Abstract
A perennial plant Nepeta multifida L. (syn. Schizonepeta multifida (L.) Briq.) is one of the most common species of the Lamiaceae family growing in Eastern Siberia and used in traditional oriental medicine. The chemical composition of N. multifida has not been sufficiently studied. Chromatographic separation of phenolic compounds of N. multifida leaves using column chromatography and preparative HPLC made it possible to isolate 16 compounds, including a new flavonoid identified as luteolin-7-O-(3″,6″-di-O-acetyl)-β-D-glucopyranoside, according to UV, NMR spectroscopy and mass spectrometry. Known compounds were luteolin and apigenin O-glycosides, rosmarinic acid, salvianolic acids A and B, and schizotenuin A. Quantitative analysis of N. multifida leaves in various phases of plant development by HPLC-UV assay showed the high content of rosmarinic acid (8.36–35.71 mg/g), luteolin-7-O-glucuronide (2.03–14.18 mg/g) and schizotenuin A (5.29–9.56 mg/g). The highest level of phenolic compounds was found in the flowering and fruiting phases. Using Ellman’s spectrophotometric method, it was found that N. multifida leaf extract and some compounds had antiacetylcholinesterase activity and luteolin glycosides being the most active showed the level of concentration of half-maximal enzyme inhibition (IC50) 29.03– 58.36 μg/mL. Thus, as a result of the present study, it was found that the leaves of N. multifida contain various groups of phenolic compounds capable of inhibiting the activity of acetylcholinesterase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Central nervous system diseases are widespread in the modern world and are among the pathological factors leading to disability of a wide range of the population. For the treatment and prevention of these diseases, both synthetic drugs and phytotherapeutic procedures are used. Among the many herbal remedies, drugs from the Lamiaceae family are particularly effective, which have a positive therapeutic effect in cerebral hypoxia and anoxia [1] and in cerebral ischemia and neuronal damage caused by excitotoxicity [2], and also demonstrate neuroprotective and anticholinesterase effects in Alzheimer’s disease [3, 4]. The most commonly used include more than 50 species, among which Nepeta species with anticholinesterase, antinociceptive and anticonvulsant effects should be especially distinguished [5].
In Siberia, genus Nepeta L. is represented by six species, two of which, N. multifida L. and N. annua Pall., belong to the Schizonepeta Benth subsection, which separated into a separate genus Schizonepeta (Benth.) Briq. [6]. In Eastern Siberia, only N. multifida grows, occupying significant territories of steppified slopes and dry meadows [7]. The known literature data on the chemical composition of N. multifida relate primarily to essential oil, the main components of which are β-ocimene, 1,8-cineol, limonene, pulegone, phellandrene and menthone [8]. Docosanoic, tetracosanoic, succinic, deoxyoleanolic acids [9] and 3-imino-N-(α-iminoethylamino) have been identified in the composition of nonvolatile compounds of N. multifida butyrolactam [10]. The study of phenolic compounds of N. multifida flowers from the Baikal region revealed the presence of luteolin and apigenin glycosides, as well as rosmarinic acid and other benzofuran lignans [10, 11]; phenolic compounds of leaves of this species have not been studied before.
In Tibetan medicine, N. multifida herb was used for the treatment of skin diseases as an antibacterial and wound healing agent, as well as for gastrointestinal diseases as an appetizing, antitumor and anthelmintic medicine [12]. Dried plants tincture was used for whooping cough and various types of cough. Of particular interest is the use of the N. multifida herb for the treatment of “planetary” diseases, the description of which resembles the clinical picture of a stroke. The studies of the biological activity of N. multifida extracts are few, but it is known that these extracts have neuroprotective, stress-protective, antioxidant [13] and antihypoxic effects [14]. This paper presents information on the chemical composition and quantitative content of individual compounds in the N. multifida leaves, as well as the results of the some phenolic components anticholinesterase activity examination.
EXPERIMENTAL
Plant raw materials. N. multifida plants were collected in 2020 in the Republic of Buryatia (Mukhorshibirsky district, 51°02′46.4″ N, 107°46′52.7″E, 830 m in.u.m.) at the beginning of vegetation (June 2–5; 30 samples), vegetation phases (June 28–30, 28 samples), flowering (July 15–18, 45 samples), fruiting (August 10–14, 38 samples) and dying (August 28–31, 22 samples). The sample consisted of leaves collected from one plant. The species is determined by Doctor of Pharmaceutical Sciences, N.K. Chirikova (North-Eastern Federal University, Yakutsk). A sample of plant raw materials is stored in the herbarium of the IGEB SB RAS (no. BU/LAM-0720/36-114). The leaves were separated and dried in a convection cabinet at 45°C to a humidity of 4–5%.
General experimental conditions. For column chromatography (CC) we used polyamide, normal-(SiO2) and reversed-phase silica gel (RP–SiO2) and Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA). Spectrophotometric studies were carried out on the SF-2000 spectrophotometer (OKB Spectrum, St. Petersburg, Russia). Mass spectra were recorded on the LCMS-8050 TQ mass spectrometer (Shimadzu, Columbia, MD, USA) [15], NMR spectra were recorded on the VXR 500S spectrometer (Varian, Palo Alto, CA, USA). Preparative HPLC was performed on an LC-20 Prominence liquid chromatograph (Shimadzu) equipped with a Shim-pak PREP-ODS column (20 × 250 mm, d 15 microns) and a SPD-M30A photodiode detector (Shimadzu); v 1.0 mL/min, column temperature 20°C.
Extraction and isolation of compounds 1–16 from N. multifida leaves. The crushed raw materials of N. multifida (850 g) were extracted with 70% ethanol (1: 12, 70°C, three times; ultrasonic bath), after which the alcohol extract was concentrated dry in vacuum (yield 272 g). The dry extract was treated in a Soxlet apparatus with hexane and chloroform until depleted to remove lipophilic components. The fat-free extract was separated in a two-phase ethyl lipid-water system (1 : 1) and then the aqueous phase was extracted with n-butanol. Organic extracts were concentrated (extract yield: ethyl acetate 66 g, n-butanol 98 g) and applied to polyamide for CC (1 : 20), which was eluted with water (eluate 1), 50% ethanol (eluate 2) and 0.5% NH3 in 90% ethanol (eluate 3). Eluates 2 (E2) and 3 (E3) of the ethyl acetate fraction and eluate 2 of the n-butanol fraction (B2) were subjected to chromatographic separation. Eluate E2 (9 g) was separated using flash chromatography into SiO2 (2 × 40 cm, EtOAc-Me2CO 100 : 0 → 60 : 40), RP–SiO2 (1 × 30 cm, H2O–MeCN 95 : 5 → 60 : 40) and Sephadex LH-20 (1 × 60 cm, MeOH–H2O 80 : 20 → 30 : 70), which made it possible to isolate luteolin-7-O-glucoside (19 mg, 2) [16] and apigenin-7-Oglucoside (8 mg, 3) [16]. To separate the E3 fraction (11 g), flash chromatography was used on SiO2 (2 × 60 cm, EtOAc–Me2CO 100 : 0 : 0 → 70 : 30), RP–SiO2 (1 × 20 cm, H2O–MeCN 100 : 0 → 20 : 80), Sephadex LH-20 (1 × 60 cm, MeOH–H2O–AcOH 90 : 5 : 5 → 20 : 75 : 5) and preparative or prep. HPLC (eluent A—MeOH, eluent B—H2O; gradient mode, % B: 0–30 min, 5–15%, 30–45 min, 15–38%, 45–90 min, 38–58%, 90–120 min, 58–85%). As a result of separation, 1 (33 mg), luteolin-7-O-glucuronide (7.5 g, 4) [16], apigenin-7-O-glucuronide (10 mg, 5) [16], luteolin-7-O-(6″-O-acetyl)-glucoside (1.4 g, 6) [17], apigenin-7-O-(6″-O-acetyl)-glucoside (5 mg, 7) [17], rosmarinic acid (9.1 g, 8) [18], salvianolic acid A (24 mg, 9) [18], salvianolic acid B (33 mg, 10) [18], schizotenuin A (820 mg, 11) [18] and nepetamultin A (8 mg, 12) [11]. Eluate B2 was separated in a manner similar to that for E2, which led to the isolation of luteolin-7-O-neohesperidoside (7 mg, 13) [16], luteolin-7-O-rutinoside (28 mg, 14) [16], apigenin-7-O-neohesperidoside (6 mg, 15) [16] and apigenin-7-O-rutinoside (5 mg, 16) [16].
Luteolin-7-O-(3″,6″-di-O-acetyl)-β-D-glucopyranoside (1). C25H24O13. UV spectrum (MeON, λmax, nm): 254, 269, 347. HR-ESI-MS, m/z 531.4273 (Calculation 531.4064 for C25H23O13 [M –H]–); ESI-MS, m/z (%): 531 [M – H]–; ESI-MS2 [531]: 489 [(M – H)–42]– (9), 447 [(M – H)–42 × 2]– (12), 285 [(M – H)–42 × 2–162]– (100); NMR spectrum of 1H (500 MHz, DMSO-d6, 298 K, δH, ppm) (Table 1). NMR spectrum 13C (125 MHz, DMSO-d6, 298 K, δC, ppm) (Table 1).
Micro-column HPLC-UV. Quantitative analysis of phenolic compounds was carried out using a microcolumn liquid chromatograph Milichrome A-02 (Econova, Novosibirsk, Russia) on a ProntoSIL column120-5-C18 AQ (2 × 75 mm, Ø5 microns; MetrohmAG, Herisau, Switzerland); mobile phase: 0.2 M LiClO4 in 0.006 M HClO4 (A), MeCN (B). Gradient conditions (%B): 0–15 min 5–100%; in 150 µL/min; column temperature 35°C; UV detector, λ 330 nm. The content of phenolic compounds was calculated reference standards ChemFaces (Wuhan, Hubei, PRC)—luteolin-7-O-glucuronide (cat. no. CFN98512, ≥98%), luteolin-7-O-rutinoside (cat. no. CFN93556, ≥98%), apigenin-7-O-glucuronide (cat. no. CFN98500, ≥98%); and Sigma-Aldrich (St. Louis, MO, USA) —luteolin-7-O-glucoside (cat. no. 74284, ≥98%), apigenin-7-O-glucoside (cat. no. 44692, ≥97%), rosmarinic acid (cat. no. R4033, ≥98%), salvianolic acid A (cat. no. 97599, ≥95%), salvianolic acid B (cat. no. PHL89783, ≥90%). For the quantitative analysis of some compounds, external comparison substances were used: luteolin-7-O-glucoside for luteolin-7-O-(6″-O-acetyl)-glucoside and luteolin7-O-(3″,6″-di-O-acetyl)-β-D-glucopyranoside, apigenin7-O-glucoside for apigenin-7-O-(6″-O-acetyl)-glucoside, rosemary acid for schizotenuin A. To prepare a solution of a standard substance, 5 mg of the compound was dissolved in a measuring flask (5 mL) in 70% acetonitrile and the volume of the solution was brought to the mark with the same solvent. Next, a series of dilutions with a concentration of 5–500 μg/mL was prepared and analyzed by HPLC-UV. The results were used to construct calibration graphs in the coordinates “concentration, μg/mL—chromatographic peak area,” which were used for further calculation. The data are presented as an average of three parallel definitions (± standard deviation, S.D.).
Sample preparation of plant samples. To carry out quantitative analysis of phenolic compounds in N. multifida leaves, an exact sample of crushed plant raw materials (200 mg) was placed in an extraction container (5 mL) with a screw cap, 2 mL of 70% ethanol was poured and extracted in an ultrasonic bath (100 W, 35 kHz) at 50°C for 20 min. The resulting sample was centrifuged (3000 g, 15 min) and the supernatant was transferred to a measuring flask with a capacity of 5 mL. The extraction was repeated under the same conditions again. The volume of the combined extract was adjusted to the label with 70% ethanol. Before the HPLC procedure, the test solution was filtered through a PTFE filter (0.22 microns) and used for analysis without prior dilution. The extract for the study of biological activity was obtained in a similar way, after which the extractant was removed in vacuum and the dry residue was crushed.
Biological activity. Inhibitory effect of N. multifida extract and pure compounds on acetylcholinesterase from Electrophorus electricus (Sigma-Aldrich, cat. no. C3389, type VI-S, 1000 units/mg) was studied using the Ellman method, in which thiocholine formed from an enzyme reacts with 5.5′-dithiobis(2nitrobenzoic acid) to form a colored complex recorded by spectrophotometric method at a wavelength of 412 nm. The Acetylcholinesterase Activity Assay Kit (Sigma-Aldrich, cat. no. MAK119). The composition of N. multifida extract metabolites was studied before and after incubation with acetylcholinesterase under the HPLC-UV conditions described above. The extract sample (10 mg) was dissolved in a 20 mM tris-HCl buffer (pH 7.5; 5 mL), centrifuged (6000 g, 10 min), the supernatant (50 mL) was mixed with 50 mL of acetylcholinesterase solution in a 20 mM tris-HCl buffer (1 mg/mL) and incubated at 37° from (30 min). After that, 100 mL of acetonitrile was poured into the sample, centrifuged (6000 g, 5 min) and the supernatant was analyzed by HPLC-UV.
Statistical analysis was performed using single-factor analysis of variance (ANOVA). The significance of the mean differences was determined using the Duncan multi-rank test. Differences at p <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
As a result of chromatographic separation of methanol extract from N. multifida leaves using CC on polyamide, normal- and reversed-phase silica gel, Sephadex LH-20 and preparative HPLC, known compounds 2–16 and a new flavonoid 1 were isolated.
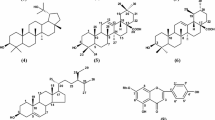
Compound 1 was determined by the formula C25H24O13 according to MS (HR-ESI-MS, m/z 531.4273; calculation 531.4064 for the ion C25H23O13 [M – H]–) and NMR spectroscopy 13C. UV spectroscopy data indicated that 1 is a flavonoid of the luteolin group (Fig. 1a), and in the mass spectrum of MS2, ions were detected due to the removal of two acetyl groups (m/z 531 → 489, 447) and a hexose fragment (m/z 447 → 285) (Fig. 1b) [19].
Luteolin and D-glucose were found in the products of acid hydrolysis of compound 1 with TFA. The signal of the anomeric proton of the carbohydrate fragment was in the region of 5.10 ppm (δH, d, J = 8.1 Hz), which is typical for the β-anomer of glucose [20]. NMR spectra were close to those of luteolin-7-O-β-D-glucopyranoside (2) [16] with the exception of additional signals of acetyl groups in the spectra of 1H (δH 1.95/2.07) and 13C (δC 20.5/21.4 ppm, 170.0/172.5 ppm) (Table 1). The signals of protons H-3″ and H-6″ of glucopyranose in 1 were shifted to a weak down in comparison with those of 2 (δH 3.40 → 4.98 for H-3″; 3.57/3.79 → 4.22/4.59 for H-6″), which was also observed for C-3″ carbon signals (δC 77.0 → 78.2) and C-6″ (δC 60.8 → 63.9) with simultaneous high-field shift of signals of neighboring carbon atoms (δC C-2″ 74.5 → 72.8; C-4″ 70.9 → 69.5; C-5″ 78.0 → 75.6). These features of the NMR spectra indicated the presence of substitution in C-3″ and C-6″ glucopyranose [20], which confirmed the existing correlations in the HMBC spectrum between the signals of the proton H-3″ c δH 4.98 ppm and H-6″with δH 4.22/4.59 ppm and carbons of acetyl carbonyls with δC 172.5 and 170.0 ppm, respectively (Fig. 1c).
Thus, compound 1 was a diacetylated analogue of 2, which determined the structure of luteolin-7-O-(3″,6″-diO-acetyl)-β-D-glucopyranoside, which is a new natural flavonoid (Fig. 1d).
One natural acetate of luteolin-7-O-glucoside is known—luteolin-7-O-(6″-O-acetyl)-glucoside isolated from Salix gilgiana Seemen (Salicaceae) [17]. The presence of acetic acid fragment in luteolin glycosides was also detected in luteolin-3′-O-glucuronide (3″- and 4″-O-monoacetates), luteolin-7-O-(2″-O-apiosyl)xyloside (3‴-O-acetate), luteolin-7-O-(2″-O-allosyl)glucoside (6‴-O-acetate) and luteolin-7-O-sophoroside (6‴-O-acetate) [21].
Among the known compounds in the leaves of N. multifida, luteolin-7-O-glucoside (2), apigenin-7-Oglucoside (3), luteolin-7-O-glucuronide (4), apigenin-7-Oglucuronide (5), luteolin-7-O-(6″-O-acetyl)-glucoside (6), apigenin-7-O-(6″-O-acetyl)-glucoside (7), rosemary acid (8), salvianolic acid A (9), salvianolic acid B (10), schizotenuin A (11), nepetamultin A (12), luteolin-7O-neohesperidoside (13), luteolin-7-O-rutinoside (14), apigenin-7-O-neohesperidoside (15) and apigenin-7-Orutinoside (16) were identified (Fig. 2). The presence of compounds 2–12 was previously shown in the flowers of N. multifida [10, 11], and 13–16 were detected for the first time for the species. The studied species belongs to the subsection Schizonepeta Benth. the genus Nepeta, which in addition to N. multifida includes N. annua Pall. and N. tenuifolia Benth. In the composition of phenolic compounds of N. tenuifolia, 2, 3, 4, 8, 11, and 14 were found, and in N. annua—2 [5], which indicates the widespread distribution of luteolin, apigenin and rosmarinic acid derivatives in the species of this subsection.
The study of the quantitative profile of phenolic compounds of N. multifida leaves was carried out using HPLC-UV on raw material samples collected during various phases of vegetation (Fig. 3).
Chromatogram (HPLC-UV) of N. multifida leaf extract (λ 330 nm; raw material sample collected in the flowering phase) before (straight line) and after incubation with acetylcholinesterase from Electrophorus electricus (dotted line). The numbers indicate the position of the compounds: 1, luteolin-7-O-(3″,6″-di-O-acetyl)-β-D-glucopyranoside; 2, luteolin-7-O-glucoside; 3, apigenin-7-Oglucoside; 4, luteolin-7-O-glucuronide; 5, apigenin-7-O-glucuronide; 6, luteolin-7-O-(6″-O-acetyl)-glucoside; 7, apigenin-7-O-(6″O-acetyl)-glucoside; 8, rosemary acid; 9, salvianolic acid A; 10, salvianolic acid B; 11, schizotenuine A; 14, luteolin-7-O-rutinoside.
Analysis of the content of twelve compounds revealed a change in the total concentrations of phenolic compounds during seasonal plant growth from 16.21 to 69.69 mg/g (Table 2). The dominant compound of N. multifida leaves was rosmarinic acid, the content of which varied from 8.36 mg/g at the beginning of the growing season to the highest in the flowering and fruiting phases (32.68–35.71 mg/g), decreasing to 14.01 mg/g by the end of the growing season. The content of other hydroxycinnamates, including schizotenuine A, salvianolic acids A and B, was 5.29–9.56, 0.59–2.35, and 0.53–1.40 mg/g, respectively, and the highest concentration of this group of phenols was detected in the flowering phase (46.38 mg/g). The main group of flavonoid compounds were luteolin glucosides, with luteolin-7-O-glucuronide and its 6″-O-acetyl derivative being the main flavones of N. multifida leaves. The nature of the accumulation of compounds was similar: there was an increase from the beginning of the growing season to the phases of flowering and fruiting and a decrease by the end of the growing season. The variation in the concentrations of luteolin-7-O-glucuronide and luteolin-7-O-(6″-O-acetyl)-glucoside was 2.03–14.18 and 0.53–6.93 mg/g, respectively. The content of apigenin glycosides in N. multifida leaves did not exceed 2.16 mg/g. The obtained results indicated that the optimal time for collecting N. multifida leaves is the flowering and fruiting period of the plant.
A study of the biological activity of N. multifida leaf extract showed the presence of an inhibitory effect on acetylcholinesterase with a concentration of half-maximal inhibition of the enzyme (IC50) 105.33 ± 4.73 μg/mL. Chromatographic analysis of the extract before and after incubation with acetylcholinesterase from Electrophorus electricus revealed a significant decrease in the peak area of two compounds – luteolin-7-O-glucuronide (4) and luteolin-7-O-(6″-O-acetyl)-glucoside (6), which indicated the formation of insoluble complexes between the enzyme and flavonoids (Fig. 3). The IC50 values for pure compounds 4 and 6 were 32.10 ± 1.61 and 35.14 ± 1.65 μg/mL, respectively. Minor luteolin glycosides also demonstrated pronounced inhibition of the enzyme—luteolin-7-O-(3″,6″-di-O-acetyl)-β-D-glucopyranoside (1; IC50 43.69 ± 1.74 μg/mL), luteolin-7O-glucoside (2; IC50 29.03 ± 1.39 μg/mL), luteolin-7 -O-neohesperidoside (13; IC50 57.45 ± 2.98 μg/mL) and luteolin-7-O-rutinoside (14; IC50 58.36 ± 2.80 μg/mL). Apigenin derivatives (3, 5, 7) and hydroxycinnamates were less active (8–11), showing efficacy with IC50 >200 μg/mL. Thus, luteolin glycosides are the components responsible for the manifestation of the antiacetylcholinesterase effect of N. multifida leaf extract. It was previously shown that luteolin [22], luteolin-7-Oglucoside [23] and luteolin-7-O-rutinoside [24] have an inhibitory effect on cholinesterases, which is due to the influence of the ortho-di-hydroxy-substituted ring B in the structure of these flavonoids. The presence of luteolin glycosides is characteristic of many Nepeta species, as well as representatives of the Nepetinae subtribe of the Lamiaceae family [5], which is probably a chemical sign of the presence of anticholinesterase activity in these species.
CONCLUSIONS
Nepeta multifida leaves contain benzofuran lignans from the rosemary acid group, as well as luteolin and apigenin O-glycosides, including the new flavonoid luteolin-7-O-(3″,6″-di-O-acetyl)-β-D-glucopyranoside.
The accumulation of phenolic compounds in the leaves of N. multifida depends on the phase of plant development, with the highest content revealed during flowering and fruiting.
Luteolin glycosides found in N. multifida leaves have an inhibitory effect on acetylcholinesterase.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Amirzargar, N., Heidari-Soureshjani, S., Yang, Q., Abbaszadeh, S., and Khaksarian, M., Nat. Prod. J., 2020, vol. 10, pp. 550–565. https://doi.org/10.2174/2210315509666190820103658
Pineda-Ramírez, N., Calzada, F., Alquisiras-Burgos, I., Medina-Campos, O.N., Pedraza-Chaverri, J., OrtizPlata, A., Pinzón Estrada, E., Torres, I., and Aguilera, P., Antioxidants, 2020, vol. 9, 253. https://doi.org/10.3390/antiox9030253
Hanafy, D.M., Prenzler, P.D., Burrows, G.E., Gurusinghe, S., Thejer, B.M., Obied, H.K., and Hill, R.A., Nutrients, 2020, vol. 12, Article ID: 1366. https://doi.org/10.3390/nu12051366
Topcu, G. and Kusman, T., Bezmialem Sci., 2014, vol. 1, pp. 1–25. https://doi.org/10.14235/bs.2014.233
Sharma, A., Cooper, R., Bhardwaj, G., and Cannoo, D.S., J. Ethnopharmacol., 2021, vol. 268, Article ID: 113679. https://doi.org/10.1016/j.jep.2020.113679
Flora of Siberia. Pyrolaceae-Lamiaceae, Ed. Malyshev, L.I. CRC Press: Boca-Raton, USA, 2006, vol. 11, 310 p.
Flora of Central Siberia, Ed. Malyshev, Z.D. and Peshkova, G.A. Novosibirsk, 1979, 536 p.
Liu, Z.L., Chu, S.S., and Jiang, G.H., J. Sci. Food Agricult., 2011, vol. 91, pp. 905–909. https://doi.org/10.1002/jsfa.4263
Liu, J.-T., Yu, J.-C., Jiang, H.-M., Zhang, L.-Y., Zhao, X.-J., and Fan, S.-D., Chin. J. Chem., 2008, vol. 26, pp. 1129–1132. https://doi.org/10.1002/cjoc.200890201
Olennikov, D.N., Chem. Nat. Comp., 2021, vol. 57, pp. 818–822. https://doi.org/10.1007/s10600-021-03488-7
Kashchenko, N.I. and Olennikov, D.N., Chem. Nat. Comp., 2022, vol. 58, pp. 274–278. https://doi.org/10.1007/s10600-022-03658-1
Batorova, S.M., Yakovlev, G.P., and Aseyeva, T.A., Reference Book of Medicinal Plants of Traditional Tibetan Medicine, Novosibirsk, 2013, 293 p.
Razuvayeva, Ya.G., Kharzheyev, D.V., Toropova, A.A., and Olennikov, D.N., Voprosy Biologicheskoy, Meditsinskoy i Farmatsevticheskoy Khimii, 2018, vol. 21, pp. 5–10. https://doi.org/10.29296/25877313-2018-07-02
Razuvaeva, Y.G., Toropova, A.A., Olennikov, D.N., and Kharzheev, D.V., Nat. Prod. Rep., 2021, vol. 36, pp. 3105–3109. https://doi.org/10.1080/14786419.2021.1935932
Olennikov, D.N., Nikolaev, V.M., and Chirikova, N.K., Antioxidants, 2021, vol. 10, Article ID: 863. https://doi.org/10.3390/antiox10060863
Malikov, V.M. and Yuldashev, M.P., Chem. Nat. Comp., 2002, vol. 38, pp. 358–406. https://doi.org/10.1023/A:1021638411150
Mizuno, M., Kato, M., Iinuma, M., Tanaka, T., Kimura, A., Ohashi, H., and Sakai, H., Phytochemistry, 1987, vol. 26, pp. 2418–2420. https://doi.org/10.1016/S0031-9422(00)84739-1
Matsuta, M., Kanita, R., Saito, Y., and Yamashita, A., Nat. Med., 1996, vol. 50, pp. 204–211.
Olennikov, D.N., Chirikova, N.K., Kashchenko, N.I., Nikolaev, V.M., Kim, S.-W., and Vennos, C., Front. Pharmacol., 2018, vol. 9, Article ID: 756. https://doi.org/10.3389/fphar.2018.00756
Bock, K. and Pedersen, C., Adv. Carbohydr. Chem. Biochem., 1983, vol. 41 (C), pp. 27–66. https://doi.org/10.1016/S0065-2318(08)60055-4
Flavonoids: Chemistry, Biochemistry, and Applications, Eds. Andersen, Ø.M. and Markham, K.R. CRC Press, Boca Raton, USA, 2006, 1197 p.
Tundis, R., Bonesi, M., Menichini, F., Loizzo, M.R., Conforti, F., Statti, G., and Menichini, F., Nat. Prod. Commun., 2012, vol. 7, pp. 1015–1020. https://doi.org/10.1177/1934578x1200700814
Olennikov, D.N., Chirikova, N.K., and Vennos, C., Nat. Prod. Commun., 2017, vol. 12, pp. 55–56. https://doi.org/10.1177/1934578x1701200115
Orhan, I., Kartal, M., Tosun, F., and Şener, B., Z. Naturforsch. C, 2007, vol. 62, pp. 829–832. https://doi.org/10.1515/znc-2007-11-1210
Funding
The research was carried out with the support of the Ministry of Science and Higher Education of the Russian Federation within the framework of scientific project no. 121030100227-7.
Author information
Authors and Affiliations
Contributions
The authors NIK and DNO—selected the literature data on the review topic, contributed to manuscript preparation. All authors participated in the discussions.
Corresponding author
Ethics declarations
This article does not contain any studies involving patients or animals as test objects. Informed consent was not required for this article. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kashchenko, N.I., Olennikov, D.N. Flavonoids and Lignans of Nepeta multifida (Lamiaceae) Leaves and Their Biological Activity. Russ J Bioorg Chem 49, 1689–1698 (2023). https://doi.org/10.1134/S1068162023070695
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162023070695