Abstract
The research is aimed at removing uranium from low uranium content solutions using the ion flotation technique. The ion flotation process is an excellent technique for uranium separation from its solutions with low content or trace levels of uranium after optimizing carbonate–bicarbonate concentration for such solutions. It can also be applied to collecting uranium efficiently from all raffinates of the uranium separation or purification projects involving low-grade ore instead of other conventional long tedious methods such as ion exchange or solvent extraction, especially at low U levels. In this study, cetyltrimethylammonium bromide was used as a collector. The factors that can affect the flotation process (uranium concentration, gas flow rate, concentration of collector, and flotation time) were studied, and the best conditions were chosen: uranium concentration 0.02 g/L, carbonate concentration 10 g/L, gas flow rate 52 cm3/min, collector concentration 5 × 10–4 M, ethanol concentration 0.2% v/v, and flotation time 40 min. Under these conditions, the uranium flotation percentage reached more than 99%. A sample representing a sandy carbonaceous rock of Allouga area, southwestern Sinai, was prepared for alkaline leaching of uranium because of the high content of the carbonate which will consume large amounts of acids. The results of the experiments have shown that the optimum Na2CO3/NaHCO3 ratio is 1/1 at a total concentration of 80 g/L and S/L = 1/2, with 4-h agitation at room temperature. Under these conditions, the uranium leaching efficiency reached 94.2%, and the leachability increased to 98.7% at 80°C. The produced carbonate alkaline uranium-bearing leachate was subjected to the flotation process. A simplified sketch for the uranium separation from the carbonate solutions with a cationic collector is presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Ion flotation is a comparatively novel separation technology that uses special properties of interfaces in order to concentrate ions or other charged species contained in aqueous solutions [1]. In 1959, Felix Sebba first presented ion flotation as a possible method to enrich inorganic ions from aqueous solution, even very dilute [2]. Usually, flotation is classified into two submethods [3]: precipitate flotation and ion flotation. Flotation is an efficient industrial wastewater treatment technology and includes ion flotation, precipitation flotation, and adsorption flotation [4]. Ion flotation originates from minerals beneficiation (usually named as froth flotation or foam flotation) and is recognized as one of the most promising methods for removing inorganic and organic anions and cations from aqueous solution [5]. Due to its simplicity, flexibility, low energy consumption, small space requirements, small volume of sludge, and selectivity, ion flotation is highly efficient in industrial applications [6, 7]. Ion flotation is a method for recovery of inactive, as a rule, inorganic ions using surfactants. Surfactants are molecules containing hydrophobic and hydrophilic moieties, commonly represented by an ionogenic group and an organic radical with a large number of carbon atoms. In the course of flotation, the surfactant interacts with the inorganic ion and can be removed from the solution together with the foam [8]. Biosurfactants, such as chemically synthesized surfactants, have a hydrophobic end and a hydrophilic end. In general, the hydrophilic group is a polar group, usually composed of peptides, amino acids, monosaccharides, or polysaccharides, while the hydrophobic end is usually composed of unsaturated or saturated hydrocarbon chains or fatty acids [9].
Ion flotation was used to remove copper(II), lead(II), and chromium(III) from simulated wastewater using a new biodegradable biosurfactant, sodium N-lauroylsarcosinate (LS), and a synthetic surfactant, sodium dodecyl sulfate (SDS), as collectors [10].
The precipitate flotation involves firstly the immobilization of ions as precipitates; e.g., raising the respective concentrations may lead to precipitation of the ion as a surfactant-floatable product before air is passed. This means that it is not a solution anymore but rather dispersion [11, 12].
Uranium is radioactive and chemically toxic; it can cause serious health hazards and can be life-threatening once ingested. Unfortunately, uranium has been released into the environment due to uranium mining, nuclear accidents, nuclear experiments and research, etc. [13–15]. Uranium is the key element and the raw material to fuel nuclear reactors for the use of nuclear energy [16]. Sodium uranate precipitation is currently used to separate uranium from alkaline solutions. The usual method is to raise the pH to 12 or higher, whereby uranium is precipitated as a mixture of uranates. This method, however, gives satisfactory results only when the uranium concentration is high (>2.5 g U3O8/L). For solutions with low uranium content, other procedures should be used. Ion flotation furnishes an attractive possibility for recovering traces of uranium from carbonate media. Generally, ion flotation differs from foam fractionation in that the ion flotation technique utilizes a low gas flow rate and short columns. The separation occurs at the gas–liquid interface and not in the foam phase as it is the case in foam fractionation. Uranium forms two anionic complexes with either carbonate or bicarbonate ions, namely, a dicarbonate complex UO2(CO3)2(H2O)22– and a tricarbonate complex UO2(CO3)34–, and it would be expected that these compounds could be floated by cationic collectors. In the presence of excess carbonate or bicarbonate ions, the dicarbonate complex is transferred quantitatively into the tricarbonate form, and under such conditions uranium exists entirely as the tetraanionic tricarbonate complex [17], which is expected to be more susceptible to flotation by reason of its high electric charge.
Although there is some work on the foam fractionation of uranium from carbonate media [18–20], there are no data in the literature on the separation of uranium from carbonate solutions by the ion flotation technique. In the present work, the surfactant, cetyltrimethylammonium bromide, CTAB [(C16H33)N(CH3)3]Br, is employed as a cationic collector for studying the ion flotation of uranium in the form of uranyl tricarbonate complex, UO2(CO3)34−, to reach the highest uranium recovery from its low-level carbonate solution and the highest uranium purity instead of other conventional time-consuming procedures. The effects of some parameters such as gas flow rate, collector concentration, and flotation time have been examined.
The uranium(VI) ion flotation data were expressed as the removal percentage, which was calculated using the following equation:
Floatability F or removal (%) = (Ci – Cf) /Ci × 100%,
where Ci and Cf are the initial and final concentrations (mg/L) of uranium(VI), respectively.
Alkali (sodium or less commonly ammonium) carbonate is sometimes used for uranium leaching from its ores that are high in carbonate gangue minerals like calcite, dolomite, etc. This is due to the fact that the carbonate anion forms with uranium stable soluble uranyl carbonate complexes [UO2(CO3)n]2–2n [21]. It can be applied to both primary and secondary mineral deposits, however, after oxidation of the former. Alkali carbonate leaching has several important advantages over acid leaching [22–24], including the selectivity (comparatively pure solutions are readily obtained) and noncorrosive nature. Also, the consumption of the reagent by the ore is low, and uranium can be readily recovered from the leach liquors. Finally, the carbonate solutions can be regenerated for further leaching recycle [25, 26]. However, there are some limitations to using carbonate leaching: Under mild conditions of the process, some uranium minerals are not solubilized by carbonate leach solutions. Moreover, it requires fairly fine grinding of the ore to obtain reasonable reactions rates. Alternatively, carbonate leaching can be performed at relatively high pressure and temperature in suitable autoclaves [27].
The chemistry of uranium alkaline leaching can be summarized as follows:
UO2+ 3CO32– + H2O + 0.5O2 → [UO2(CO3)3]4– + 2OH–,
UO3 + 3Na2CO3 + H2O → Na4UO2(CO3)3 + 2NaOH.
As can be seen, OH– is generated during the leaching process, so that the presence of sodium bicarbonate is required. The bicarbonate has the important function of buffering the leaching solution and preventing the pH from rising to the point where diuranate would precipitate. The uranium precipitation reaction is chemically shown as:
UO2 + 1/2O2 + Na2CO3 + 2NaHCO3→ Na4UO2(CO3)3 + H2O,
2Na4UO2(CO3)3 + 6NaOH→ Na2U2O7 + 6Na2CO3 + 3H2O.
Sodium uranate precipitation is currently used to separate uranium from alkaline solutions. The usual method [28] is to raise the pH to 12 or higher, whereby uranium is precipitated as a mixture of uranates. This method, however, gives satisfactory results only when the uranium concentration is high. For solutions with low uranium content, other procedures should be used. Ion flotation furnishes an attractive possibility for recovering traces of uranium from carbonate media.
EXPERIMENTAL
Materials and Methods
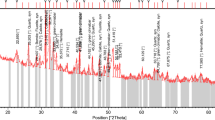
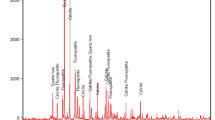
The collected technological sample representing a sandy carbonaceous rock of Allouga area, southwestern Sinai, was first subjected to complete chemical analysis for the major oxides as well as for some interesting trace elements. The loss on ignition (LOI) was determined gravimetrically. In this type of leaching, a weighed ore sample (5 g) was agitated in Na2CO3 and NaHCO3 solutions. The main relevant agitation leaching conditions were studied including the concentration of sodium carbonate and sodium bicarbonate, S/L ratio, and leaching time and temperature. At the end of each leaching experiment, the ore slurry was filtered, the residue was properly washed, and the obtained leach liquor and washings were completed to a proper volume for U analysis.
To determine the chemical composition, an approximately 50-g sample was separated by quartering from the finely divided representative sample. The sample was then analyzed using the conventional wet chemical procedures [29]. SiO2 and Al2O3 were analyzed spectrophotometrically; Na and K were determined by flame photometry; CaO and MgO were determined titrimetrically using Murexide and Eriochrome Black T indicators, respectively; Fe2O3 was determined titrimetrically using sulfosalicylic acid as indicator. The trace elements Cu, Zn, Sr, and V were analyzed by X-ray fluorescence technique (XRF).
To evaluate the leaching and recovery efficiency, stream solutions were subjected to U analysis. The oxidimetric titration method against ammonium metavanadate was used after the uranium reduction [30, 31].
Recovery Procedures
The flotation system used consisted of a pure nitrogen cylinder connected to a flotation cell. The ion flotation installation was a cylindrical tube 15 cm long and 4.5 cm i.d., provided with a stopcock at the bottom and a stopper at the top. The pH values of the solutions were measured using WTW Inolab Level 1 device. A 450-mL sample was taken into a beaker. The collector (cetyltrimethylammonium bromide, CTAB [32]) solutions were freshly prepared just before use, and the collector itself was evaporated twice with reagent-grade absolute ethanol before dissolution to convert any micelles into the ionic form. Absolute ethanol was used as a solvent for the collector. 1 mL of ethanol per 450 mL of uranyl solution was used except for collector concentrations higher than 8 × 10–4 M, where 2 mL was used to ensure complete dissolution.
A 450-mL portion of the uranyl carbonate solution was taken into the flotation cell, and nitrogen gas was passed at a rate (R) of 28 cm3/min unless otherwise specified. A small volume of the solution was withdrawn for uranium analysis. The solution of the collector in ethanol was injected with a syringe in one injection while vigorously stirring the solution. That instant was recorded as the zero time. The uppermost layer of the foam was regularly skimmed to prevent flooding. Small samples of the bulk solution were withdrawn at predetermined intervals for uranium analysis. After each experiment, the flotation cell was washed with ethanol, concentrated nitric acid, and distilled water.
RESULTS AND DISCUSSION
Material Characteristics
Data on the average major chemical composition of the working technological sample are given in Table 1.
The content of trace elements is given in Table 2, where uranium (2500 ppm) is the main target.
Uranium Recovery
Effect of flotation time. As seen from the removal percentage/time curves obtained at three different collector to uranium molar ratios (C/U) at a constant uranium concentration of 0.02 g/L (Fig. 1), the removal percentage increases with an increase in the bubbling time until a maximum is reached. This maximum removal, or ultimate percentage removed, strongly depends on the collector concentration.
Effect of collector concentration. To determine the collector concentration at which the best uranium removal can be obtained, additional flotation experiments were run using different collector ratios at two levels of uranium concentration. All other conditions were kept constant. The removal percentages obtained after 40 min flotation were plotted vs. the collector ratios. The results obtained (Fig. 2) show that the recovery increases with an increase in the collector–uranium ratio until a certain collector concentration (critical collector concentration) is reached at which a further increase in the collector–uranium ratio results in a decrease in the removal percentage. The magnitude of this decrease depends on the uranium concentration, being more pronounced at high uranium concentrations, as indicated by the steepness of the descending parts of the curves. Also, the collector concentration beyond which the removal percentage decreases (critical collector concentration) seems to vary with the concentration of uranium in the solution. Under our experimental conditions, the optimum collector concentration is about 5 × 10–4 and 7 × 10–4 M for uranium concentrations of 0.02 and 0.01 g/L, respectively.
The decrease in the uranium removal above critical collector concentration may be due to the fact that its critical micelle concentration (CMC) is exceeded with the consequent formation of micelles, which have a deleterious effect on the ion flotation. The fact that the CMC is affected by the concentration of ions of charges opposite to that of the collector [33] accounts for the dependence of the critical collector concentration on the concentrations of both uranyl and carbonate ions in the solution. As the concentration of these anions is increased, CMC decreases with the corresponding decrease in the critical collector concentration.
Effect of the concentration of carbonate and bicarbonate ions. The removal percentage of uranium from solutions of different concentrations of either sodium carbonate or sodium bicarbonate is plotted in Figs. 3 and 4, respectively. As seen from the curves, the concentration of these salts significantly affects both the ultimate removal and the removal rate. The ultimate fraction removed decreases with an increase in the sodium carbonate or bicarbonate concentration, being e.g., about 70 and 97% for 10 and 1 g/L carbonate solutions, respectively.
Under all our experimental conditions, no significant pH changes were caused by the collector addition, and only the uranyl tricarbonate complex was present because of the high carbonate–uranium ratio applied; the effects of the concentration of sodium carbonate cannot therefore be ascribed to changes in the uranium speciation. At a constant carbonate ion concentration and a constant ratio of the collector to the uranyl tricarbonate complex, the removal percentage is affected by the carbonate–uranium ratio. Because it is unlikely that the removal of collector (and, consequently, of the uranium–collector product) is significantly affected by slight changes in the carbonate strength of the solution, the strong effects of the carbonate ion concentration on the uranium removal cannot be mainly caused by changes of the activities of solutes due to changes in the salt concentration. Therefore, these strong effects can be mostly related to changes in the competition between carbonate anions and the anionic uranyl tricarbonate complex for the collector cations. The uranyl tricarbonate complex, having a higher electric charge than either the carbonate or bicarbonate ions, is preferentially attracted to the collector cations. At high carbonate or bicarbonate concentrations, the probability of attraction between the CO32– or HCO3– ions and the collector cations increases and, consequently, the uranium removal decreases as a direct function of the ratio of [CO32–] or [HCO3–] to the collector. From equimolar solutions of carbonate and bicarbonate, the ultimate removal of uranium was found to be greater from the bicarbonate solutions because of the lower charge of the bicarbonate anion.
Effect of uranium concentration. The effect of the concentration of uranyl ion on the removal percentage was studied at two levels of sodium carbonate concentration and a constant collector–uranium molar ratio of 5. The results obtained (Fig. 5) show that, for each carbonate concentration, the maximum removal percentage is achieved in a certain interval of uranium concentrations. As expected, the removal percentage decreases at higher uranium concentrations because the critical collector concentration is exceeded. At lower uranium concentrations, the removal percentage decreases also. The carbonate concentration was kept constant throughout the experiments, and a decrease in the uranium concentration would thereby be accompanied by the corresponding increase in the carbonate–uranium ratio; hence, the low removal percentage observed at low uranium concentrations can be attributed to an increase in the carbonate–uranium ratio. Because the removal percentage decreases at uranium concentrations higher than approximately 0.02 g/L for 10 g/L carbonate solution and the decrease in the removal percentage is due to the fact that the critical collector concentration is exceeded, it can be concluded that the critical collector concentration depends on the carbonate concentration and is shifted to lower values as the CO32– concentration is increased.
The maximum uranium concentration in the solution to be successfully treated by the ion flotation technique is limited by the critical micelle concentration, and it was naturally desired to shift the CMC to a higher value by adding, e.g., alcohol, so that a higher collector concentration can be safely utilized. As the hydrophobic portions of a surfactant are, in general, more soluble in organic liquids than in water, the addition of considerable quantities of ethanol, which is water-miscible, to the system under investigation was expected to have a disaggregating effect on the micelles, perhaps leading to a substantial increase of the CMC as has been found for another collector [34].
Effect of the ethanol concentration. The data on the uranium removal percentage from 10 g/L carbonate solutions containing different concentrations of ethanol are given in Fig. 6. They show that an increase of the alcohol concentration leads to a decrease in the ultimate removal. This is probably due to easy dissolution of the uranium–collector product in alcohol.
Effect of gas flow rate. Three levels of the gas flow rate were chosen: 28, 52, and 77 cm3/min. The results obtained (Fig. 7) show that, at low gas flow rates, the ultimate removal is not significantly affected, but the removal rate considerably increases with an increase in the gas flow rate, as indicated by the steepness of the initial slopes of the curves at each gas flow rate. At high gas flow rates, the initial removal rate is very high, but the ultimate removal is lower than that at lower gas flow rates. This may be due to the back redispersion into the bulk solution of some of the uranium from the foam phase.
Uranium Leaching
Effect of different alkaline reagents. The effect of different alkaline leaching reagents, either single or in combination, on the uranium leaching efficiency has been studied. In these experiments, the other leaching factors were fixed: –60 mesh size ore finess, 3 h agitation time, room temperature (25°C), 1/2 solid/liquid (S/L) ratio, and 100 g/L concentration of the leaching agent. The obtained data on the uranium leaching efficiency are given in Table 3. They show that the best reagent is a 1 : 1 mixture of Na2CO3 and NaHCO3 in a total concentration of 100 g/L.
Effect of Na2CO3/NaHCO3 weight ratio. The U leaching efficiency when using Na2CO3 alone is moderate, and it is necessary to add NaHCO3 to neutralize the formed NaOH. Accordingly, a series of leaching experiments were performed in which sodium bicarbonate was mixed with a 100 g/L sodium carbonate solution in Na2CO3/NaHCO3 weight ratios of 1/1, 2/1, and 3/1 at 25°C. The results are shown in Fig. 8.
As can be seen, the uranium leaching efficiency increased on adding NaHCO3, and this increase is the more pronounced, the lower is the Na2CO3/NaHCO3 weight ratio. On decreasing the of Na2CO3/NaHCO3 weight ratio from 3/1 to 1/1, the leaching efficiency increased from 25.43 to 89%, respectively.
Effect of Na2CO3/ NaHCO3 concentration. To study the effect of the Na2CO3/NaHCO3 concentration on the efficiency of uranium leaching from the ore material studied, a set of leaching experiments were performed with different Na2CO3/NaHCO3 concentrations ranging from 20 to 100 g/L. The other leaching conditions were fixed: 25°C, 3 h, S/L = 1/2, and Na2CO3/NaHCO3 weight ratio 1/1. The results are plotted in Fig. 9. They show that the best concentration for uranium leaching is 80 g/L. Under these conditions, the uranium leaching efficiency reached 92.55%.
Effect of liquid/solid ratio (L/S). The effect of L/S ratio (mL/g) on the leaching of uranium from the ore material was studied at L/S varied from 1/1 to 2.5/1; the other conditions were fixed: Na2CO3/NaHCO3 weight ratio 1/1, Na2CO3/NaHCO3 concentration of 80 g/L, 3 h, and 25°C. The results are plotted in Fig. 10.
As can be seen, with increasing L/S ratio from 1/1 to 2/1 the uranium leaching efficiency increases from 35.76 to 92.55%, respectively. Further increase in the L/S ratio to 2.5/1 had an adverse effect on the U leaching efficiency, which decreased to 86.92 %. This decrease can be attributed to reprecipitation of uranium due to the formation of NaOH. Thus, the L/S ratio of 2/1 can be considered as an optimum for reaching 92.55% uranium leaching efficiency under the applied conditions.
Effect of agitation time. To study the effect of agitation time on the uranium leaching efficiency, a set of leaching experiments were performed at different time periods ranging from 1 to 6 h. The other leaching conditions were fixed: Na2CO3/NaHCO3 weight ratio 1/1, Na2CO3/ NaHCO3 concentation 80 g/L, L/S = 2/1, and 25°C. The results are plotted in Fig. 11.
The results obtained show that the efficiency of U leaching from the working ore increased from 30.38 to 94.15% with increasing agitation time from 1 to 4 h, respectively. Further increase in the leaching time to 5 and 6 h has not further improved the leaching efficiency; thus, the agitation time of 4 h can be considered as an optimum ensuring 94.15% uranium leaching efficiency under the applied conditions.
Effect of leaching temperature. The effect of leaching temperature on the efficiency of uranium leaching from the working ore was studied at 40–100°C; the other conditions were fixed: Na2CO3/NaHCO3 weight ratio 1/1, Na2CO3/NaHCO3 concentration 80 g/L, L/S = 2/1, and leaching time 4 h. The results are plotted in Fig. 12.
As can be seen, with increasing leaching temperature to 80°C, the uranium leaching efficiency increases to 98.72%, whereas at 100°C it decreases. This is most probably due to increased NaOH formation and U reprecipitation.
Case Study (Allouga Uranyl Tricarbonate Complex, UO2(CO3)3 4−)
The partially purified uranyl tricarbonate compound is produced after alkaline filtration experiments and diluted to obtain the optimum concentrations of uranium, carbonate, and bicarbonate. Foreign ions can also interfere with the removal process. To reach the optimum flotation conditions, these interferences were overcome by adding 6 × 10–4 M cetyltrimethylammonium bromide (CTAB) instead of 5 × 10–4 M CTAB. Then, the ion flotation was performed, and the uranium flotation percentage reached more than 99%. The summarized flow diagram of the process is shown in Fig. 13.
CONCLUSIONS
The performance of the ion flotation process for uranium(VI) removal from carbonate solutions is evaluated. The uranium(VI) flotation strongly depends not only on the collector concentration but also on the uranium and carbonate concentrations. Uranium(VI) is efficiently removed (> 99%) in presence of 10 g of sodium carbonate using 5 × 10–4 M CTAB and 0.2% v/v ethanol in 40 min at a nitrogen flow rate of 52 cm3/min. The high degree of removal achieved in the presence of carbonate is attributed to the electrostatic attraction between UO2(CO3)34−, present under our experimental conditions, and CTA+. Thus, the addition of CTAB to uranyl tricarbonate solution resulted in the formation of UO2(CO3)3–CTAB soluble product.
If it is required to recycle the collector, then the latter can be easily recovered from the scum first by heating it with dilute acid to destroy the carbonate and then by boiling with an alkali solution [2]; in the process, uranium is precipitated as an alkali metal uranate and the collector is obtained in solution as the quaternary ammonium hydroxide which can be converted into the bromide.
The ion flotation is an excellent technique for uranium separation from its solutions of low content or trace levels after optimizing carbonate and bicarbonate concentrations in such solutions. Also, it can be applied to collect uranium efficiently from all raffinates of the uranium separation or purification projects involving low-grade ores instead of other conventional long tedious methods such as ion exchange or solvent extraction, especially at low U levels.
Sample representing a sandy carbonaceous rock of Allouga area, southwestern Sinai was prepared for alkaline leaching of uranium because of the high carbonate content, leading to high acid consumption. The experimental results show that the optimum conditions are as follows: Na2CO3/NaHCO3 ratio 1/1, total concentration 80 g/L, S/L = 1/2, agitation time 4 h. Under these conditions at room temperature, the uranium leaching efficiency reached 94.2%, and at 80°C it increased to 98.7%.
REFERENCES
Tavera, F.J., Escudero, R, Uribe, A., and Finch, J.A., Afinidad, 2000, vol. 490, p. 415.
Sebba, F., Nature, 1959, vol. 184, p. 1062.
Mizuike, A. and Hiraide, M., Pure Appl. Chem., 1982, vol. 54, p. 1566.
Hoseinian, S., Rezai, B., Safari, M., Deglon, D., and Kowsari, E., J. Environ. Manag., 2019, vol. 244, pp. 408–414.
Grieves, R.B. and Wilson, T.E., Nature, 1965, vol. 205, p. 1066.
Deliyanni, E.A., Kyzas, G.Z., and Matis, K.A., J. Mol. Liq., 2017, vol. 225, p. 260.
Salmani, M.H., Davoodi, M., Ehrampoush, M.H., Ghaneian, M.T., and Fallahzadah, M.H., Iran. J. Environ. Health Sci. Eng., 2013, vol. 10, p. 16.
Chirkst, D.E., Lobacheva, O.L., Berlinskii, I.V., and Sulimova, M.A., Russ. J. Appl. Chem., 2009, vol. 82, no. 8, pp. 1273−1276.
Drakontis, C.E. and Amin, S., Curr. Opin. Colloid Interface Sci., 2020, vol. 48, pp. 77–90.
Kai Jia, Yuxia Yi, Wuju Ma, Yijun Cao, Guosheng Li, Shiqiang Liu, Taojin Wang, and Nan An, Miner. Eng., 2022, vol. 176, ID 107338.
Alexandrova, L. and Grigorov, L., Int. J. Miner. Process., 1996, vol. 48, pp. 111–125.
Zouboulis, A.I., Matis, K.A., and Stalidis, G.A., Innovations in Flotation Technology, Mavros, P., and Matis, K.A., Eds., Dordrecht: Kluwer, 1992.
Riegel, M., Tokmachev, M., and Hoell, W.H., React. Funct. Polym., 2008, vol. 68, pp. 1072–1080.
Bhalara, P.D., Punetha, D., and Balasubramanian, K., J. Environ. Chem. Eng., 2014, vol. 2, pp. 1621–1634.
Zhang, M., Yuan, M., Zhang, M., Wang, M., Chen, J., Li, R., Qiu, L., Fenga, X., Hu, J., and Wu, G., Radiat. Phys. Chem., 2020, vol. 171, ID 108742.
Tan, K., Li, C., Liu, J., Qu, H., Xia, L., Hu, Y., and Li, Y., Hydrometallurgy, 2014, vol. 150, pp. 99–106.
Bullwinkel, E.P., US Atomic Energy Commission, RMO, 1954, vol. 2614.
Jacebelli-Turi, C., Barocas, A., and Salvetti, F., Gazz. Chim. Ital., 1963, vol. 93, p. 1493.
Barocas, A., Jacobelli-Turi, C., and Salvetti, F., J. Chromatogr., 1964, vol. 14, p. 291.
Jacobelli-Turi, C., Barocas, A., and Terenzi, S., Ind. Eng. Chem. Process Des. Develop., 1967, vol. 6, p. 161.
Kunin, R., Ion Exchange Resins, Wiley, 1971, pp. 190–197.
Narayan, K., Village, W., and Pick, R., US Patent, 4092399, 1978.
Lyaudet, G., Mazarin, C., and Vial, J., US Patent, 4256702, 1981.
Riegel, M., Tokmachev, M., and Hoell, W., React. Funct. Polym., 2008, vol. 68, pp. 1072–1080.
Merritt, R.C., Extractive Metallurgy of Uranium, Merritt, R C., Ed.; Colorado School of Mines Research Inst., 1971.
Clark, D.L., Hobart, D.E., and Neu, M.P., Chem. Rev., 1995, vol. 95, p. 25.
Gupta, C.K. and Singh, H., Uranium Resource Processing: Secondary Resources, Mumbai, India: Bhabha Atomic Research Centre, 2001.
Forward, F.A. and Halpern, J., J. Met., 1954, vol. 6, p. 1408.
Shapiro, L. and Brannock, N.W., Rapid Analysis of Silicate, Carbonate and Phosphate Rocks, US Geological Survey Bulletin, 1962, no. 1144A.
Mathew, K.J., Bürger, S., Ogt, S.V., Mason, P.M.E.M., and Narayanan, U.I., in Eighth Int. Conf. on Methods and Applications of Radioanalytical Chemistry (Marc VIII), Kailua-Kona, Hawaii, 2009, vol. 5.
Yang Hu, Chunguang Li, Jiang Liu, Huiqiong Qu, Liangshu Xia, and Yongmei Li, Hydrometallurgy, 2014, vol. 150, pp. 99–106.
Mahmoud, M.R. and Othman, S.H., Radiochim. Acta, 2018, vol. 106, no. 6, pp. 465–476.
Corrin, M.L. and Harkins, W.D., J. Am. Chem. Soc., 1947, vol. 69, p. 683.
Ralston, A. and Hoerr, C.W., J. Am. Chem. Soc., 1946, vol. 68, p. 2460.
ACKNOWLEDGMENTS
The author is grateful to Prof. Dr. Kamal Abd Elbaki Ali Rabee, Nuclear Materials Authority (NMA), for his continuous and constructive assistance and for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Dayem, S.M.A.E. Studies for Ultimate Uranium Separation from Its Low-Content Carbonate Leachate Solutions by Ion Flotation. Radiochemistry 64, 193–202 (2022). https://doi.org/10.1134/S1066362222020116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222020116