Abstract
Paeonol (Pae) is the main active ingredient from the root bark of Paeonia moutan and the grass of Radix Cynanchi Paniculati. Numerous reports indicate that Pae effectively inhibits several types of cancer lines. In this study, we report that Pae hinders prostate cancer growth both in vivo and in vitro. Human prostate cancer lines DU145 and PC-3 were cultured in the presence of Pae. The xenograft tumor in mice was established by subcutaneous injection of DU145 cells. Cell growth was measured by MTT, and the apoptosis was detected by the flow cytometry. Expression of Bcl-2, Bax, Akt, and mTOR were tested by western blotting assay. DU145 and PC-3 showed remarkable sensitivity to Pae, and exposure to Pae induced dose-and time-dependent growth inhibitory responses. Moreover, treatment of Pae promoted apoptosis and enhanced activities of caspase-3, caspase-8, and caspase-9 in DU145. Further work demonstrated Pae reduced expression of Bcl-2 and increased expression of Bax in DU145. Interestingly, we observed that Pae significantly decreased phosphorylated status of Akt and mTOR, and inhibitory effects of Pae and PI3K/Akt inhibitor on DU145 proliferation were synergistic. Finally, we confirmed that oral administration of Pae to the DU145 tumor-bearing mice significantly lowered tumor cell proliferation and led to tumor regression. Pae possesses inhibitory effects on prostate cancer cell growth both in vitro and in vivo, and the anti-proliferative effect may be closely related to its activation of extrinsic and intrinsic apoptotic pathway and inhibition of the PI3K/Akt pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second leading cause of cancer mortality in men around the world, and its incidence and mortality rates have increased substantially in recent years [16]. In the USA, there were an estimated 240,890 new cases of prostate cancer and 33,720 deaths in 2011 [24]. In Lebanon, the prostate cancer incidence increased from 29.9 cases per 100,000 persons in 2003 to 39. cases per 100,000 in 2008, and the value is expected to increase from 42 cases per 100,000 in 2009 to 64 cases per 100,000 in 2018 [14]. Radical prostatectomy, hormone ablation therapy, and radiotherapy are suggested treatments for prostate cancer patients. However, no curative treatment exists once the cancer invades beyond the gland and metastasizes to bone and lymph nodes, which become androgen refractory [10, 17]. Therefore, novel approaches are urgently required.
Research indicates that cytotoxic drugs cause adverse effects. In recent years, natural products have received increasing attention, with some proving to be promising alternatives for preventing the progression of prostate cancer. Paeonol (Pae; Fig. 1d) is one of such agents that can inhibit cancer progression. Pae is the main active component extracted from the root bark of Paeonia moutan and the grass of Radix Cynanchi Paniculati, which is widely used as a nutrient supplement in traditional Chinese medicine. Pae has been confirmed to possess extensive pharmacological activities, including hypnosis, anti-pyresis, analgesia, anti-oxidation, anti-inflammation, and anti-bacteria [13]. Additionally, it has also been previously demonstrated that Pae has a wide anti-neoplastic spectrum and exhibits anti-proliferative effects and apoptosis-inducing activities in hepatoma cell lines (HepG2 and SMMC-7721), esophageal cancer cell lines (SEG-1 and Eca-109), colorectal cancer cell line (HT-29), colon cancer cell lines (DLD-1), gastric cancer cells(MFC and SGC-790 cells), and ovarian cancer cell line (SKOV3) [21, 26, 28, 30, 32–34]. Moreover, Pae effectively reverses paclitaxel resistance in MCF-7/PTX cells [5, 36]. Apoptosis-inducing activity of Pae is expected to provide a novel method of chemoprevention in cancer treatment. However, there is no existing research which addresses the effects of Pae on prostate cancer development. Thus, the objective of this research was to determine whether Pae could inhibit prostate cancer proliferation in vivo and in vitro and focus on its potential mechanisms.
Effects of Pae on the viability of human prostate cancer cells lines, PC-3 (a) and DU145 (b). Cell growth was measured by MTT assay. Data are expressed as the percentage of the control treated with vehicle. IC50 of Pae created by GraphPad Prism (version 5.0, GraphPad Software Inc., CA) is shown in c. Values are shown as means ± SD, **P < 0.01. Chemical structure of Pae is presented in d
Materials and methods
Cell lines and culture
Human prostate carcinoma cell lines (DU145 and PC-3) were obtained from American Type Culture Collection (Manassas, VA), maintained in RPMI 1640 (Life Technologies, Inc., Grand Island, NY), and supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) at 37 °C in a 5% CO2 atmosphere.
Cells growth assay
Cells growth was measured by MTT assay as previously described [21]. Briefly, the cells were seeded into 96-well plate with a density of 2000 cells per well and subsequently treated with Pae (0–1000 μM, Sigma-Aldrich, St. Louis, MO) or LY290042 (PI3K/Akt inhibitor, Sigma-Aldrich, St. Louis, MO) for the indicated time. After Pae treatments, the cells were centrifuged and washed with ice-cold PBS, and liquid supernatant were discarded. Next, the cells were lysed in ice-cold cell lysis buffer for 15 min to prepare supernatant, and 20 μL of MTT solution (5 mg/ml, Sigma-Aldrich, St. Louis, MO) was added to each well; next, the cells were incubated for additional 4 h. Finally, the addition of DMSO-induced formation of color was detected using an ELISA plate reader at 570 nm. Each treatment and time point had three plates.
Cells apoptosis assay [20]
The apoptotic rate of DU145 was tested using an Annexin-V apoptosis detection kit from Molecular Probes, Inc. (Eugene, OR, USA). The cells were cultured in medium containing Pae (100, 200, and 500 μM). Following treatment, the cells were collected and washed twice with ice-chilled PBS and then were harvested and suspended in Annexin-V binding buffer, and stained with FITC-conjugated Annexin-V for 10 min at room temperature. Following the dye of propidium iodide (PI) (1 mg/ml), the apoptotic cells were quantified analysis using a Becton Dickinson FACS Calibur system (Becton Dickinson, San Jose, CA, USA). Three independent experiments were performed, and the average results were recorded.
Caspase activity assay
The cells (1 × 106 cells/ml) were incubated in medium in the presence of Pae (200 and 500 μM) for 48 h. At the end of treatment, the cells were centrifuged (600×g, 4 °C, 5 min) and washed with ice-cold PBS. Later, the cells were lysed in ice-cold cell lysis buffer (Sigma-Aldrich, USA) for 15 min and centrifuged at 10,000×g for 10 min to separate supernatant. Caspase activity was measured by a colorimetric caspase assay kit (Roche, Germany) according to the manufacturer’s instructions. Briefly, the supernatant was incubated with the supplied reaction buffer containing 10 mM DTT and DEAD-pNA (caspase-3), IETD-pNA (caspase-8), or LEHD-pNA (caspase-9) as substrates at 37 °C. Lastly, formation of p-nitroanilide in the analytes was measured using an ELISA microplate reader at 405 nm. The increases in activity of caspase-3, caspase-8, and caspase-9 were determined by comparing the results with the control.
Western blot analysis
At the end of treatment, DU145 were harvested and washed thrice with cold PBS. Cells were lysed in ice-cold lysis buffer containing (50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 50 mM NaF, 5 mM EDTA, 0.1% Triton X-100, 0.1 mM Na3VO4). After centrifugation at 14,000×g for 10 min, the supernatants were removed, and the protein levels were estimated using a Bio-Rad protein assay (BioRad, Munich, Germany) according to the manufacturer’s protocol. Following this, equal amounts of proteins were denatured in SDS-PAGE buffer and subjected to SDS-PAGE on 12% Tris-glycine gel. Membranes were probed for the protein levels of Bcl-2, Bax, Akt, p-Akt, mTOR, and p-mTOR using specific primary antibodies, followed by peroxidase-conjugated secondary antibody, and visualized by the ECL detection system (Amersham Corp., Arlington Heights, IL). The primary antibodies to Bcl-2, Bax, Akt, p-Akt, and β-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The primary antibodies to mTOR and p-mTOR were from Abcam (Cambridge, UK).
Induction of prostate tumor in mice and pathological examination
The study was approved by the ethics committee of the Quzhou People’s Hospital. A total of 24 male, six-week-old BALB/c nude mice were purchased from the animal department of Zhejiang University. Establishment of the mice with tumors was performed using existing methods [27]. Briefly, the nude mice were subcutaneously injected with DU145 suspension (100 μL, 8 × 107 cells/ml). The size of tumor was measured using the following formula: V = maximal length (mm) × (perpendicular width) (mm)2 / 2. When the tumors reached an average size greater than 160 mm3, the animals were randomly divided into four groups, with six in each, and the mice were orally treated with 0.9% saline (control) or Pae (50, 100, 200 mg/kg, dissolved in 0.9% saline) for eight consecutive weeks. Tumor sizes were monitored until the end of the study. Subsequently, mice were killed and tumor tissues were dissected, measured, and evaluated histopathologically. The tumor tissues were embedded in paraffin and cut into 4-μm sections. These sections were then stained with hematoxylin and eosin and observed using a BH2 optical microscope (Olympus, Tokyo, Japan).
Detection of Pae levels in mice with xenografted tumor
For quantification of Pae levels in the tumor mice, we established an analytical method according to previously published work [29]. Briefly, an Agilent 1200 series HPLC system (Agilent Technologies, CA, USA) consisting of a vacuum degasser, a quaternary pump, a column compartment, a multi-wavelength UV detector, and Agilent Chemstation software was employed for the present study. Additionally, an Agilent HC-C18 column (4.6 mm × 250 mm, 5 µm) was used. The detection was performed at a wavelength of 230 nm under a constant temperature of 25 °C. The mobile phase consisted of methanol (60%) and aqueous solution (40%), and the flow rate was 1 ml/min. Injection volume was 50 μL. The validation of the methodology is shown in the supplementary materials (Figs. 1S–4S). At different times after finally oral administration (Pae, 100 mg/kg), the mice were killed, and blood was collected. The preparation of plasma samples was conducted according to published methods [29].
Statistical analysis
The in vitro study was performed in triplicate. Data were presented as means ± SD. The statistical significance of results was evaluated by one-way and two-way analyses of variance (ANOVA) and by the Dunnett’s T test. A P value <0.05 was being considered to be statistically significant.
Results
Pae decreased the growth of DU145 and PC-3 in vitro
To measure the effects of Pae on proliferation of human prostate carcinoma cells, DU145 and PC-3 were utilized, receiving a treatment of Pae. As shown in Fig. 1a–c, Pae inhibited the growth of DU145 and PC-3 in the dose-dependent and time-dependent manners. For example, after stimulation with Pae (100 and 500 μM) for 48 h, DU145 viability decreased by approximately 21.3 and 59.5%, respectively, compared to the control group. Moreover, Pae treatment (200 μM) resulted in a 19.6 and 30.2% decrease in DU145 and an 11.7 and 23.0% decrease in PC-3 growth, in comparison with the untreated controls after 24 and 48 h, respectively. In addition, IC50 of Pae is shown in Fig. 1c, and appeared that DU145 cells were more sensitive to Pae than PC3. Therefore, the later experiments were conducted with DU145 cells.
Pae induced apoptosis of DU145 in vitro
As we observed, Pae treatment caused significant cytotoxicity in prostate carcinoma cells; later testing was performed to assess whether the inhibitory effects of Pae involved induction of apoptosis. As seen in Fig. 2, Pae caused a strong apoptotic death of DU145 cells. For example, compared with DU145 treated with vehicle displaying 4.6% apoptotic cells, treatment with Pae (100 μM and 200 μM) for 48 h resulted in 7.2 and 12.4% induction of apoptosis in DU145 cells (P < 0.01), which means the apoptotic cells population accounts for a 1.56- to 2.70-fold increase over the control, respectively.
Effects of Pae on the apoptosis of DU145. DU145 cells received Pae or vehicle treatment for 48 h, and then Annexin V/PI staining was performed. Representative images of apoptosis are shown in a (control), b (Pae 100 μM), c (Pae 200 μM), and d (Pae 500 μM); treatment of Pae (200 and 500 μM) significantly promoted apoptosis of DU145 (e). **P < 0.01 compared with the control (Con; vehicle). Data are expressed as the means ± SD, n = 3
Effects of Pae on the expression of Bax and Bcl-2 in DU145 cells. DU145 cells were treated with Pae for 48 h. a The representative images of protein bands; b Pae (200 and 500 μM) significantly reduced Bcl-2 expression and meanwhile increased Bax expression. The data are expressed as the means ± SD, n = 3. **P < 0.01 compared with DU145 without treatment of Pae
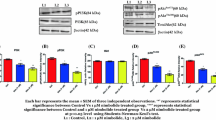
Effects of Pae on the PI3K/Akt/mTOR pathway in DU145 cells. DU145 cells were treated with Pae for 48 h. a Representative protein bands of Akt and p-Akt; b Pae (200 and 500 μM) significantly reduced phosphorylated status of Akt and had no effects on expression of Akt. c Representative protein bands of mTOR and p-mTOR; d Pae (200 and 500 μM) significantly reduced phosphorylated status of mTOR and had no effects on expression of mTOR; the data are expressed as the means ± SD, n = 3. **P < 0.01 compared with DU145 without treatment of Pae; e PI3K/Akt inhibitor (LY290042) obviously inhibited the growth of DU145 and enhanced cytotoxic sensitivity of DU145 in response to Pae. IC50 of Pae, LY290042, and Pae in the combined group were determined as 387.2, 267.8, and 96.4 μM, respectively. The data are expressed as the means ± SD, n = 3
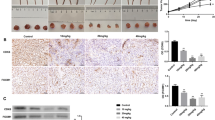
Inhibitory effects of Pae on DU145 induced tumor growth in nude mice. Hematoxylin-eosin (HE)-stained sections from untreated control (a) showed numerous tumor cells, and Pae administration (100 mg/kg: b; 200 mg/kg: c) prominently reduced the cells proliferation as indicated by fewer numbers of the cells (×400). Oral administration of Pae caused a significant reduction in both tumor volume (d) and tumor weight (e) in the tumor-bearing mice. The data are expressed as the means ± SD, n = 6. **P < 0.01 compared to the mice receiving vehicle
Mean plasma concentration-time profiles of Pae in mice plasma after oral administration of Pae. Typical HPLC chromatograms are shown in a (mobile phase), b (blank plasma sample), c (mobile phase spiked with Pae), and d (plasma from mice receiving Pae administration). The black arrows show the peaks of Pae. e Mean plasma concentration-time profiles of Pae. The data are expressed as the means ± SD, n = 5
Pae induced DU145 cells apoptosis through caspase-dependent pathway
After treatment with Pae for 48 h, activities of caspase-3, caspase-8, and caspase-9 were detected (Fig. 3). The results demonstrated Pae significantly increased caspase-3, caspase-8, and caspase-9 activities (P < 0.01). For example, the activity of caspase-3 increased 2.95-fold in cells treated with 500 μM Pae compared to the control. The activities of caspase-8 and caspase-9 increased from 100% in the control to 168 and 142%, respectively, after treatment with Pae (200 μM).
Pae treatment modulated Bcl-2 family proteins in DU145 cells
It is well known that Bcl-2 family plays a critical role in the regulation of apoptosis by functioning as promoters (Bax) or inhibitors (Bcl-2) of cell death process [1]. Therefore, we determined effects of Pae on levels of Bax and Bcl-2 in DU145 cells. As presented in Fig. 4, expression of Bax had obviously increased (P < 0.01), and Bcl-2 expression had significantly decreased after treatment of Pae (P < 0.01).
Pae inactivated the PI3K/Akt pathway in DU145 cells
To investigate whether the PI3K/Akt pathway was involved in Pae-induced apoptosis, we assessed phosphorylated-Akt (p-Akt) levels in DU145 cells. The results in Fig. 5a, b indicate that Akt levels were unaffected in Pae pretreated DU145, while p-Akt levels were significantly reduced in response to Pae treatment (P < 0.01). Similarly, the downstream of Akt (mTOR) was unaltered in Pae pretreated DU145, while that of p-mTOR was significantly reduced (Fig. 5c, d; P < 0.01). Moreover, to confirm involvement of Akt pathway, a representative PI3K/Akt inhibitor (LY290042) was used. As shown in Fig. 5e, the cells pretreated with LY294002 and Pae resulted in a distinct increase in the growth inhibition. These findings suggest that PI3K/Akt pathway plays an important role in Pae-induced inhibitory effects on DU145 cells.
Pae-inhibited DU145 growth in vivo
Inhibitory effects of Pae were further evaluated by examining the growth of DU145 tumor xenografts in the nude mice. As seen in Fig. 6b, there were no significant inhibitory effects 4 weeks after Pae treatment. However, remarkable inhibition on the tumor growth was observed after 5 weeks of the treatment (P < 0.01; Fig. 6b). Moreover, we measured the weight of the tumor tissues. The results showed that Pae treatment significantly reduced tumor weight (P < 0.01; Fig. 6c). Finally, histological examination of the tumor tissues revealed obvious tumor cell proliferation, which was significantly repressed after treatment of Pae (Fig. 6a–c).
Pae has a better absorption in the nude mice with tumor xenografts
The results in Fig. 7 indicate that Pae has a good absorbent process in the gastrointestinal tract of nude mice. The maximum plasma concentration of Pae was 3.24 μg/mL with a dosage of 100 mg/kg. Our result suggests that Pae has a direct anti-tumor activity in vivo.
Discussion
Pae is a natural compound occurring in P. moutan Sim and Pycnostelma paniculatum K. Schum. Pae is a safe medicine with a LD50 value of 3430 mg/kg when it is orally administered to mice [32]. As discussed previously, Pae possesses extensive pharmacological activities, including sedation, hypnosis, anti-pyresis, analgesic, anti-oxidation, and anti-inflammation [13]. Therefore, Pae has been on the market in China for treating several illnesses, such as eczema, dermatitis, and allergic rhinitis. In recent years, a number of works [21, 26, 28, 30, 32–34] suggest that Pae is a potent anti-neoplastic drug for cancer therapy. In short, Pae shows significant inhibitory effects on the growth of hepatoma cell lines, esophageal cancer cell lines, colorectal cancer cell lines, colon cancer cell lines, gastric cancer cell lines, ovarian cancer cell lines, and colorectal cancer cell lines. These findings indicate Pae has a wide anti-neoplastic spectrum. However, Pae’s effects on prostate carcinoma are not clearly understood. To the best of our knowledge, this work is the only to fully emphasize effects of Pae on human prostate cancer cell lines. In the present study, we found that Pae inhibited the growth of DU145 and PC-3 in dose-dependent and time-dependent manners, and these results are consistent with previous conclusion research [7, 21] in human gastric cancer cell lines and human hepatocellular carcinoma cell lines. Moreover, previous studies [21, 26, 34] noted that Pae has mild anti-tumor activities. For instance, IC50 of Pae for treating SEG-1 and Eca-109 cells were 57.73 and 124.77 mg/L, for treating SKOV3 cells was 200.06 mg/L, and for treating MFC and SGC-790 cells were 60.10 and 82.60 mg/L, respectively. In our study, we noticed similar results as reflected by IC50 of Pae (PC-3 858.40 μM; DU145 395.60 μM). To further confirm anti-carcinoma activity of Pae in vivo, we examined the effects of Pae on the tumor formation in DU145-treated nude mice. As expected, the results demonstrated that Pae reduced tumor growth and tumor cell proliferation in vivo. Moreover, we also found that Pae has a good absorbent process in the gastrointestinal tract of the nude mice, which suggested Pae has a direct anti-tumor activity in vivo.
Although the precise mechanism of growth inhibitory effect is not clear, several studies have shown that apoptosis is involved [26, 31]. It is believed that apoptosis is typically a physiological process that regulates cell numbers in order to maintain homeostasis in multi-cellular organisms. So far, two major pathways, the death receptor pathway (extrinsic pathway), and the mitochondrial pathway (intrinsic pathway), have been discussed in regards to induction of cell apoptosis [11, 12]. The extrinsic pathway is normally defined by activation of caspase-8 and caspase-3. In the intrinsic pathway, pro-apoptotic Bax causes mitochondrial disruption and cytochrome-c release, activating caspase-9 and its downstream caspase-3. Interestingly, a recent report described activation of caspase-8 through engagement of the death receptor can also trigger the mitochondrial pathway via a member of the Bcl-2 family [6]. It should be noted that Pae shows induction of apoptosis via modulate caspase activity and expression of apoptotic related proteins. For instance, Pae reduces expression of Bcl-2 and increases expression of Bax in a concentration-related manner in gastric cancer cell lines MFC and SGC-790 [21]. Moreover, Pae [18] has also been shown to increase the activity of caspase-3, caspase-8, and caspase-9 in SKOV3, HCT116, SW620, and LoVo. In the present work, we noted that Pae significantly increased caspase-3, caspase-8, and caspase-9 activity in vitro and restored the balanced levels of Bcl-2/ Bax. These results indicate that both extrinsic and intrinsic apoptotic pathways are involved in Pae-mediated DU145 apoptosis.
Several studies have been performed to better understand cellular pathways involved in Pae-mediated apoptosis. Zhang L et al. [35] found Pae inhibits B16F10 melanoma metastasis in vitro and in vivo via disrupting pro-inflammatory cytokine-mediated NF-κB and STAT3 pathways; Horng CT et al. [15] reported Pae inhibits migration and invasion of human chondrosarcoma via up-regulation of miR-141 and c-Src pathway; and Li et al. [19] demonstrated that inhibition of PGE2 synthesis and COX-2 expression are related to Pae-induced tumor cell apoptosis. Additionally, PI3K/Akt is an important transduction pathway that regulates cell survival, proliferation, metabolism, and apoptosis [9]. Activated PI3K causes Akt phosphorylation and transfer from membrane to cytoplasm and nucleus, where it controls various cellular functions through phosphorylation of key proteins [3]. mTOR is one of the key proteins involved in the progression of cells growth and proliferation. Activated AKT can directly activate mTOR by phosphorylating mTOR [3]. The roles of PI3K-Akt in tumor progression are well established. Akt directly phosphorylates anti-apoptotic proteins such as Bad at Ser136 [31]; caspase-9, as an initiator of the mitochondrial apoptotic pathway and an important substrate of Akt, could be phosphorylated and inactivated by Akt; and the former study showed a significant correlation between constitutive p-Akt with caspase-9 phosphorylation in gastric tumor tissues [23]. Furthermore, several apoptosis-related signaling pathways or proteins, such as the NF-κB pathway, FoxO family of transcription factors, and surviving, have been affected by Akt [2, 4, 8]. In the former research, we noted that Pae evidently exerted its ability to suppress the promoting effects of TNF-alpha on phosphorylation of PI3K/Akt and activation of NF-κB pathway in fibroblast-like synoviocytes [22]. In the present study, we tested the effects of Pae on Akt/mTOR cascade in prostate cancer cells. The results showed that Pae reduced the phosphorylated status of Akt and mTOR, and the growth inhibitory effects of Pae and PI3K/Akt inhibitor are synergistic, which suggests modulation of p-Akt contributed to the inhibitory effects of Pae.
In summary, we conclude that Pae causes growth inhibition and apoptosis induction in the prostate tumor cell lines. The apoptotic alteration can be explained by the activation of extrinsic and intrinsic apoptotic pathways, which is modulated by balanced Bax/Bcl-2 and blockade of the PI3K/Akt/mTOR cascade. Our results provide evidence that Pae may be a promising medicine for prostate tumor treatment.
References
Adams JM, Cory S (2001) Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci 26:61–66
Bai D, Ueno L, Vogt PK (2009) Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer 125:2863–2870
Bartholomeusz C, Gonzalez-Angulo AM (2012) Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets 16:121–130
Benayoun BA, Caburet S, Veitia RA (2011) Forkhead transcription factors: key players in health and disease. Trends Genet 27:224–232
Cai J, Chen S, Zhang W, Hu S, Lu J, Xing J, Dong Y (2014) Paeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2. Phytomedicine 21:984–991
Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG (2004) Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol 24:6592–6607
Chunhu Z, Suiyu H, Meiqun C, Guilin X, Yunhui L (2008) Antiproliferative and apoptotic effects of paeonol on human hepatocellular carcinoma cells. Anti-Cancer Drugs 19:401–409
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Davis WJ, Lehmann PZ, Li W (2015) Nuclear PI3K signaling in cell growth and tumorigenesis. Front Cell Dev Biol 3:24
Feldman BJ, Feldman D (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45
Goldar S, Khaniani MS, Derakhshan SM, Baradaran B (2015) Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev 16:2129–2144
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7(12) pii: a006080. doi:10.1101/cshperspect.a006080
He CN, Peng Y, Zhang YC, Xu LJ, Gu J, Xiao PG (2010) Phytochemical and biological studies of paeoniaceae. Chem Biodivers 7:805–838
Hilal L, Shahait M, Mukherji D, Charafeddine M, Farhat Z, Temraz S, Khauli R, Shamseddine A (2015) Prostate cancer in the Arab world: a view from the inside. Clin Genitourin Cancer 13:505–511
Horng CT, Shieh PC, Tan TW, Yang WH, Tang CH (2014) Paeonol suppresses chondrosarcoma metastasis through up-regulation of miR-141 by modulating PKCδ and c-Src signaling pathway. Int J Mol Sci 15:11760–11772
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Kar S, Palit S, Ball WB, Das PK (2012) Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 17:735–747
Li M, Tan SY, Zhang J, You HX (2013) Effects of paeonol on intracellular calcium concentration and expression of RUNX3 in LoVo human colon cancer cells. Mol Med Rep 7:1425–1430
Li M, Tan SY, Wang XF (2014) Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE2 synthesis and COX-2 expression. Oncol Rep 32:2845–2853
Lima AP, Pereira FC, Almeida MA, Mello FM, Pires WC, Pinto TM, Delella FK, Felisbino SL, Moreno V, Batista AA, de Paula S-LE (2014) Cytoxicity and apoptotic mechanism of ruthenium(II) amino acid complexes in sarcoma-180 tumor cells. PLoS One 9:e105865
Li N, Fan LL, Sun GP, Wan XA, Wang ZG, Wu Q, Wang H (2010) Paeonol inhibits tumor growth in gastric cancer in vitro and in vivo. World J Gastroenterol 16:4483–4490
Li Y, Li P, Lin SH, Zheng YQ, Zheng XX (2014) Paeonol inhibited TNF-alpha-induced GM-CSF expression in fibroblast-like synoviocytes. Int J Clin Pharmacol Ther 52:986–996
Sangawa A, Shintani M, Yamao N, Kamoshida S (2014) Phosphorylation status of Akt and caspase-9 in gastric and colorectal carcinomas. Int J Clin Exp Pathol 7:3312–3317
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236
Singh SS, Yap WN, Arfuso F, Kar S, Wang C, Cai W, Dharmarajan AM, Sethi G, Kumar AP (2015) Targeting the PI3K/Akt signaling pathway in gastric carcinoma: a reality for personalized medicine? World J Gastroenterol 21:12261–12273
Sun GP, Wan X, Xu SP, Wang H, Liu SH, Wang ZG (2008) Antiproliferation and apoptosis induction of paeonol in human esophageal cancer cell lines. Dis Esophagus 21:723–729
Tang L, Jin T, Zeng X, Wang JS (2005) Lycopene inhibits the growth of human androgen- independent prostate cancer cells in vitro and in BALB/c nude mice. J Nutr 135:287–290
Tan S, Ye J, Qian C, Ji C, Liu C, Wang J (2007) Paeonol inhibits the proliferation of human colorectal carcinoma cells and synergic with chemotherapeutic agents. Saudi Med J 28:642–643
Xie Y, Zhou H, Wong YF, Xu HX, Jiang ZH, Liu L (2008) Study on the pharmacokinetics and metabolism of paeonol in rats treated with pure paeonol and an herbal preparation containing paeonol by using HPLC-DAD-MS method. J Pharm Biomed Anal 46:748–756
Xing G, Zhang Z, Liu J, Hu H, Sugiura N (2010) Antitumor effect of extracts from moutan cortex on DLD-1 human colon cancer cells in vitro. Mol Med Rep 3:57–61
Xu SP, Sun GP, Shen YX, Wei W, Peng WR, Wang H (2007) Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J Gastroenterol 13:250–256
Xu SP, Sun GP, Shen YX, Peng WR, Wang H, Wei W (2007) Synergistic effect of combining paeonol and cisplatin on apoptotic induction of human hepatoma cell lines. Acta Pharmacol Sin 28:869–878
Ye JM, Deng T, Zhang JB (2009) Influence of paeonol on expression of COX-2 and p27 in HT-29 cells. World J Gastroenterol 15:4410–4414
Yin J, Wu N, Zeng F, Cheng C, Kang K, Yang H (2013) Paeonol induces apoptosis in human ovarian cancer cells. Acta Histochem 115:835–839
Zhang L, Tao L, Shi T, Zhang F, Sheng X, Cao Y, Zheng S, Wang A, Qian W, Jiang L, Lu Y (2015) Paeonol inhibits B16F10 melanoma metastasis in vitro and in vivo via disrupting proinflammatory cytokines-mediated NF-κB and STAT3 pathways. IUBMB Life 67:778–788
Zhang W, Cai J, Chen S, Zheng X, Hu S, Dong W, Lu J, Xing J, Dong Y (2015) Paclitaxel resistance in MCF-7/PTX cells is reversed by paeonol through suppression of the SET/phosphatidylinositol 3-kinase/Akt pathway. Mol Med Rep 12:1506–1514
Acknowledgements
This work was financially supported by technology projects in Quzhou City (No. 2014156).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the Quzhou People’s Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 56 kb)
Rights and permissions
About this article
Cite this article
Xu, Y., Zhu, Jy., Lei, Zm. et al. Anti-proliferative effects of paeonol on human prostate cancer cell lines DU145 and PC-3. J Physiol Biochem 73, 157–165 (2017). https://doi.org/10.1007/s13105-016-0537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0537-x