Abstract
A simple one-pot synthesis of N-substituted benzimidazoles from aromatic carboxylic acids and aromatic 1,2-diamines in the presence of 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate/hydroxybenzotriazole (HATU/HOBT) and N,N-diisopropylethylamine in DMF at 100°C, featuring good to excellent yields and easy workup, is developed. The protocol can be scaled to gram quantities, and no chromatographic purification is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Benzimidazoles are regarded as an important class of the heterocyclic compounds due to the wide range of biological activities associated with this core. A great number of benzimidazole derivatives have reached the clinical testing stage and are also present in marketed drugs. Albendazole, Mebendazole, and Fenbendazole are used as antigiardiasis agents as effective as Metronidazole. The benzimidazole core is also present in such blockbuster drugs as Omeprazole and Lansprazole [1–5].
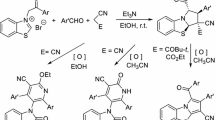
Due to the importance of benzimidazoles in medicinal chemistry many methods were reported in the literature for their synthesis. One of the most common synthetic approaches to benzimidazole derivatives involves the condensation–dehydration of o-phenylenediamine with acids, carbonyl compounds, or acid chlorides, etc. Furthermore, esters, lactones, and anhydrides can generate N-substituted benzimidazoles via condensation followed by oxidative cyclization [6, 7]. Aromatic esters react with arylenediamines under Weinreb conditions to give 2-arylbenzimidazoles. 2-Methylbenzimidazoles were synthesized from acid anhydrides of monobasic acids. Cyclic anhydrides of dibasic acids, too, were used for the synthesis of benzimidazoles, but, however, to convert the intermediate N-(o-aminophenyl)imide to target benzimidazoles generally required high temperatures and strong acids [8, 12]. Yang et al. [9, 10] developed an efficient one-step protocol for the synthesis of benzimidazoles via the reductive cyclization of o-nitroaniline with aromatic or aliphatic aldehydes under the action of Na2S2O4 in methanol at 60°C [9, 10]. The direct Pd-catalyzed arylation of 1-methyl-1H-benzo[d]imidazole with aryl halides has also been reported [11, 13].
In the present work we have developed a one-pot protocol for the synthesis of N-methyl-benzimidazoles, starting from N-methyl-o-phenylenediamine and aromatic carboxylic acids in the presence of 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) and hydroxybenzotriazole (HOBT). We have also found that aliphatic, heterocyclic, and some carbocyclic acids fail to react in similar conditions.
The advantage of the developed procedure over previously reported methods consists in that it does not require isolation of the intermediate amide and also does not require harsh conditions, such as use of strong mineral acids (HCl, H2SO4, hot glacial acetic acid, or polyphosphoric acid), high temperatures, or in microwave irradiation for cyclization [6, 7].
RESULTS AND DISCUSSION
We began our studies with 3-chlorobenzoic acid as a model substrate. Initially, we attempted one-pot synthesis of N-substituted benzimidazoles. To this end, we screened a variety of coupling reagents, specifically, propanephosphonic acid anhydride (T3P), N,N,N',N'-tetramethyl-O-(benzotriazol-1-yl)uranium tetrafluoroborate (TBTU), 3-[bis(dimethylamino)methylene]-3H-benzotriazol-1-oxide hexafluorophosphate (HBTU), N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC∙HCl), and HATU. However, we observed formation of an amide intermediate and no cyclized product (Table 1). Moreover, the reactions of aromatic carboxylic acids with N-substituted o-phenelyenediamines in the presence of HATU or HOBT along, too, gave an intermediate amide and no cyclized product. However, the reactions with both HATU and HOBT (HATU–HOBT) in DMF at 100°C in the presence of diisopropylethylamine (DIPEA) resulted in the exclusive formation of benzimidazoles. Notably, in the reactions with HATU–HOBT at room temperature we isolated an amide intermediate, but, as the reaction temperature was raised, the benzimidazole yield gradually increased from traces at 50°C to 20% at 70°C, and, finally, 80% at 100°C (Table 1).
With the optimized reaction conditions in hand, we checked the substrate scope of the reaction and obtained good results with a variety of aromatic carboxylic acids (Scheme 1 and Experimental).
However, the reactions with heterocyclic carboxylic acids 4 and 5 and substituted phenylacetic acids 6 gave no cyclization products (Scheme 2).
Further, we have studied the scope of optimized method on N-ethyl and N-phenyl substituted o-phenylenediamine but observed formation of the amide intermediate only and no cyclization products (Scheme 3).
2-Aminophenol and 2-thiophenol were treated with benzoic acid in same conditions. The first reaction gave an amide intermediate and no the expected cyclization product 2-phenylbenzo[d]oxazole, whereas the second reaction formed neither the intermediate carbamate nor 2-phenylbenzo[d]thiazole (Scheme 4).
Our method provides easy access to N-substituted 2-arylbenzimidazoles under mild conditions by a scalable eco-friendly procedure using commercially available reagents. The resulting products are easily isolable and require no chromatographic purification. Under the optimized conditions, successful reaction takes place with differently substituted aromatic carboxylic acids. However, monosubstituted aromatic carboxylic acids with electron-withdrawing substituents give better yields of N-substituted-2-aryl benzimidazoles compared with carboxylic acids with electron-donor substituents.
In view of the data in [9, 10], we can propose a plausible reaction pathway, which involves amide formation with an aryl benzoic acid and subsequent cyclodehydration to yield the corresponding N-substituted benzimidazole (Scheme 5).
In a summary, we have developed an efficient one-pot protocol for the synthesis of 2-aryl-1-methyl-1H-benzo[d]imidazoles using a 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate–hydroxybenzotriazole system (HATU–HOBT) in DMF in the presence of DIPEA. This method works well with a wide range of aromatic carboxylic acids. The method provides an alternative protocol for the synthesis of benzimidazoles in good yields, which requires no chromatographic purification of the target products and can be used on a gram scale.
EXPERIMENTAL
All reagents were of reagent grade and used as received unless specified otherwise. Solvents for reactions were distilled prior to use. All reagents were of reagent grade and used as received unless specified otherwise. Solvents for reactions were distilled prior to use. All reactions were conducted under nitrogen or argon in flame-dried or oven-dried glassware with magnetic stirring. Thin-layer chromatography was performed on analytical silica gel 60 F254–coated TLC plates (Purchased from Merck Chemicals), eluent 50% EtOAc in n-hexane, and were visualized with UV light or by treatment with the TLC reagents such as KMnO4, ninhydrin, Dragandroff’s or phosphomolybdic acid (PMA). Column chromatography was carried out on silica gel (60–120 mesh). Technical grade ethyl acetate and hexane used for column chromatography were distilled prior to use. Commercially available starting materials were used without further purification.
The 1H and 13C NMR spectra were recorded in DMSO-d6 on a Bruker Avance-400 MHz at ambient temperature. The high-resolution mass spectral analysis was performed on Agilent 1290 UHPLC connected to an Agilent 6538 QTOF (Santa Clara, CA, USA).
Benzimidazoles 3a–3l (general procedure). 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (1.227 mmol), HOBT (1.227 mmol), and DIPEA (2.454 mmol) were added to a magnetically stirred solution of N-1-methylbenzene-1,2-diamine (1, 0.818 mmol) and arylcarboxylic acid 2 (0.818 mmol) in DMF (3 mL) in a Biotage reaction tube. The tube was sealed with an aluminum cap with a septum, and the reaction mixture was stirred at 100°C for 16 h. Progress of the reaction was monitored by TLC of samples of the reaction mixture, taken with a 1-mL syringe through the cap septum. Used the sealed tube which having aluminium cap with septa (purchased from Biotage) and sample took via 1 mL syringe. Upon completion of the reaction, the reaction mixture was cooled to room temperature and poured into water (15 mL) and treated with ethyl acetate (3 × 15 mL), and the combined organic extracts were dried over anhydrous sodium sulphate and filtered. The solvent was distilled off from the filtrate under reduced pressure to leave a crude product, which was purified by recrystallization or trituration with diethyl ether or pentane.
2-(3-Chlorophenyl)-1-methyl-1H-benzo[d]imidazole (3a). Yield 83.2%, brown solid, mp 92–94°C, Rf 0.63. 1H NMR spectrum (DMSO-d6), δ, ppm: 3.89 s (3H), 7.24–7.33 m (2H), 7.59–7.65 m (3H), 7.68–7.70 d (1H, J 7.6 Hz), 7.91 s (1H), 7.84–7.82 m (1H). 13C NMR spectrum, δ, ppm: 32.14, 111.19, 119.62, 122.59, 123.15, 128.40, 129.38, 130.02, 131.06, 132.65, 133.86, 137.07, 142.8, 151.9. Mass spectrum (ESI-HRMS), m/z: 243.0689 [M + H]+. C14H11ClN2. [M + H]+ 243.0695.
2-(3,4-Dichlorophenyl)-1-methyl-1H-benzo[d]imidazole (3b). Yield 75.4%, brown solid, mp 102–103°C, Rf 0.70. 1H NMR spectrum, δ, ppm: 3.92 s (3H), 7.26–7.36 m (2H), 7.65–7.67 d (1H, J 8.0 Hz), 7.70–7.72 d (1H, J 8.0 Hz), 7.85–7.87 m (2H), 8.14 s (1H). 13C NMR spectrum, δ, ppm: 32.16, 111.25, 119.68, 122.71, 123.31, 129.86, 131.21, 131.41, 131.44, 132.03, 133.03, 137.12, 142.76 151.05. Mass spectrum (ESI-HRMS), m/z: 277.0299 [M + H]+. C14H10Cl2N2. [M + H]+ 277.0302.
2-(3,4-Dimethoxyphenyl)-1-methyl-1H-benzo[d]imidazole (3c). Yield 70.5%, brown solid, mp 200°C, Rf 0.68. 1H NMR spectrum, δ, ppm: 3.89 s (3H), 3.92 s (3H), 3.92 s (3H), 4.06 s (2H), 7.31–7.33 d (1H, J 8.0 Hz), 7.53–7.66 m (4H), 7.85 d (1H, J 8.0 Hz), 8.03 d (1H, J 8.0 Hz). 13C NMR spectrum, δ, ppm: 32.21, 56.08, 56.12, 110.87, 111.97, 113.18, 119.16, 122.31, 122.45, 122.57, 122.86, 137.08, 142.80, 149.14, 150.50, 153.53. Mass spectrum (ESI-HRMS), m/z: 269.1290 [M + H]+. C16H16N2O2. [M + H]+ 269.1299.
1-Methyl-2-[3-(trifluoromethyl)phenyl]-1H-benzo[d]imidazole (3d). Yield 73.8%, yellow solid, mp 97°C, Rf 0.65. 1H NMR spectrum, δ, ppm: 3.92 s (3H), 7.27–7.33 m (2H), 7.64 d (1H, J 8.0 Hz), 7.71 d (1H, J 8.0 Hz), 7.83–7.85 m (1H), 7.93–7.95 d (1H, J 8.0 Hz), 8.18–8.19 m (2H). 13C NMR spectrum, δ, ppm: 32.15, 111.25, 119.69, 122.67, 123.26, 125.81, 126.29, 126.34, 126.37, 130.50, 133.59, 131.72, 137.15, 142.85, 151.91. Mass spectrum (ESI-HRMS), m/z: 277.09471 [M + H]+. C15H11F3N2. [M + H]+ 277.09476.
2-(3-Fluorophenyl)-1-methyl-1H-benzo[d]imidazole (3e). Yield 75.6%, off-white solid, mp 90°C, Rf 0.6. 1H NMR spectrum, δ, ppm: 3.92 s (3H), 7.26–7.35 m (2H), 7.41–7.45 m (1H), 7.62–7.67 m (2H), 7.70–7.74 m (3H). 13C NMR spectrum, δ, ppm: 32.15, 111.19, 117.17, 119.58, 122.61, 123.15, 125.99, 131.34, 132.80, 137.05, 142.69, 152.07, 161.25, 163.67. Mass spectrum (ESI-HRMS), m/z: 227.0979 [M + H]+. C14H11FN2. [M + H]+ 227.09780.
4-(1-Methyl-1H-benzo[d]imidazol-2-yl)benzonitrile (3f). Yield 75.9%, off-white solid, mp 205°C, Rf 0.43. 1H NMR spectrum, δ, ppm: 3.93 s (3H), 7.28–7.38 m (2H), 7.67–7.69 d (1H, J 8.0 Hz), 7.73–7.74 d (1H, J 7.6 Hz), 8.05–8.11 m (4H). 13C NMR spectrum, δ, ppm: 32.30, 111.34, 112.54, 119.02, 119.83, 122.80, 123.49, 130.54, 133.06, 135.03, 137.24, 142.89, 151.66. Mass spectrum (ESI-HRMS), m/z: 234.10257 [M + H]+. C15H11N3. [M + H]+ 234.10252.
2-(4-Ethylphenyl)-1-methyl-1H-benzo[d]imidazole (3g). Yield 76.5%, off-white solid, mp 94°C, Rf 0.63. 1H NMR spectrum, δ, ppm: 1.26 t (3H), 2.70–2.76 m (2H), 3.89 s (3H), 7.23–7.37 m (2H), 7.43 d (2H, J 8.0 Hz), 7.62 d (1H, J 8.0 Hz), 7.68 d (1H J 7.6 Hz), 7.79 d (2H, J 8 Hz). 13C NMR spectrum, δ, ppm: 15.90, 28.51, 32.14, 110.96, 119.36, 122.32, 122.68, 128.05, 128.54, 129.77, 137.07, 142.97, 145.96, 153.58. Mass spectrum (ESI-HRMS), m/z: 237.13863 [M + H]+. C16H16N2. [M + H]+ 237.13839.
2-(4-Isopropylphenyl)-1-methyl-1H-benzo[d]imidazole (3h). Yield 76.6%, brown solid, mp 112°C, Rf 0.68. 1H NMR spectrum, δ, ppm: 1.26 d (6H, J 6.8 Hz), 2.96–3.03 m (1H), 3.87 s (3H), 7.21–7.30 m (2H), 7.45 d (2H, J 8.0 Hz), 7.60 d (1H, J 7.6 Hz), 7.66 d (1H, J 8.0 Hz), 7.78 d (2H, J 8.0 Hz). 13C NMR spectrum, δ, ppm: 24.20, 32.15, 33.71, 110.95, 119.37, 122.32, 122.67, 126.41, 126.76, 127.08, 128.20, 129.79, 137.08, 142.99, 150.50, 153.56, Mass spectrum (ESI+), m/z: 251.3 [M + H]+.
2-(4-Ethoxyphenyl)-1-methyl-1H-benzo[d]imidazole (3i). Yield 82.3%, yellow solid, mp 84°C, Rf 0.45. 1H NMR spectrum, δ, ppm: 1.37–1.40 m (3H), 3.87 s (3H), 4.11–4.16 m (2H), 7.12 d (2H, J 8.0 Hz), 7.21–7.29 m (2H), 7.58–7.60 m (2H), 7.79 d (2H, J 12.0 Hz). 13C NMR spectrum, δ, ppm: 15.02, 15.09, 32.14, 63.75, 110.84, 119.19, 122.23, 122.48, 122.71, 132.72, 137.06, 142.96, 153.50, 160.05. Mass spectrum (ESI-HRMS), m/z: 253.13354 [M + H]+. C16H16N2O. [M + H]+ 253.13348.
2-(4-Isopropoxyphenyl)-1-methyl-1H-benzo[d]imidazole (3j). Yield 75.6%, yellow solid, mp 128°C, Rf 0.54. 1H NMR spectrum, δ, ppm: 1.20–1.32 m (6H), 3.86 s (3H), 4.70–4.76 m (1H), 7.09 d (2H, J 8.0 Hz), 7.20–7.28 m (2H), 7.57 d (1H, J 8.0 Hz), 7.63 d (1H, J 4.0 Hz), 7.77 d (2H, J 8.0 Hz). 13C NMR spectrum, δ, ppm: 22.27, 32.13, 32.17, 69.82, 110.84, 115.91, 119.17, 122.23, 122.48, 122.52, 131.29, 137.05, 142.95, 153.51, 159.03. Mass spectrum (ESI-HRMS), m/z: 267.14919 [M + H]+. C17H18N2O. [M + H]+ 267.14921.
2-(3-Chloro-5-fluorophenyl)-1-methyl-1H-benzo[d]imidazole (3k). Yield 74.9%, yellow solid, mp 62°C, Rf 0.78. 1H NMR spectrum, δ, ppm: 3.93 s (3H), 7.27–7.37 m (2H), 7.66–7.76 m (4H), 7.81 s (1H). 13C NMR spectrum, δ, ppm: 32.12, 111.29, 115.77, 117.77, 119.76, 123.43, 125.91, 133.98, 134.91, 137.09, 142.66, 150.76, 161.22, 163.69. Mass spectrum (ESI-HRMS), m/z: 261.05893 [M + H]+. C14H10ClFN2. [M + H]+ 261.05901.
2-(4-Chloro-3-methoxyphenyl)-1-methyl-1H-benzo[d]imidazole (3l). Yield 73.0%, mp 110°C, Rf 0.78, yellow solid. 1H NMR spectrum, δ, ppm: 3.91 s (3H), 3.97 s (3H), 7.25–7.34 m (2H), 7.44 d (1H, J 8.0 Hz), 7.58 s (1H), 7.63 d (2H, J 4.0 Hz), 7.71 d (1H, J 8.0 Hz). 13C NMR spectrum, δ, ppm: 32.20, 56.81, 111.12, 114.04, 119.54, 122.55, 122.59, 123.05,123.13, 130.50, 130.75, 137.12, 142.81, 152.51,155.01. Mass spectrum (ESI-HRMS), m/z: 273.07892 [M + H]+. C15H13ClN2O. [M + H]+ 273.07896.
REFERENCES
Heers, J., Backx, L.J.J., Mostmans, J.H., and Van, J., J. Med. Chem., 1979, vol. 22, p. 1003. https://doi.org/10.1021/jm00194a023
Hunkeler, W., Mohler, H., Pieri, L., Polc, P., Bonetti, E.P, Cumin, R., Schaffner, R., and Haefely, W., Nature, 1981, vol. 290, p. 514. https://doi.org/10.1038/290514a0
Brimblecombe, R.W., Duncan, W.A.M., Durant, G.J., Emmett, J.C., Ganellin, C.R., and Parsons, M.E., J. Int. Med. Res., 1975, vol. 3, p. 86. https://doi.org/10.1177/030006057500300205
Tanigawara, Y., Aoyama, N., Kita, T., Shirakawa, K., Komada, F., Kasuga, M., and Okumura, K., Clin. Pharmacol. Ther., 1999, vol. 66, p. 528. https://doi.org/10.1016/S0009-9236(99)70017-2
Wauquier, A., Van Den Broeck, W.A.E., Verheyen, J.L., and Janssen, P.A.J., Eur. J. Pharmacol., 1987, vol. 47, p. 367. https://doi.org/10.1016/0014-2999(78)90117-6
John, B., Chem. Rev., 1951, vol. 48, p. 397. https://doi.org/10.1021/cr60151a002
Preston, P.N., Chem. Rev., 1974, vol. 74, p. 279. https://doi.org/10.1021/cr60289a001
Donglai, Y., Demosthenes, F., Jingzhou, L., Libing, Y., and Baldino, C.M., Synthesis, 2005, vol. 1, p. 47. https://doi.org/10.1055/s-2004-834926
Turner, G.L., Morris, J.A., and Michael, F., Angew.Chem. Int. Ed., 2007, vol. 46, p. 7996. https://doi.org/10.1002/anie.200702141
Zhang, W., Tian, Y., Zhao, N., Wang, Y., Li, J., and Wang, Z., Tetrahedron, 2014, vol. 70, p. 6120. https://doi.org/10.1016/j.tet.2014.04.065
Bellini, F., Calandri, C., Cauteruccio, S., and Rossi, R., Tetrahedron, 2007, vol. 63, pp. 1970. https://doi.org/10.1016/j.tet.2006.12.068
Czarny, A., Wilson, W.D., and Boykin, D.W., J. Heterocycl. Chem., 1996, vol. 33, p. 1393. https://doi.org/10.1002/jhet.5570330463
Zhang, W., Zeng, Q., Zhang, X., Tian, Y., Yue, Y., Guo, Y., and Wang, Z., J. Org. Chem., 2011, vol. 76, p. 4741. https://doi.org/10.1021/jo200452x
ACKNOWLEDGMENTS
Authors are highly grateful to Kadi Sarva Vishwavidyalaya Gandhinagar, Gujarat, India for providing infrastructure and laboratory facilities.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81903623), China Postdoctoral Science Foundation (grant no. 2019M652586), Postdoctoral Research Grants in Henan Province nos. 1902001 and 19030008, and Henan Medical Science and Technology Program (grant no. 2018020601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Duan, Y.T., Parmar, T.H., Sangani, C.B. et al. 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-Oxide Hexafluorophosphate (HATU)/Hydroxybenzotriazole (HOBT)-based One-Pot Cyclization of N-Substituted 2-Arylbenzimidazole Derivatives. Russ J Org Chem 56, 856–862 (2020). https://doi.org/10.1134/S107042802005019X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042802005019X