Abstract
A series of novel 2-(oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamide derivatives have been prepared by one-pot three-component synthesis using 2-aminopyridin-3-ol, dimethyl phthalate, and anilines in 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4) at 80–85°C for 90–120 min. This method offers remarkable advantages of good yields, straightforward protocol, environmental friendliness, short reaction times, and mild reaction conditions. The need in catalysts and solvents is avoided by using a catalytically active ionic liquid as a medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Multicomponent reactions (MCRs) are an important technique for successful and swift synthesis of a broad range of composite heterocyclic frameworks [1, 2]. Such reactions are specifically focused on organic synthesis due to their ability to make composite molecular functionalities from three or more starting materials via one-pot reaction [3–5]. The development of new, environmentally benign multicomponent reactions has received ample attention in view of the prospect of compliance with green chemistry principles [6].
The rising environmental consciousness of chemical industry creates a demand for clear and clean methods that avoid use of hazardous and harmful organic solvents. Ionic liquids (ILs) have become popular as novel and promising solvents for heterocyclic synthesis [7, 8]. The main useful property of ILs is their negligible vapor pressure at temperatures below the decomposition point. For this reason, ILs are considered as green chemistry solvents. The thermal stability of ILs is limited by the strength of their heteroatom–carbon and heteroatom–hydrogen bonds [9].
Over the past years special attention has been focused on 1-butyl-3-methyl imidazolium salts as environmentally sustainable and efficient media for in various heterocyclic transformations including hydrogenations [10], as well as Heck [11], Friedel–Crafts [12], and Bishler–Napieral reactions [13]. Among representatives of this family of ILs, 1-butyl-3-methyl imidazolium tetrafluoroborate ([BMIM]BF4) has found the most marvelous applications in various organic conversions [14]. The weak electrostatic interactions of the tetrafluoroborate anion with the imidazolium cation impart good thermal and electrochemical stabilities to [BMIM]BF4. The other favorable physical and chemical properties, like mildness, lack of inflammability, neutral nature, commercial availability, volatility, environmental sustainability, and ability to dissolve many organic products, make this ionic liquid preferred over the others [15].
Oxazolopyridines are significant heterocyclic structures incorporated in various biologically active substances, which have found applications as anti-inflammatory, analgesic, anti-pyretic, and antifungal agents [16]. Oxazolopyridine derivatives can also act as inhibitors of fatty acid amide hydrolaze (FAAH) [17, 18], inhibitors of monoterpenoidindole alkaloid production in catharanthus roseus cells [19], inhibitors of acetyl- and butyrylcholinesterases [20], and SIRTI activators [21]. The fact that many oxazolopyridine derivatives fluoresce in the visible spectral range makes them good candidates for application in LED devices or nonlinear optical systems [22].
In the present work we prepared a series of 2-(oxazolo[5,4-b]pyridin-2-yl)-N-arylbenzamide derivatives with good yields by one-pot three-component synthesis using dimethyl phthalate, anilines, and 2-aminopyridin-3-ol in a [BMIM]BF4 medium at 80–85°C for 90–120 min.
RESULTS AND DISCUSSION
The conditions of the synthesis of 2-(oxazolo[5,4-b]pyridin-2-yl)-N-arylbenzamide derivatives were optimized in the model one-pot synthesis of 2-(oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamide 4a in the reaction of equimolar amounts of dimethyl phthalate (1), aniline (2a) and 2-aminopyridin-3-ol (3) (Scheme 1, Tables 1 and 2) by varying the nature and amount of the ionic liquid and the reaction temperature. Of the four tested ionic liquids, specifically [BMIM]BF4, [BMIM]Br, [BMIM]Cl, and [BMIM]OH, the highest yield of compound 4a was obtained in [BMIM]BF4 (Table 1, entry 1).
The effect of the temperature was studied in the model reaction of 1 with 2a and 3 in [BMIM]BF4. It was found that the reaction at 95–100°C was complete in 75 min, yielding 75% of compound 4a, while the reaction at 60–65°C gave 80% of compound 4a, but the reaction time was as long as 8 h. The highest yield of compound 4a (85%) in a relatively shorter time (90 min) was obtained at 80–85°C (Table 1, entry 1).
In a continuation of the optimization of the reaction conditions, the model reaction between equimolar amounts of 1, 2a, and 3 to form product 4a was performed with different amounts of [BMIM]BF4 at 80–85°C (Table 2). The best results were obtained with 6 equiv of [BMIM]BF4 as a medium (Table 2, entry 2).
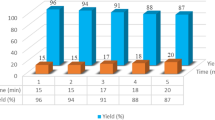
Thus, as the optimal conditions for the model one-pot synthesis of compound 4a we chose 6 equiv of [BMIM]BF4 as a medium and the reaction temperature 80–85°C. Under these conditions we reacted from dimethyl phthalate (1) and 2-aminopyridin-3-ol (3) with anilines 2a–2f to obtain a series of novel 2-(oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamide derivatives 4a–4f in yields of 81–85% (Scheme 1, see Experimental). The compositions and structures of the products were confirmed by elemental analysis and 1H and 13C NMR spectroscopy and mass spectrometry.
EXPERIMENTAL
The melting points were determined on in open capillary tubes in an H2SO4 bath. The FTIR spectra were recorded on a Bruker VERTEX 80 spectrometer in KBr. The 1H and 13C NMR spectra were obtained on a Bruker DRX-400 spectrometer at 400 and 100 MHz, respectively, in DMSO-d6; internal standard TMS. The mass spectra were recorded on an Agilent LCMS instrument. Silica gel-G plates (Merck) were used for thin layer chromatography (TLC) analysis with a mixture of n-hexane and ethyl acetate (6 : 4) as eluent.
Synthesis of 2-(oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamides 4a–4f (general procedure). A mixture of 10 mmol of dimethyl phthalate (1), 10 mmol of aniline 2a–2f, and 10 mmol of 2-aminopyridin-3-ol (3) was heated in 6 equiv of [BMIM]BF4 at 80–85°C for 90–120 min. After the reaction had been complete (by TLC), the reaction mixture was cooled to 40–45°C, diluted with 50 mL of water, stirred for 15–20 min at the same temperature, and cooled to 25–30°C. The colorless precipitate that formed was filtered off, washed with 50 mL of water, and dried at 60–65°C for 12 h. The crude product was recrystallized from ethanol to obtain the target product.
2-(Oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamide (4a) was synthesized by the general procedure from compounds 1, 3, and aniline (2a), reaction time 90 min. Yield 85%, mp > 220°C. 1H NMR spectrum, δ, ppm: 7.40–8.40 m (12H, Harom), 10.8 s (1H, CO–NH). 13C NMR spectrum, δ, ppm: 114.0, 121.5, 121.9, 124.2, 126.9, 128.3, 128.5, 129.9, 130.3, 130.7, 131.9, 134.3, 136.7, 138.9, 139.7, 149.0, 149.2, 169.9. Mass spectrum, m/z: 316. Found, %: C 72.48; H 4.19; N 13.54. C19H13N3O2. Calculated, %: C 72.37; H 4.16; N 13.33.
N-(4-Chlorophenyl)-2-(oxazolo[5,4-b]pyridin-2-yl)benzamide (4b) was synthesized by the general procedure from compounds 1, 3, and 4-chloroaniline (2b), reaction time 120 min. Yield 82%, mp > 220°C. 1H NMR spectrum, δ, ppm: 7.40–8.40 m (12H, Harom), 10.8 s (1H, CO–NH). 13C NMR spectrum, δ, ppm: 114.5, 120.5, 121.4, 124.0, 127.5, 128.3, 128.7, 129.5, 129.9, 130.2, 131.3, 134.6, 136.9, 138.6, 139.9, 151.3, 152.5, 169.5. Mass spectrum, m/z: 349. Found, %: C 65.15; H 3.54; N 12.12. C19H12ClN3O2. Calculated, %: C 65.24; H 3.46; N 12.01.
2-(Oxazolo[5,4-b]pyridin-2-yl)-N-(p-tolyl)benzamide (4c) was synthesized by the general procedure from compounds 1, 3, and 4-methylaniline (2c), reaction time 110 min. Yield 83%, mp > 220°C. 1H NMR spectrum, δ (400 MHz, DMSO-d6, TMS): 7.40–8.40 m (12H, Harom), 10.8 s (1H, CO–NH). 13C NMR spectrum, δ, ppm: 19.5, 114.2, 119.0, 120.4, 122.2, 125.5, 127.3, 128.5, 129.0, 129.9, 130.5, 130.9, 131.5, 134.6, 135.9, 137.5, 149.7, 149.9, 167.3. Mass spectrum, m/z: 330. Found, %: C 72.83; H 4.50; N 12.85. C20H15N3O2. Calculated, %: C 72.94; H 4.59; N 12.76.
N-(4-Methoxyphenyl)-2-(oxazolo[5,4-b]pyridin-2-yl)benzamide (4d) was synthesized by the general procedure from compounds 1, 3, and 4-methoxyaniline (2d), reaction time 110 min. Yield 82%, mp > 220°C. 1H NMR spectrum, δ, ppm: 7.40–8.40 m (12H, Harom), 10.8 s (1H, CO–NH). 13C NMR spectrum, δ, ppm: 54.2, 117.2, 121.2, 121.9, 123.4, 126.8, 128.3, 128.9, 129.2, 129.5, 130.7, 130.8, 133.1, 136.4, 137.5, 138.5, 150.0, 150.1, 167.9. Mass spectrum, m/z: 346. Found, %: 69.43; H 4.26; N 12.19. C20H15N3O3. Calculated, %: 69.56; H 4.38; N 12.17.
N-(4-Nitrophenyl)-2-(oxazolo[5,4-b]pyridin-2-yl)benzamide (4e) was synthesized by the general procedure from compounds 1, 3, and 4-nitroaniline (2e), reaction time 90 min. Yield 83%, mp > 220°C. 1H NMR spectrum, δ, ppm: 7.40–8.40 m (12H, Harom), 10.8 s (1H, CO–NH). 13C NMR spectrum, δ, ppm: 113.9, 121.2, 121.8, 122.9, 127.5, 127.9, 128.0, 128.9, 130.0, 130.7, 133.0, 134.9, 137.0, 138.7, 148.0, 148.9, 169.9. Mass spectrum, m/z: 361. Found, %: 63.44; H 3.23; N 15.43. C19H12N4O4. Calculated, %: 63.33; H 3.36; N 15.55.
N-(4-Fluorophenyl)-2-(oxazolo[5,4-b]pyridin-2-yl)benzamide (4f) was synthesized by the general procedure from compounds 1, 3, and 4-fluoroaniline (2f), reaction time 100 min. Yield 81%, mp > 220°C. 1H NMR spectrum, δ, ppm: 7.40–8.40 m (12H, Harom), 10.8 s (1H, CO–NH). 13C NMR spectrum, δ, ppm: 113.9, 120.9, 121.8, 122.9, 127.3, 127.9, 128.5, 129.0, 129.5, 130.5, 130.9, 132.9, 135.7, 137.5, 148.0, 148.7, 169.7. Mass spectrum, m/z: 334. Found, %: 68.36; H 3.69; N 12.78. C19H12N3O2. Calculated, %: 68.46; H 3.63; N 12.61.
CONCLUSIONS
In summary, we developed an efficient and environmentally benign protocol for the synthesis of 2-(oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamides in [BMIM]BF4 as a medium. This one-pot four- component reaction proceeds in short time with high yields and features a straightforward work-up procedure and no need of column purification.
REFERENCES
Maddila, S., Gangu, K.K., Maddila, S.N., and Jonnalagadda, S.B., Mol. Divers., 2017, vol. 21, p. 247. https://doi.org/10.1007/s11030-016-9708-5
Shabalala, S., Maddila, S., Zyl, W.E., and Jonnalagadda, S.B., ACS Ind. Eng. Chem. Res., 2017, vol. 56, p. 11372. https://doi.org/10.1021/acs.iecr.7b02579
Gangu, K.K., Maddila, S., and Jonnalagadda, S.B., New J. Chem., 2017, vol. 41, p. 6455. https://doi.org/10.1039/C7NJ00855D
Bhaskaruni, S.V.H.S., Maddila, S., Zyl, W.E., and Jonnalagadda, S.B., Catal. Commun., 2017, vol. 100, p. 24. https://doi.org/10.3390/molecules23071648
Shabalala, S., Maddila, S., Zyl, W.E., and Jonnalagadda, S.B., Catal. Commun., 2016, vol. 79, p. 21. https://doi.org/10.1039/C8RA01994K
Gangu, K.K., Maddila, S., Maddila, S.N., and Jonnalagadda, S.B., RSC Adv., 2017, vol. 7, p. 423. https://doi.org/10.1039/c6ra25372e
Wasserscheid, P. and Keim, W., Angew. Chem., Int. Ed., 2000, vol. 39, p. 3773. https://doi.org/10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO,2-5
Corma, A. and Garcia, H., Chem. Rev., 2003, vol. 103, p. 4307. https://doi.org/10.1021/cr030680z
Olivier-Bourbigou, H. and Magna, L., J. Mol. Catal. A, 2002, vol. 182, p. 419. https://doi.org/10.1016/S1381-1169(01)00465-4
Dyson, P.J., Ellis, D.J., Parker, D.G., and Welton, T., Chem. Commun., 1999, p. 25. https://doi.org/10.1039/A807447J
Bohm, V.P.W. and Herrmann, W.A., Chem. Eur. J., 2000, vol. 6, p. 1017. https://doi.org/10.1002/(SICI)1521-3765(20000317)6:6<1017::AID-CHEM1017>3.0.CO,2-8
Stark, A., MacLean, B.L., and Singer, R.D., Dalton Trans., 1999, p. 63. https://doi.org/10.1039/A806708B
Judeh, Z.M.A., Chi, B.C., Jie, B., and McCluskey, A., Tetrahedron Lett., 2002, vol. 43, p. 5089. https://doi.org/10.1016/S0040-4039(02)00998-X
Bubun, B., Chemistry Select, 2017, vol. 2, p. 8362. https://doi.org/10.1002/slct.201701700
Sowmiah, V., Srinivasadesikan, M.-C., and Tseng, Y.-H., Molecules, 2009, vol. 14, p. 3780. https://doi.org/10.3390/molecules14093780
Ziarani, G.M., Nahad, M.S., Lashgari, N., and Badiei, A., J. Nanostruct. Chem., vol. 5, p. 39. https://doi.org/10.1007/s40097-014-0129-7
Zhuravlev, F.A., Tetrahedron Lett., 2006, vol. 47, p. 2929. https://doi.org/10.1016/j.tetlet.2006.02.117
Maryanoff, B.E. and Costanzo, M.J., Bioorg. Med. Chem., 2008, vol. 16, p. 1562. https://doi.org/10.1016/j.bmc.2007.11.015
Mincheva, Z., Courtois, M., Andreu, F., Rideau, M., and Viaud-Massuard, M.-C., Phytochemistry, 2005, vol. 66, p. 1797. https://doi.org/10.1016/j.phytochem.2005.06.002
Marco, J.L., de los Ríos, C., Garćia, A.G., Villarroya, M., Carreiras, M.C., Martins, C., Eleutério, A., Morreale, A., Orozco, M., and Luque, F.J., Bioorg. Med. Chem., 2004, vol. 12, p. 2199. https://doi.org/10.1016/j.bmc.2004.02.017
Bemis, J.E., Vu, C.B., Xie, R., Nunes, J.J., Ng, P.Y., Disch, J.S., Milne, J.C., Carney, D.P., Lynch, A.V., Jin, L., Smith, J.J., Lavu, S., Iffland, A., Jirousek, M.R., and Perni, R., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 2350. https://doi.org/10.1021/jm8012954
Barni, E., Pasquino, S., Savarino, P., Di Modica, G., and Giraudo, G., Dyes Pigm., 1985, vol. 6, p. 1. https://doi.org/10.1016/0143-7208(85)80001-2
ACKNOWLEDGMENTS
The authors are highly grateful to the authorities of the Department of Chemistry, Koneru Lakshmaiah Education Foundation, Green Fields, Vaddeswaram, Guntur (Dist), Andhra Pradesh, India, for providing the research facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Satyanarayana, M.V., Mehta, B., Prasad, K.R.S. et al. 1-Butyl-3-methylimidazolium Tetrafluoroborate ([BMIM]BF4): An Efficient Ionic Liquid Medium for the Synthesis of Novel 2-(Oxazolo[5,4-b]pyridin-2-yl)-N-phenylbenzamides. Russ J Org Chem 57, 237–240 (2021). https://doi.org/10.1134/S1070428021020147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021020147