high input impedance amplifier Model 1600 (A-MSystems, Sequim, WA, USA). The signal was digitized and analyzed using specific software (L-card, Russia; Synaptosoft, USA). Stable impalements were maintained during the entire period of drug application. Changes in the resting potential, AP upstroke velocity (dV/dtmax) and AP duration at 50 % (APD50) and 90 % of repolarization (APD90) were determined.
Ap4A or Ap5A (10 μM) were administrated for 5 min after 5 min of control recording. P1 purinergic receptors antagonist DPCPX (0.1 μM), P2 purinergic receptors antagonists suramin (10 μM), or pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (100 μM) were applied for 10 min before and during the application of Ap4A or Ap5A.
Drugs
Ap4A, Ap5A, and DPCPX were purchased from Sigma (St. Louis, MO, USA). Suramin was purchased from Tocris (Bristol, UK). PPADS was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA).
Statistical analysis
All data in the text and figures except the original recordings are presented as means ± SEM for n experiments. Statistica 6 (StatSoft Inc.) was used for statistical analysis of the data. Effects of Ap(n)As on registered parameters relative to the respective basal value of these parameters were analyzed by Wilcoxon test. The effects of Ap(n)As in the absence and presence of DPCPX or suramin were compared by Mann-Whitney test. P < 0.05 was adopted as the level of statistical significance.
Results
Effects of Ap4A and Ap5A on isolated rat heart contractile function
Both Ap4A and Ap5A affected the isolated rat heart rate and contractile activity. Slight non-significant effects were induced by 1 μM of Ap4A and Ap5A. However, only highest tested concentration (10 μM) of Ap(n)As altered heart rate and mechanical function significantly (Fig. 1).
Effects of Ap4A (continuous line) and Ap5A (broken line) (0.1–10 μM) in the isolated Langendorff-perfused rat heart. Both Ap4A and Ap5A decrease heart rate (a) and rate pressure production (b) in isolated non-paced heart and induce the rise of diastolic pressure (d) and reduction of contractility (c, e–h). Here and in the following figures: LVDP left ventricular developed pressure, LVEDP left ventricular end diastolic pressure, dP/dt max or dP/dt min maximal rate of contraction and relaxation, respectively, (dP/dtmax)/LVDP, (dP/dtmax)/LVDP normalized maximal rate of contraction and relaxation. Asterisk and ampersand indicate significant effect of Ap4A and Ap5A, respectively, p < 0.05, Wilcoxon test
Ap(n)As showed different time course of developed effects. Representative traces of LVDP in control conditions and during the treatment with 10 μM Ap4A and Ap5A are shown at Fig. 2. Effect of Ap4A rapidly reached the peak after start of infusion and became negligible to the end of fifth minute of infusion (Fig. 2, left). In contrast to Ap4A, Ap5A produced permanent depression of contractile activity observed throughout the period of drug application (Fig. 2, right). Termination of A4pA or A5pA application led to the recovery of heart rate and mechanical activity in 10–15 min. However, washout was accompanied by extrasystoles and rhythm disturbances (Fig. 2, top) both in spontaneously beating and paced hearts.
Representative traces of contractile activity in isolated paced rat heart during the application of 10 μM Ap4A (left panel) or Ap5A (right panel). Downward and upward arrows mark the onset and termination of Ap(n)As application. LVDP insets demonstrate fragments of contractile activity traces marked by red frames, superimposed on the control traces
Ap4A and Ap5A (10 μM) induced reduction of the heart rate in isolated spontaneously beating hearts from 285 ± 5 and 282 ± 7 beats per minute at baseline to 260 ± 8 (by 9 %, n = 6, p < 0.05) and 256 ± 10 (10 %, n = 7, p < 0.05) beats per minute, respectively (Fig. 1a). Both polyphosphates also significantly decreased heart performance calculated as a product of heart rate and LVDP. Ap4A and Ap5A reduced heart performance by 40 and 73 %, respectively (Fig. 1b).
Paced hearts showed decreasing of LVDP in response to the Ap4A and Ap5A application by 42.6 and 80.5 % of control, respectively (Fig. 1c). Thus, reduction of LVDP induced by Ap5A was significantly bigger than the same induced by Ap4A (p < 0.05, n = 6 for both drugs). LVDP reduction occurred predominantly due to the dramatic increase of LVEDP in response to both Ap4A and Ap5A (Fig. 1d; Fig. 2, top).
In few experiments (n = 3), application of Ap4A and Ap5A induced moderate elevation of LVDP (5 ± 3 %) that was always transient and followed by decrease of this parameter. Thus, stable positive inotropic effect was not assessed in our experiments.
Maximal rate of contraction and relaxation of paced hearts were strongly affected by Ap(n)As application (Fig. 1e, f; Fig. 2). One micromolar Ap4A induced moderate increase of dP/dtmax; however, effect was very transient and not statistically significant. Unlike Ap4A, 1 μM Ap5A failed to alter dP/dtmax. Administration of 10 μM Ap4A induced only decrease of dP/dtmax by 19.5 % and dP/dtmin by 18.5 % of control. Application of Ap5A also produced reduction of dP/dtmax and dP/dtmin by 75.2 and 70 %, respectively. Therefore, application of 10 μM of Ap5A resulted in significantly (p < 0.05) bigger drop of dP/dtmax and dP/dtmin in comparison with the effects of 10 μM Ap4A.
In addition, normalized maximal rate of contraction and relaxation (dP/dtmax/LVDP, dP/dtmin/LVDP) of paced hearts was also decreased under 10 μM Ap4A and Ap5A (Fig. 1g, h).
Effects of Ap4A and Ap5A on AP waveform in isolated myocardial preparations
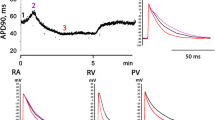
Both Ap4A and Ap5A produced qualitatively similar effects on AP configuration in all tested types of atrial and ventricular myocardial preparations (Fig. 3). Control APD50 and APD90 in supraventricular preparations were as follows: LA—25 ± 4 and 59 ± 5 ms (n = 15), RA—21 ± 4 and 45 ± 4 ms (n = 12), IAS—26 ± 5 and 54 ± 6 (n = 10), respectively. Application of 10 μM Ap4A induced significant reduction of both APD50 and APD90 in LA (by 15 and 23 %, n = 8, p < 0.05), in RA (36 and 22 %, n = 6, p < 0.05), and in IAS (13 and 11 %, n = 5, p < 0.05). Ten micromolar Ap5A also decreased APD50 and APD90 in LA by 26 and 10 % (n = 7, p < 0.05), in RA by 30 and 29 % (n = 6, p < 0.05), in IAS by 5 and 7 % (n = 5, p < 0.05), respectively. Thus, most pronounced effect was observed in RA in response to Ap5A, while IAS demonstrated minimal AP alteration in response to both compounds.
Changes in AP waveform induced by Ap4A and Ap5A (10 μM) in various regions of rat heart. a Alteration of APD50; b alteration of APD90; representative examples of APs recorded in preparations from different regions of the heart in control conditions and during c Ap4A or d Ap5A application. The insets demonstrate the time course of APD90 reduction in right ventricular wall preparation during Ap4A (c) or Ap5A (d) application. LA left auricle, RA right auricle, IAS interatrial septum, PVs pulmonary veins, Ventr right ventricular wall. Downward and upward arrows mark the onset and termination of Ap5A application. Ampersand indicates significant difference from the control values, p < 0.05, Wilcoxon test. Asterisk indicates significant difference between effect of Ap5A and Ap4A, p < 0.05, Mann-Whitney test
In ventricular preparations, control APD50 and APD90 were 18 ± 4 and 36 ± 5 ms (n = 12), respectively. Application of Ap4A led to APD50 and APD90 reduction by 27 and 28 % (p < 0.05). Thus, effect of Ap4A in the supraventricular and ventricular myocardium was qualitatively similar but significantly greater in ventricle (p < 0.05) than in each of supraventricular regions. Ap5A reduced APD50 and APD90 by 47 and 55 % (p < 0.05), respectively.
Separate series of experiments was performed using PV preparations. Paced PV myocardium demonstrates atrial-like APs with overshoot, fast upstroke, and resting potential around −75 ± 3 mV (Fig. 3c, d). Control APD50 and APD90 in PVs preparations were 27 ± 3 and 72 ± 4 ms (n = 11), respectively. Administration of Ap4A (n = 5) and Ap5A (n = 6) resulted in significant (p < 0.05) decreasing of APD50 (by 27 and 29 %, respectively) and APD90 (by 11.5 and 32 %, respectively) in PVs (Fig. 3). Both diadenosine tetra- and pentaphosphates were unable to initiate proarrhythmic events like early or delayed postdepolarizations in paced PV preparations. However, extra APs were observed in PV preparations during washout of both compounds.
Both Ap(n)As demonstrated similar dynamics of effect in all types of supraventricular myocardium preparations. Start of Ap4A and Ap5A application led to the rapid (in less then 1 min) AP shortening, following that AP waveform remained stable during the entire period of drug application. Termination of Ap4A or Ap5A infusion led to the slow recovery of APD in 5–15 min. In few experiments, AP duration exceeded control values during the washout. Interestingly, we observed strikingly different time courses of AP(n)As effect in ventricular preparations (Fig. 3, inserts). Ap4A produced quick decrease of APD, which then stabilized on more or less constant level until the drug was washed out. Ap5A induced gradual continuous AP shortening during the entire period of drug application without stabilization of AP waveform parameters. Washout of Ap4A or Ap5A led to the gradual recovery of APD.
Modulation of Ap4A and Ap5A effects by antagonists of purine receptors
Effects of P1 and P2 blockers were tested in LA preparations. P1 receptor antagonist DPCPX (0.1 μM) failed to suppress effects of Ap4A or Ap5A significantly (n = 10). On the other hand, AP shortening induced by Ap4A (n = 6) and Ap5A (n = 6) was almost abolished in the presence of P2 blocker 10 μM suramin (Fig. 4). Another P2 blocker PPADS (100 μM) tended itself to induce AP shortening, but in the presence of PPADS, neither Ap4A (n = 5) nor Ap5A (n = 6) decreased APD (Fig. 4) Representative traces of APs during application of 10 μM Ap4A in the presence of different blockers are shown at Fig. 4b. The same for Ap5A is shown in Fig. 4d.
Alteration of Ap(n)As effect in the left auricle preparation by selective blockers of P1- and P2-purinoreceptors. Decrease of APD90 induced by Ap4A (a) or Ap5A (c) alone and in the presence of P1 blocker DPCPX (0.1 μM), P2 blocker suramine (sur, 10 μM), or P2 blocker PPADS (100 μM). PPADS’ own effect is also shown. NS nonsignificant. Asterisk indicates significant difference from the control values, p < 0.05, Wilcoxon test. b, d Representative traces of APs in control conditions and in the presence of different blockers
Discussion
Effects of Ap(n)As in the rat heart
The main objective of this study was to reveal effects of diadenosine polyphosphates in the rat heart. We have demonstrated that two dinucleotide polyphosphates, Ap4A and Ap5A, altered mechanical and electrical properties of the rat myocardium. In Langendorff-perfused heart, tested compounds reduced spontaneous beating rate, depressed cardiac performance and contractility, and elevated the end-diastolic pressure. Both positive and negative inotropic effects were reported for various Ap(n)As in several previous studies.
While Ap5A was shown to stimulate contractility of the guinea pig isolated papillary muscles (Stavrou et al. 2001a, b), Ap4A failed to affect mechanical function in similar preparations (Vahlensieck et al. 1999). In addition, negative inotropic effects of both compounds were described in the guinea pig atrial myocardium (Hoyle et al. 1996; Vahlensieck et al. 1999). Also, Ap5A-induced negative chronotropy was reported from experiments with perfused guinea pig hearts (Brandts et al. 1998). In canine atrial and ventricular trabeculae, Ap4A produced suppression of contractility (Neumann et al. 1999), while in the human ventricular tissue, both the same and another group of authors observed positive inotropic effect of Ap4A (Knapp et al. 1999; Vahlensieck et al. 1999). Ap5A produced similar effect in human atrial preparations (Arvola et al. 2004). In contrast to human, rat atrial and ventricular trabeculae showed negative contractile response to Ap4A (Vahlensieck et al. 1999). Such discrepancies may result from differences in sensitivity to Ap(n)As between different species and tissues. Moreover, the used concentrations of compounds varied from 0.01 to 100 μM among the studies.
Our data obtained in isolated heart experiments are concordant with the findings of Vahlensieck et al. (1999). Negative inotropy may be mediated by various intracellular mechanisms, including reduction of L-type calcium current (ICaL). In the latter case, alteration of contractility should be accompanied by changes in electrical activity. The earlier data concerning electrophysiological effects of Ap5A and Ap4A are extremely controversial. While in the rabbit heart (Brandts et al. 2003), authors observed the Ap5A-induced reduction of effective refractory period, which might point to shortening of APs, low concentrations (0.01–1 μM) of Ap4A and Ap5A produced AP prolongation in guinea pig ventricular myocardium (Stavrou et al. 2001a, b). Therefore, to reveal the mechanism underlying the described effects of Ap(n)As on cardiac contractility, we have investigated electrophysiological effects of Ap4A and Ap5A in several types of supraventricular and ventricular rat myocardium.
Application of both compounds led to the significant reduction of AP duration measured at 50 and 90 % AP repolarization in paced myocardium of LA, RA, and IAS as well as in PV preparations. Interestingly, the extent of this effect was almost similar in all studied types of supraventricular myocardium. In ventricular myocardium, Ap4A and especially Ap5A induced even more pronounced AP shortening than in the atrial preparations. Since it is widely accepted that AP shortening leads to depression of contractile activity, we can assume that negative inotropic effects of Ap4A and Ap5A results from decrease of AP duration. Moreover, the difference in time course of Ap4A and Ap5A effects, which we have noticed in isolated right ventricular wall preparations, may partly underlie the appropriate difference in Ap(n)As effects on contractile activity of isolated hearts. Ap4A, which abruptly decreases ventricular APD, produces short-lasting depression of contractility, while Ap5A induces continuous shortening of APs and stable long-lasting negative inotropic effect. However, the fact that negative inotropic effect of Ap4A fades while the drug is still present in perfusing solution indicates that effect on contractility is not a simple derivative of AP waveform alteration and additional factors should be taken into account. Suppression of ICaL by Ap(n)As, which was demonstrated in guinea pig ventricular cardiomyocytes (Vahlensieck et al. 1999), may partly explain both AP shortening and attenuation of contractions, although this assumption should be directly checked in further experiments with isolated rat myocytes.
It is well known, that supraventricular arrhythmias, including atrial fibrillation (AF), are characterized by hemodynamic disturbances, endothelial damage, hypercoagulability, and platelet activation (Iwasaki et al. 2011; Schotten et al. 2011). Clots are frequent in atrial chambers of patients affected by AF. As has been mentioned earlier, Ap(n)As are extensively released during platelet activation and participate in blood clotting. Thus, Ap(n)As may contribute to the proarrhythmical alterations of supraventricular bioelectrical activity maintaining the persistent AF. Similar effects including AP shortening and atrial refractoriness reduction have been reported for other purine nucleotides (Mallet 2004; van der Heyden et al. 2005).
It is known that pulmonary veins contain so-called myocardial sleeves that extend from the atria to the distal regions of the vessels. Starting from pioneering studies of Haissaguerre et al. (1998), PV myocardium was considered as a main source for AF initiation and maintenance. In the present study, we demonstrate the first evidence of AP shortening in PV myocardium induced by Ap(n)As. This effect should be considered as proarrhythmic, although no postdepolarizations or episodes of fibrillation were observed in Ap4A- and Ap5A-treated PV preparations. Interestingly, washout of PV preparations leads to extrasystoles, which may also point to arrhythmogenic activity of Ap(n)As. Thus, effects of Ap(n)As in PVs shown in our experiments should encourage future investigators for more detailed study of Ap(n)As putative arrhythmogenic properties.
Molecular mechanism of Ap(n)As cardiotropic effects
While intracellular Ap(n)As are proposed as inhibitory ligands of the ATP-sensitive K+ channels (Jovanovic et al. 1996; 1997; 1998), extracellular Ap(n)As are considered as agonists of purine receptors (Laubinger et al. 2003; Lewis et al. 2000; Nahum et al. 2006). Earlier studies provided the data supporting P1 or P2 purine receptors as a main target for extracellular Ap(n)As in various tissues including myocardium (Arvola et al. 2004; Brandts et al. 1998; 2003; Conant et al. 2000; McDonald et al. 2002; Neumann et al. 1999; Vahlensieck et al. 1999). Also, simultaneous activation of both P1 and P2 purine receptors by Ap(n)A was suggested as a complex signal required for alteration of inotropy (Hoyle et al. 1996).
It is well known that soluble and membrane-binded ectohydrolases and ectonucleotidases (NT5 or CD73, CD39, CD203) may hydrolyze such nucleotides as NAD, ATP, and ADP down to AMP, adenosine, and inorganic phosphates. Extracellular degradation of dinucleotide polyphosphates has been also shown in various tissues. Central role in hydrolysis of Ap(n)As belongs to pyrophosphatases/phosphodiesterases (Vollmayer et al. 2003). Therefore, activation of P1 purine receptors by Ap(n)As-derived adenosine has been suggested as a mechanism of Ap(n)As action (Brandts et al. 2003). Also, specific non-P1 and non-P2 diadenosine polynucleotide receptors were suggested to mediate Ap(n)As action (Arvola et al. 2004; Pintor Miras-Portugal 2000). In addition, blockade of Ap(n)As effects by P2 antagonist suramin in one species and, at the same time, insensitivity to the blocker in another were shown in the same study (Arvola et al. 2004). Therefore, receptor mechanism of Ap(n)As effects remains questionable.
Cardiotropic effects of Ap4A and Ap5A demonstrated in our study were strongly antagonized by P2 antagonist suramin, while P1 blocker DPCPX was not effective. Since suramin was shown to have numerous off-target effects including the disruption of receptor-G protein coupling (Chung and Kermode, 2005), additional P2 blocker PPADS was tested. The high concentration of this drug (100 μM) was required to assure the blocking effect on P2Y-receptors, which are less sensitive to PPADS than P2X (von Kügelgen and Hoffmann, 2015; von Kügelgen, 2006). PPADS completely abolished Ap4A- and Ap5A-induced AP shortening. Thus, two widely used P2 blockers antagonize electrophysiological effects of studied Ap(n)As. In addition, AP shortening induced by Ap4A and Ap5A appears to be very similar to the effects of ATP, well-known P2 agonist (Vassort 2001). Therefore, we suggest that P2 purine receptors play the central role in mediation of Ap4A and Ap5A inhibitory effects in the rat heart. However, we are unable to completely exclude contribution of the P1 agonists produced from Ap(n)As by nucleotidases and another enzymes. It is unlikely that these compounds were produced in sufficient quantity particularly in our experiments.
The family of P2 receptors consists of ionotropic P2X receptors and metabotropic P2Y receptors associated with Gq/11 proteins. Activation of P2X receptors induces transient inward cationic current leading to the transient membrane depolarization. Since no decrease of resting membrane potential associated with Ap(n)As application was observed in electrophysiological experiments, the involvement of P2X in mediation of Ap(n)As effects is very unlikely.
Stimulation of P2Y receptors leads to the activation of phosphoinositide signaling cascade. Moreover, coupling of P2Y receptors to the NO/cGMP cascade has been shown in several studies (Buvinic et al. 2002). NO stimulates soluble guanylate cyclase leading to accumulation of intracellular cGMP, which in turn stimulates phosphodiesterase 2 and reduces intracellular cAMP content. Decrease of cAMP level leads to attenuation of ICaL (see Steinmetz et al., 2002; 2005 for review) resulting in depression of contractility and AP shortening (van der Heyden et al. 2005). The ability of Ap4A to decrease cAMP content has been previously demonstrated (Edgecombe et al. 1999). Inhibition of NOS affects Ap(n)A-induced alterations of contractility and AP waveform in guinea pig hearts (Stavrou 2003). Therefore, inhibitory effects of Ap(n)As in rat myocardium are likely to be mediated by NO/cGMP pathway, although this hypothesis should be directly checked in future studies.
Conclusion
This study provides the first detailed description of Ap4A and Ap5A effects in the rat heart. Both compounds produce pronounced shortening of APs in three types of atrial myocardium, in myocardial sleeves of pulmonary veins, and especially in ventricular tissue. The described changes in AP waveform may be considered as potential proarrhythmic influence in atrial myocardium. In ventricular myocardium, Ap(n)A-induced AP shortening definitely underlies depression of contractile activity, which both drugs produced in the isolated Langendorff-perfused rat heart. Activation of P2 purine receptors is suggested as the main mechanism of described Ap(n)A effects. Thus, our data widens the knowledge about diadenosine polyphosphates as novel endogenous signaling compounds and potential regulators of cardiac function.
Limitations
The present study has several important limitations due to the possibility of indirect effects of studied compounds. Isolated heart and multicellular preparations of myocardium have autonomic innervation, which could be affected by Ap(n)As. Since there is no input from the central nervous system driving the postganglionic parasympathetic neurons and sympathetic postganglionic fibers, we may assume the absence of evoked neurotransmitter release in the preparations. However, presence of non-quantal acetylcholine release, which does not require impulse activity of postganglionic neurons, has been shown recently in the rat heart (Abramochkin et al. 2010; 2012). This process might be regulated by neurotransmitters, and effects of Ap(n)As on non-quantal acetylcholine secretion leading to changes in cardiac performance cannot be excluded.
Abbreviations
- Ap(n)As:
-
Diadenosine polyphosphates
- Ap4A:
-
Diadenosine tetraphosphate
- Ap5A:
-
Diadenosine pentaphosphate
- AP:
-
Action potential
- PVs:
-
Pulmonary veins
- P1 receptors:
-
Purine receptors type 1
- P2 receptors:
-
Purine receptors type 2
- dV/dtmax :
-
Upstroke velocity
- APD50:
-
Action potential duration at 50 % repolarization
- APD90:
-
Action potential duration at 90 % repolarization
- LV:
-
Left ventricle
- LVDP:
-
Left ventricle developed pressure
- LVEDP:
-
Left ventricle end-diastolic pressure
- RA:
-
Right auricle
- LA:
-
Left auricle
- IAS:
-
Interatrial septum
- Ventr:
-
Ventricle
- AF:
-
Atrial fibrillation
References
Abramochkin DV, Borodinova AA, Rosenshtraukh LV (2012) Effects of acetylcholinesterase inhibitor paraoxon denote the possibility of non-quantal acetylcholine release in myocardium of different vertebrates. J Comp Physiol B 182(1):101–108. doi:10.1007/s00360-011-0602-2
Abramochkin DV, Nurullin LF, Borodinova AA, Tarasova NV, Sukhova GS, Nikolsky EE, Rosenshtraukh LV (2010) Non-quantal release of acetylcholine from parasympathetic nerve terminals in the right atrium of rats. Exp Physiol 95(2):265–273. doi:10.1113/expphysiol.2009.050302
Arvola L, Bertelsen G, Hassaf D, Ytrehus K (2004) Positive inotropic and sustained anti-beta-adrenergic effect of diadenosine pentaphosphate in human and guinea pig hearts. Role of dinucleotide receptors and adenosine receptors. Acta Physiol 182(3):277–285. doi:10.1111/j.1365-201X.2004.01363.x
Brandts B, Borchard R, Dirkmann D, Wickenbrock I, Sievers B, van Bracht M, Prull MW, Trappe HJ (2003) Diadenosine-5-phosphate exerts A1-receptor-mediated proarrhythmic effects in rabbit atrial myocardium. Br J Pharmacol 139(7):1265–1272. doi:10.1038/sj.bjp.0705361
Brandts B, Brandts A, Wellner-Kienitz MC, Zidek W, Schluter H, Pott L (1998) Non-receptor-mediated activation of IK(ATP) and inhibition of IK(ACh) by diadenosine polyphosphates in guinea-pig atrial myocytes. J Physiol 512(2):407–420. doi:10.1111/j.1469-7793.1998.407be.x
Buvinic S, Briones R, Huidobro-Toro JP (2002) P2Y(1) and P2Y(2) receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol 136(6):847–856. doi:10.1113/jphysiol.2006.105882
Chung WC, Kermode JC (2005) Suramin disrupts receptor-G protein coupling by blocking association of G protein alpha and betagamma subunits. J Pharmacol Exp Ther 313(1):191–198. doi:10.1124/jpet.104.078311
Conant AR, Fisher MJ, McLennan AG, Simpson AW (2000) Diadenosine polyphosphates are largely ineffective as agonists at natively expressed P2Y(1) and P2Y(2) receptors on cultured human saphenous vein endothelial cells. J Vasc Res 37(6):548–555. doi:10.1159/000054088
Conant AR, Theologou T, Dihmis WC, Simpson AW (2008) Diadenosine polyphosphates are selective vasoconstrictors in human coronary artery bypass grafts. Vasc Pharmacol 48(4–6):157–164. doi:10.1016/j.vph.2008.01.005
Delicado EG, Miras-Portugal MT, Carrasquero LM, León D, Pérez-Sen R, Gualix J (2006) Dinucleoside polyphosphates and their interaction with other nucleotide signaling pathways. Pflugers Arch 452(5):563–572. doi:10.1007/s00424-006-0066-5
Edgecombe M, McLennan AG, Fisher MJ (1999) Diadenosine polyphosphates and the control of cyclic AMP concentrations in isolated rat liver cells. FEBS Lett 457(3):455–458. doi:10.1016/S0014-5793(99)01099-6
Flores NA, Stavrou BM, Sheridan DJ (1999) The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc Res 42(1):15–26. doi:10.1016/S0008-6363(99)00004-8
García-Villalón AL, Monge L, Fernández N, Salcedo A, Narváez-Sánchez R, Diéguez G (2009) Coronary response to diadenosine pentaphosphate after ischaemia-reperfusion in the isolated rat heart. Cardiovasc Res 81(2):336–343. doi:10.1093/cvr/cvn321
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339(10):659–666. doi:10.1056/NEJM199809033391003
Hoyle CH, Ziganshin AU, Pintor J, Burnstock G (1996) The activation of P1- and P2-purinoceptors in the guinea-pig left atrium by diadenosine polyphosphates. Br J Pharmacol 118(5):1294–1300
Iwasaki Y, Nishida K, Kato T, Nattel S (2011) Atrial fibrillation pathophysiology: implications for management. Circulation 124(20):2264–2274. doi:10.1161/CIRCULATIONAHA.111.019893
Jankowski J, Jankowski V, Laufer U, van der Giet M, Henning L, Tepel M, Zidek W, Schlüter H (2003) Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler Thromb Vasc Biol 23(7):1231–1238. doi:10.1161/01.ATV.0000075913.00428.FD
Jovanovic A, Alekseev AE, Terzic A (1997) Intracellular diadenosine polyphosphates: a novel family of inhibitory ligands of the ATP-sensitive K+ channel. Biochem Pharmacol 54(2):219–225. doi:10.1016/S0006-2952(97)00262-1
Jovanovic A, Jovanovic S, Mays DC, Lipsky JJ, Terzic A (1998) Diadenosine 5′,5″-P1,P5-pentaphosphate harbors the properties of a signaling molecule in the heart. FEBS Lett 423(3):314–318. doi:10.1016/S0014-5793(98)00114-8
Jovanovic A, Zhang S, Alekseev AE, Terzic A (1996) Diadenosine polyphosphate-induced inhibition of cardiac KATP channels: operative state-dependent regulation by a nucleoside diphosphate. Pflugers Arch 431(5):800–802. doi:10.1007/BF02253848
Knapp J, Bokník P, Linck B, Läer S, Müller FU, Neumann J, Vahlensieck U, Schlüter H, Zidek W, Schmitz W (1999) Inositol-1,4,5-trisphosphate increase by diadenosine tetraphosphate in preparations from failing human myocardium. Naunyn Schmiedeberg’s Arch Pharmacol 360(3):354–357. doi:10.1007/s002109900076
Laubinger W, Wang H, Welte T, Reiser G (2003) P2Y receptor specific for diadenosine tetraphosphate in lung: selective inhibition by suramin, PPADS, Ip5I, and not by MRS-2197. Eur J Pharmacol 468(1):9–14. doi:10.1016/S0014-2999(03)01624-8
Lewis CJ, Gitterman DP, Schlüter H, Evans RJ (2000) Effects of diadenosine polyphosphates (Ap(n)As) and adenosine polyphospho guanosines (Ap(n)Gs) on rat mesenteric artery P2X receptor ion channels. Br J Pharmacol 129(1):124–130. doi:10.1038/sj.bjp.0702993
Luo J, Jankowski J, Knobloch M, Van der Giet M, Gardanis K, Russ T, Vahlensieck U, Neumann J, Schmitz W, Tepel M, Deng MC, Zidek W, Schlüter H (1999) Identification and characterization of diadenosine 5′,5‴-P1,P2 -diphosphate and diadenosine 5′,5‴-P1,P3-triphosphate in human myocardial tissue. Faseb j 13(6):695–705
Luo J, Jankowski V, Güngär N, Neumann J, Schmitz W, Zidek W, Schlüter H, Jankowski J (2004) Endogenous diadenosine tetraphosphate, diadenosine pentaphosphate, and diadenosine hexaphosphate in human myocardial tissue. Hypertension 43(5):1055–1059. doi:10.1161/hyp.0000126110.46402.dd
Lüthje J, Miller D, Oqilvie A (1987) Unproportionally high concentrations of diadenosine triphosphate (Ap3A) and diadenosine tetraphosphate (Ap4A) in heavy platelets. Consequences for in vitro studies with human platelets. Blut 54(4):193–200. doi:10.1007/BF00594193
Lüthje J, Oqilvie A (1983) The presence of diadenosine 5′,5‴-P1,P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun 115(1):253–260. doi:10.1016/0006-291X(83)90997-X
Mallet ML (2004) Proarrhythmic effects of adenosine: a review of the literature. Emerg Med J 21(4):408–410. doi:10.1136/emj.2004.016048
McDonald HA, Chu KL, Bianchi BR, McKenna DG, Briggs CA, Burgard EC, Lynch KJ, Faltynek C, Cartmell J, Jarvis MF (2002) Potent desensitization of human P2X3 receptors by diadenosine polyphosphates. Eur J Pharmacol 435(2–3):135–142. doi:10.1016/S0014-2999(01)01568-0
Miras-Portugal MT, Gualix J, Pintor J (1998) The neurotransmitter role of diadenosine polyphosphates. FEBS Lett 430(1–2):78–82. doi:10.1016/S0014-5793(98)00560-2
Mutafova-Yambolieva VN, Durnin L (2014) The purinergic neurotransmitter revisited: a single substance or multiple players? Pharmacol Ther 144(2):162–191. doi:10.1016/j.pharmthera.2014.05.012
Nahum V, Tulapurkar M, Lévesque SA, Sévigny J, Reiser G, Fischer B (2006) Diadenosine and diuridine poly(borano)phosphate analogues: synthesis, chemical and enzymatic stability, and activity at P2Y1 and P2Y2 receptors. J Med Chem 49(6):1980–1990. doi:10.1021/jm050955y
Neumann J, Meissner A, Bokník P, Gombosová I, Knapp J, Lüss H, Müller FU, Schlüter H, Zidek W, Rolf N, Van Aken H, Vahlensieck U, Schmitz W (1999) Inotropic effects of diadenosine tetraphosphate in isolated canine cardiac preparations. J Cardiovasc Pharmacol 33(1):151–156
Pereira MF, Hernández MD, Pintor J, Miras-Portugal MT, Cunha RA, Ribeiro JA (2000) Diadenosine polyphosphates facilitate the evoked release of acetylcholine from rat hippocampal nerve terminals. Brain Res 879(1–2):50–54. doi:10.1016/S0006-8993(00)02726-8
Pintor J, Miras-Portugal MT (2000) Receptors for diadenosine polyphosphates P2D, P2YApnA, P4 and dinucleotide receptors: are there too many? Trends Pharmacol Sci 21(4):135. doi:10.1016/S0165-6147(00)01453-X
Schlüter H, Offers E, Brüggemann G, van der Giet M, Tepel M, Nordhoff E, Karas M, Spieker C, Witzel H, Zidek W (1994) Diadenosine phosphates and the physiological control of blood pressure. Nature 367(6459):186–188. doi:10.1038/367186a0
Schotten U, Verheule S, Kirchhof P, Goette A (2011) Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91(1):265–325. doi:10.1152/physrev.00031.2009
Stavrou BM (2003) Diadenosine polyphosphates: postulated mechanisms mediating the cardiac effects. Curr Med Chem Cardiovasc Hematol Agents 1(2):151–169. doi:10.2174/1568016033477513
Stavrou BM, Lawrence C, Blackburn GM, Cohen T, Sheridan DJ, Flores NA (2001a) Coronary vasomotor and cardiac electrophysiologic effects of diadenosine polyphosphates and nonhydrolyzable analogs in the guinea pig. J Cardiovasc Pharmacol 37(5):571–584
Stavrou BM, Sheridan DJ, Flores NA (2001b) Contribution of nitric oxide and prostanoids to the cardiac electrophysiological and coronary vasomotor effects of diadenosine polyphosphates. J Pharmacol Exp Ther 298(2):531–538
Steinmetz M, Gabriëls G, Le TV, Piechota HJ, Rahn KH, Schlatter E (2003) Vasoactivity of diadenosine polyphosphates in human small renal resistance arteries. Nephrol Dial Transplant 18(12):2496–2504. doi:10.1093/ndt/gfg405
Steinmetz M, Janssen AK, Pelster F, Rahn KH, Schlatter E (2002) Vasoactivity of diadenosine polyphosphates in human small mesenteric resistance arteries. J Pharmacol Exp Ther 302(2):787–794. doi:10.1124/jpet.302.2.787
Steinmetz M, Schlatter E, Boudier HA, Rahn KH, De Mey JG (2000) Diadenosine polyphosphates cause contraction and relaxation in isolated rat resistance arteries. J Pharmacol Exp Ther 294(3):1175–1181
Steinmetz M, Van Le T, Bierer S, De Mey JG, Schlatter E (2005) Prior vasorelaxation enhances diadenosine polyphosphate-induced contractility of rat mesenteric resistance arteries. Naunyn Schmiedeberg’s Arch Pharmacol 371(5):359–363. doi:10.1007/s00210-005-1059-1
Sumiyoshi R, Nishimura J, Kawasaki J, Kobayashi S, Takahashi S, Kanaide H (1997) Diadenosine polyphosphates directly relax porcine coronary arterial smooth muscle. J Pharmacol Exp Ther 283(2):548–556
Vahlensieck U, Bokník P, Gombosová I, Huke S, Knapp J, Linck B, Lüss H, Müller FU, Neumann J, Deng MC, Scheld HH, Jankowski H, Schlüter H, Zidek W, Zimmermann N, Schmitz W (1999) Inotropic effects of diadenosine tetraphosphate (AP4A) in human and animal cardiac preparations. J Pharmacol Exp Ther 288(2):805–813
van der Heyden MA, Wijnhoven TJ, Opthof T (2005) Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res 65(1):28–39. doi:10.1016/j.cardiores.2004.09.028
Vassort G (2001) Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81(2):767–806
Vollmayer P, Clair T, Goding JW, Sano K, Servos J, Zimmermann H (2003) Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur J Biochem 270(14):2971–2978. doi:10.1046/j.1432-1033.2003.03674.x
von Kügelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110(3):415–432
von Kügelgen I, Hoffmann K (2015) Pharmacology and structure of P2Y receptors. Neuropharmacology. doi:10.1016/j.neuropharm.2015.10.030
Acknowledgments
This study was supported by the Russian Science Foundation [14-15-00268 to DVA].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pustovit, K.B., Kuzmin, V.S. & Abramochkin, D.V. Diadenosine tetra- and pentaphosphates affect contractility and bioelectrical activity in the rat heart via P2 purinergic receptors. Naunyn-Schmiedeberg's Arch Pharmacol 389, 303–313 (2016). https://doi.org/10.1007/s00210-015-1199-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1199-x