Abstract
In the present study, 1-(3,5-dimethylphenyl)-6-methyl-1H-pyrazolo[4,3-c]pyridin-4 (5H)-one (DPMPP) was investigated as an antiproliferative agent for prostate cancer cells and the mechanism of its action was studied. Cell lines 22Rv1 and SGC‑7901 were used as in vitro models of prostate cancer. The DPMPP treatment inhibited proliferation of 22Rv1 and SGC‑7901 cells in dose-depended manner. The viability of 22Rv1 and SGC‑7901 cells was reduced to 21 and 19%, respectively after treatment with 32 µM DPMPP. In DPMPP treated (16 µM) 22Rv1 and SGC‑7901 cells apoptosis increased to 62.78 and 68.51%, respectively. Moreover, DPMPP treatment caused cell cycle arrest in S phase and inhibition of PI3K/AKT activation. In the same time ROS production showed elevation and MMP (Matrix MetalloProteinase) decreased in the cells. Apparently DPMPP induces cytotoxicity through induction of oxidative response and apoptosis in prostate cancer cells in vitro. The PI3K/Akt/ERK phosphorylation was inhibited, while p21 and p53, death receptor, expression was promoted by DPMPP treatment. Therefore, DPMPP has a potential to be used as a therapeutic agent for treatment of prostate cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Prostate cancer, a worldwide common solid tumor in men, is the second most common reason for deaths associated with cancer [1]. In United States alone approximately 30.000 deaths were caused by prostate cancer in the year 2010 [1]. Over the past two decades, the incidence of prostate cancer has increased in several countries, including China [2]. Many factors such as ethnicity, age group of a person, and family history are associated with the incidence of prostate cancer patients [2]. Genetic variation is also associated with patients' susceptibility to developing prostate cancer [3]. Studies have identified about 30 sites in the genomes of various ethnic groups around the world, mutations in which are associated with the risk of developing prostate cancer [3]. Currently, early-stage prostate cancer is usually treated with surgery, radiation therapy, and androgen ablation [4]. Hormonal therapy for prostate cancer has been shown to induce hormone-unresponsive cancer [4]. Research is currently underway to identify new and effective chemotherapeutic agents for prostate cancer.
Pyrazolo[4,3-c]pyridin-4(5H)-ones were first discovered in silico as important heteroaromatic compounds in a drug development program [5, 6]. Some of these compounds showed significant anti-tumor activities against various types of cancers [5, 6]. In the present study 1-(3,5-Dimethylphenyl)-6-methyl-1H-pyrazolo[4,3-c]pyridine-4(5H)-one (DPMPP; Fig. 1), which is a member of pyrazolo[4,3-c]pyridin-4(5H)-ones, has been investigated as anti-proliferative agent for prostate cancer cells in vitro and the mechanism of its action has been studied.
MATERIALS AND METHODS
Drugs and Reagents
The drug compound, 1-(3,5-Dimethylphenyl)-6-methyl-1H-pyrazolo[4,3-c]pyridine-4(5H)-one (DPMPP; 99% purity), was provided by Dr. Zhang from University of Tsinghua University, China [6]. Its stock solution was prepared in normal saline which was diluted at the time of experiment. Dimethyl sulfoxide and other common chemicals were purchased from Sigma-Aldrich.
Cell Lines and Culture
The SGC‑7901 and 22Rv1 cell lines were provided by the American Type Culture Collection (ATCC; Manassas, VA, USA). The cell culture was performed in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and antibiotics (1% penicillin/streptomycin). Incubation of the cells was carried out at 37˚C temperature under humidified atmosphere with 5% CO2.
MTT Assay
The SGC‑7901 and 22Rv1 cells were seeded in 96‑well plates at 1 × 106 cells/well density and incubated for 24 h in DMEM containing 10% FBS. Then the medium was replaced by fresh medium containing 1.0, 2.0, 4.0, 8.0, 16.0, or 32.0 µM DPMPP and cells were incubated in it for 48 h. Afterwards, the medium was replaced by fresh one without serum and containing 5 mg/ml solution of 3‑4‑5‑Dimethylthiazol‑2‑yl‑25‑diphenyltetrazolium bromide (MTT) and cells were incubated for 4 h more. Then the medium was removed by DMSO. The absorbance was recorded at 568 nm using Multimode Reader (Model: Varioskan Flash; Thermo Fisher Scientific, Inc.).
Apoptosis Analysis by Flow Cytometry
The SGC‑7901 and 22Rv1 cells were plated at 1 × 106 cells/well concentration in 6‑well plates and treated with 32.0 µM DPMPP or normal saline as a control. After incubation for 48 h, the cells were washed three times with PBS and then stained with 5 µl Annexin V‑FITC and 10 µl PI dyes for 25 min under darkness in accordance with manufacturer’s instructions. The apoptosis induction in cells was detected using flow cytometry (Beckman Coulter Inc., Miami, FL, USA).
Cell Cycle Assay
The SGC‑7901 and 22Rv1 cells were plated in 6‑well plates at 2 × 105 cells/well concentration and treated with 32.0 µM DPMPP for 48 h. Then cells were washed twice with PBS and fixed by 70% methyl alcohol at –20°C overnight. Then cells were centrifuged for 10 min at 100× g, washed with PBS and re-suspended in 400 µl buffer containing 10 µl RNase and 25 µl Protein Inhibitors. After incubation for 25 min the DNA content of cells was detected using flow cytometer (Beckman Coulter Inc.).
Determination of ROS Production Assay
The SGC‑7901 and 22Rv1 cells were seeded in 6-well plates at 1 × 106 cells/well concentration and treated with 32.0 µM DPMPP for determination of ROS production by well-known methodology [7]. The cells were harvested, centrifuged for 10 min at 100× g and subsequently washed with PBS. The cells were dyed with 10 µM 2,7'-dichlorofluorescin diacetate (DCFDA) for 25 min at room temperature under darkness. The ROS secretion in cells was analyzed using flow cytometer (Cytomics FC 500; Beckman Coulter Inc.).
Determination of Mitochondrial Membrane Potential
The mitochondrial membrane potential was measured using a known method [7]. The SGC‑7901 and 22Rv1 cells were plated into 6‑well plates at 1 × 106 cells/well concentration and incubated with 32 µM DPMPP. The medium was mixed with NAC or without NAC and incubation was performed for 48 h. The cell incubation was followed by treatment with Rhodamine-123 dye for 15 min at 37°C. The changes in MMP were detected using flow cytometry (Beckman Coulter Inc.).
Western Blot Analysis
The 22Rv1 and SGC 7901 cells were plated in 6-well plates at 1 × 106 cells/well concentration and incubated for 48 h with 32.0 µM DPMPP. Then cells were washed with PBS, collected and lysed by RIPA buffer [40 mM Tris-HCl (pH 7.4) containing 150 mM NaCl and 1% (v/v) Triton X-100 and Protein Inhibitors cocktail (1.0 mM phenylmethylsulfonyl fluoride (PMSF), 10 µM Leupeptin, 0.1 µM Aprotinin, 1.0 µM Pepstatin)]. The concentration of proteins in lysates was measured by bicinchoninic acid protein assay. The protein samples (30 µg per lane) were separated by 10% SDS-PAGE and subsequently transferred to polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane blocking was performed for 1.5 h with 5% skimmed milk in Tris buffered saline containing 0.1% Tween-20 at room temperature. The protein samples were probed with antibodies against p-Akt (1 : 1.000; cat. no. 9272), p‑PI3K (1 : 1.000; cat. no. 4249), Bcl‑2 (1 : 1.000; cat. no. 2872), JAK2 (1 : 1.000, cat. no. 3230), p‑JAk2 (1 : 1.000; cat. no. 3771), p‑ERK (1 : 2.000; cat. no. 4370), p38 (1 : 500; cat. no. 8690), STAT3 (1 : 1.000; cat. no. 12 640), p‑STAT3 (1 : 500; cat. no. 9145), and Bax (1 : 1.000; cat. no. 2774; Cell Signaling Technology, Inc. Danvers, MA, USA) for overnight at 4oC. After 1X PBST washing thrice the blots were stained with horseradish peroxidase‑conjugated secondary antibodies (Cell Signaling Technology, Inc.) for 1 h at room temperature. Visualization of the bands was made using SignalFire™ Plus ECL Reagent.
Statistical Analysis
The expressed data is the mean ± SD of triplicate experiments performed independently. The data was analyzed statistically using the SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). The data comparison between the groups was made using one‑way ANOVA with Bonferroni post hoc test and student’s t-test. The differences were taken significant statistically at p < 0.05.
RESULTS
Cytotoxicity of DPMPP for 22Rv1 and SGC‑7901 Cells
The DPMPP treatment of 22Rv1 and SGC‑7901 cells showed its toxicity in dose-based manner (Fig. 2). The 22Rv1 cell viability was measured as 84 and 19%, respectively to the control, at 1.0 and 32 µM DPMPP. Similarly, SGC‑7901 cells viability was 91 and 21% at 1.0 and 32 µM DPMPP after 48 h incubation. Thus, the cytotoxicity of DPMPP towards 22Rv1 and SGC-7901 cells showed a significant (p < 0.05) increase with increasing DPMPP concentration.
DPMPP as an Inducer of Apoptosis for 22Rv1 and SGC – 7901 Cells
The induction of apoptosis in 22Rv1 and SGC-7901 cells by DPMPP was analyzed by annexin V / PI labeling and subsequent flow cytometry (Figs. 3a, 3b). At 32 μM DPMPP, the proportion of apoptotic 22Rv1 cells was 68.78% compared to 2.03% in untreated cells. In SGC‑7901 cells proportion of apoptotic cells raised to 62.51% at 32 µM DPMPP compared to 2.43% in control cells.
DPMPP Elevates Pro-apoptotic Proteins Expression in 22Rv1 and SGC‑7901 Cells
In 22Rv1 and SGC-7901 cells treated with 32 μM DPMPP for 48 h, changes in protein expression levels were assessed (Fig. 4). The DPMPP treatment elevated cleaved caspase‑3, Bax and cleaved-PARP expression in 22Rv1 and SGC‑7901 cells. The Bcl-2 expression in DPMPP treated cells showed a marked suppression compared to control cells.
Cell Cycle Arrest by DPMPP in 22Rv1 and SGC‑7901 Cells
The treatment with 32 µM DPMPP increased the content of cells in S-phase in 22Rv1 and SGC‑7901 cells compare to untreated (Fig. 5a). Treatment with DPMPP reduced content of cells in G1/G0 and G2/M phases in 22Rv1 and SGC‑7901 cells compared to untreated cells. Thus DPMPP treatment caused 22Rv1 and SGC‑7901 cell cycle arrest in S phase. In cells treated with DPMPP, after 48 hours, a noticeable suppression of the expression of pRb and E2F1 proteins was observed relative to the control (Fig. 5b). Expression of p21 and p53 in 22Rv1 and SGC-7901 cells was markedly increased after DPMPP treatment.
Effect of DPMPP on progress of cell cycle in 22Rv1 and SGC‑7901 cells. (a) The cells, treated with 32 µM DPMPP, were analyzed after 48 h by flow cytometry after PI staining. (b) The pRb, E2F1, p21, and p53 expression in DPMPP treated cells was assessed by western blotting. *p < 0.048; and **p < 0.186 vs. control.
DPMPP Promotes ROS Release and Suppresses MMP in 22Rv1 and SGC‑7901 Cells
ROS production in DPMPP-treated and control cells was measured 48 h after the start of the experiment (Figs. 6a, 6b). ROS production in 22Rv1 and SGC-7901 cells treated with DPMPP was significantly higher (p < 0.048) than in controls. Treatment with DPMPP at 32.0 µM suppressed MMP of 22Rv1 and SGC‑7901 cells significantly relative to control cells (Fig. 6c).
Effect of DPMPP on ROS production and MMP in 22Rv1 and SGC‑7901 cells. (a, b) In 32 µM DPMPP-treated cells ROS production was measured using DCFH‑DA labeling. (c) The level of MMP in 32 µM DPMPP-treated cells compare to untreated was assessed using Rhodamine 123 dye after an exposure to (or in absence of) NAC. *p < 0.0487 and **p < 0.0965 vs. control.
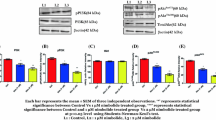
Suppression of PI3K/Akt/ERK, NF‑κB Activation and Elevation of p38 by DPMPP
The DPMPP treatment suppressed activation of PI3K/Akt in 22Rv1 and SGC‑7901 cells significantly relative to control cells (Fig. 7). The p-ERK1/2 expression in DPMPP-treated cells was also reduced in 48 h. However, 32 µM DPMPP treatment statistically significantly promoted p38 expression in 22Rv1 and SGC‑7901 cells.
DPMPP Down-regulates JAK2/STAT3 Activation in 22Rv1 and SGC‑7901 Cells
Treatment of 22Rv1 and SGC-7901 cells with 32 μM DPMPP significantly suppressed JAK2 and STAT3 activation (Fig. 8). There was negligible change in total JAK2 and STAT3 expression in these cells.
DISCUSSION
In the present study DPMPP treatment of 22Rv1 and SGC‑7901 cells exhibited anti-proliferative effect and promoted apoptosis induction. Cell cycle arrest in DPMPP-treated cells was evident from an increase in the proportion of cells in the S-phase and a corresponding decrease in cells in the G1/G0 and G2/M phases. The data obtained using western blotting showed that DPMPP treatment inhibited phosphorylation of PI3K and Akt in 22Rv1 and SGC‑7901 cells. Moreover, ERK phosphorylation in 22Rv1 and SGC‑7901 cells were also down-regulated by treatment with DPMPP. Assessment of JAK2 and STAT3 activation in DPMPP treated 22Rv1 and SGC‑7901 cells showed a marked down-regulation compared to control cells. This data demonstrates that DPMPP treatment suppresses anti-apoptotic potential of 22Rv1 and SGC‑7901 cells by targeting over-expression of p-PI3K/p-Akt/p-ERK. During cell division transition from S to G2/M phase is associated with the activation of pRb and E2F1 [8]. In DPMPP treated 22Rv1 and SGC‑7901 cells the expression of pRb and E2F1 was markedly reduced compared to control cells. The expression of death receptors, p21 and p53 was markedly elevated in DPMPP treated 22Rv1 and SGC‑7901 cells compared to control.
CONCLUSIONS
In summary, DPMPP exhibited anti-proliferation potential and promoted apoptosis in 22Rv1 and SGC‑7901 prostate cancer cells. The PI3K/Akt/ERK phosphorylation was inhibited while as death receptor, p21 and p53 expression was promoted. Thus DPMPP may be developed for treatment of prostate cancer however; in vivo studies need to be performed for ascertaining the same.
COMPLIANCE WITH ETHICAL STANDARDS The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
REFERENCES
Jemal, A., Siegel, R., and Xu, J.E., Ward Cancer statistics, 2010, Cancer J. Clin., 2010, vol. 60, pp. 277–300.
Fedorov, A., Fluckiger, J., Ayers, G.D., Li, X., Gupta, S.N., Tempany, C., Mulkern, R., Yankeelov, T.E., and Fennessy, F.M., A comparison of two methods for estimating DCE-MRI parameters via individual and cohort based AIFs in prostate cancer: a step towards practical implementation, Magn. Reson. Imaging, 2014, vol. 32, pp. 321–329.
Yu, E.Y., Massard, C., Gross, M.E., Carducci, M.A., Culine, S., Hudes, G., Posadas, E.M., Sternberg, C.N., Wilding, G., Trudel, G.C., et al., Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer, Urology, 2011, vol. 77, pp. 1166–1171.
Shetty AV, Thirugnanam S, Dakshinamoorthy G, et al: 18a-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes, Int. J. Oncol., 2011, vol. 39, pp. 635–640.
Maciej, D., Vishal, C.K., Valeria, N., Roberto, F., Kenji, S., Leonidas, E., Dominik, L., et al., Structure–activity relationship in pyrazolo[4,3-c]pyridines, first inhibitors of PEX14–PEX5 protein–protein interaction with trypanocidal activity, J. Med. Chem., 2020, vol. 63, pp. 847–879.
Smyth, L.A., Matthews, T.P., Horton, P.N., Hursthouse, M.B., and Collins, I., Synthesis and reactivity of 3-amino-1H-pyrazolo[4,3-c]pyridin-4(5H)-ones: development of a novel kinase-focused library, Tetrahedron, 2010, vol. 66, no. 15, pp. 2843–2854.
Choi, Y.-M., Kim, H.-K., Shim, W., Anwar, M.A., Kwon, J.-W., Kwon, H.-K., et al., Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation, PLoS One, 2015, vol. 10, no. 8. e0135083.
Hamid, S.M., Cicek, S., Karamil, S., Ozturk, M.B., Debelec-Butuner, B., Erbaykent-Tepedelen, B., Varisli, L., Gonen-Korkmaz, C., Yorukoglu, K., and Korkmaz, K.S., HOXB13 contributes to G1/S and G2/M checkpoint controls in prostate, Mol. Cell Endocrinol., 2014, vol. 383, pp. 38–47.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, J., Zhang, Z. & Hu, H. Prostate Cancer Growth Inhibition by 1-(3,5-Dimethylphenyl)–6-methyl-1H-pyrazolo[4,3-c]pyridin-4(5H)-one via Down-regulation of Phosphorylation PI3K/AKT and STA3/JAK2. Dokl Biochem Biophys 495, 347–353 (2020). https://doi.org/10.1134/S160767292006006X
Published:
Issue Date:
DOI: https://doi.org/10.1134/S160767292006006X