Abstract
Phosphate rock contains various gangue minerals including silicates and carbonates which need to be reduced in content in order to meet the requirements of the phosphate industry. Froth flotation has become an integral part of phosphate concentration process. In this study, double reverse flotation was applied to recover apatite from phosphate ore. H3PO4 and CaO were used as phosphate depressants, in acidic and alkaline conditions. Fatty acids and amines were added as carbonate and silicate collectors respectively. An experimental protocol devised to optimize the grade and recovery of phosphate using anionic–cationic method was found effective. Consequently, a required high quality of phosphate concentrate containing 30.1% P2O5 was obtained, with a recovery of 94%. X-ray diffraction and optical microscopy studies were performed to define the main minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphates are some of the most important minerals on Earth as they are the indispensable fertilizing raw material for agriculture and also the essential feedstock of chemical industries such as cleaning agents, dental creams, and flame retardants. A large proportion of the world’s phosphate reserves are sedimentary deposits containing a considerable amount of carbonate minerals. Phosphate beneficiation from carbonate-rich sedimentary phosphate deposits is not effortless and requires further investigation (Al-Fariss et al. 1991). Phosphate ore processing techniques are dependent on the type of phosphate minerals and associated gangue. Froth flotation is a widely used physicochemical beneficiation technique in mineral processing industry for separating finely ground valuable minerals from a mixture of gangue minerals (Wills and Nappier-Munn 2006; Miller et al. 2002). The efficiency of a flotation circuit operation depends on several factors related to nature and structure of associated minerals together with the type of instrumentation and operational variables which have been extensively investigated (Ralston and Dukhin 1999; Lima et al. 2009; Dos Santos et al. 2012; Feng and Aldrich 2004; Eigels 1967; Liu et al. 2011).
Phosphate ores are mainly accompanied with various associated gangue minerals like clays, silica, calcareous minerals (mainly calcite and dolomite), carbonaceous matter, and iron oxides. Various separation methods have been developed for phosphate ores; however, most of them have posed problems for upgrading the ores due to similarities in the apatite and associated gangue minerals in terms of physical properties (density, particle size, particle shape, etc.) and physico-chemicals properties of carbonate and apatite minerals (Abouzeid et al. 2009; Bogdanov and Maximov 1990). Various beneficiation schemes have been established for improving phosphate grade including scrubbing/washing and size classification, gravity separation, magnetic separation, and flotation (Al-Fariss et al. 1991, 2013; Unkelbach and Wasmuth 1991; Abu-Eishah et al. 1991; Abdel-Khalek 2000; Deghani et al. 2012). In order to be considered successful; the beneficiation method must be able to increase P205 content above 30%. Applying a two-stage reverse flotation process for removal of carbonates and silicates from phosphate ore using anionic and cationic collectors, this work aims at illustrating the effect of collector’s concentrations or dosage and pH on flotation performance of carbonates and quartz from phosphate ore.
Materials and methods
Materials
Phosphate ore and pure minerals
The samples were collected from Djebel Onk deposits in Algeria. The phosphate ore sample and pure minerals such apatite, dolomite, and quartz were ground below 0.16 mm for flotation studies. The phosphate analyzed 24.7% P2O5 with 9.4% insolubles.
Flotation reagents
The flotation reagents used in the first stage are fatty acid fractions of C10–C18 synthesized from the paraffin oil by catalytic oxidation using potassium permanganate (Doudenkov and Choubov 1969). The composition of the used fatty acid, determined by gas chromatography, contains a significant percentage of carbon atoms in the molecule, from C10 to C17 equals 89.8% and the remaining C7 to C9; C18 to C20 equals 10.2%. The fatty acids were used in emulsion form, with phosphoric acid as depressant for phosphate and pH modifier.
The reagent scheme in the second stage flotation included an amine-based cationic collector containing 50% dodecylamine (C12), 19% tetradecylamine (C14), 14% hexadecylamine (C16), and some other fractions in small proportions with CaO as pH modifier.
Methods
Flotation tests
Microflotation tests of pure minerals (2 g)—dolomite, quartz, and apatite—were conducted in a monobubble Hallimond tube. The Reynolds number of the bubble produced in monobubble Hallimond tube is more than 385 indicating a near turbulent flow in the layer of water behind the bubble and vertical velocity of the bubbles was found to be within 13.1 cm/s (Drzymala 1994).
Flotation experiments for phosphate ore (300 g) were conducted in a flotation cell of 1-L capacity at the impeller rate of 1200 rpm. The rate of removal particles with bubbles in flotation cell is given by Ralston and Laskowski (1992), Pyke et al. (2003). However, the effect of bubble size attachment, attachment efficiency, and velocity given by Reay and Ratcliff (1973), Kouachi et al. (2010) has shown that the bubble velocity equal 18 cm/s.
Spectroscopic methods
FTIR spectroscopy was used for the characterization of studied phosphate ore. The FTIR spectra were measured by Jasco 460 Plus spectrometer (Tokyo, Japan) with resolution 2 cm−1.
c-X-ray diffraction and optical microscopy
Identification of the minerals in the raw phosphate ore was performed using XRD Bruker diffractometer (AXS-8D) operating at 45 kV and 40 mA with CuKα radiation (1,5406 Å) and also a microscope equipped with a trinocular head, infinitely corrected lenses with transmitted and reflected light, and a 360° rotating polarizer.
Results and discussion
Characterization of phosphate ore
Mineralogical analyses
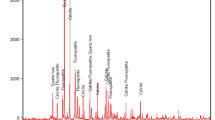
The X-ray diffraction pattern of the phosphate ore sample presented in Fig. 1 shows a significant difference between phases. The main mineralogical phases identified are apatites. On the basis of International Centre for Diffraction Data (ICDD) (Hounslow and Chao 1970; Brophy and Nash 1968; Hendricks et al. 1932) and using the X’Pert HighScore software, we were able to identify the mineralogical composition of the raw phosphate ore. All the indications shown in the X-ray diagrams concern two phases of the phosphate ore, the 45–125 μm phase and the 1–45 μm phase; − 20-μm size was removed.
Phase 1—45 μm
The studied phase on minus 45 μm showed the existence of several phases containing calcite, quartz, hematite, green cinnabar, fluorite, bornite, and calcium chloride phosphate (Tables 1 and 2 and Figs. 1 and 2).
Phase 45–125 μm
On the other hand, the 45–125-μm size range contained calcium fluoride phosphate (Howie and Broadhurst 1958); dolomite was proportionally found more prevalent than other secondary minerals than that shown on Figs. 3 and 4 and Tables 3 and 4.
Optical microscopy
The identification of the major contained minerals in the phosphate ore has been done with the use of an optical microscope. We focused on the phosphate ore particles of different sizes (45–180 μm). Some inclusions of magnetic dark red spherical minerals in Fig. 5 indicate the presence of hematite mineral.
Infrared spectroscopy characterization of phosphate ore
The infra-red phosphate spectrum (Fig. 6) shows bands in the interval of 1100–950 cm−1, characterizing the deformed oscillations of P–O of bonds PO43−; bands of average intensity in the interval 1440–1420 cm−1confirm the existence of magnesium compounds; bands of low intensity in the interval of 858–793 cm−1 and 644 cm−1 characterize the deformed oscillations of groups OH of the hydrogen bond; and doublets of average intensity in the interval 600–568 cm−1 and the bands between 465 and 420 cm−1 of relatively insignificant intensity characterize the oscillations P–O of PO43− bonds.
Chemical analyses
The chemical analysis of the studied phosphate ore is presented in Table 5.
Micro-flotation of pure minerals with fatty acids and amines
Flotation of dolomite and apatite using fatty acids
In the system CaO-P2O5-H2O system, the passage from the basic and neutral medium to the acid medium of less than 6, the solubility of the phosphate increases from 0.003 to 0.15 g/L (Shuvalova and Ratobilskaya 1984). In this case, there is a transfer of Ca 2+ cations to the liquid phase, which reduces the number of active sites causing a significant decrease in the adsorption of anionic collectors. On the other hand, the dissolution of the carbonates in the acid medium increases the number of active sites, which increases the adsorption of anionic collectors.
The solution chemistry of fatty acids is an important factor in anionic flotation where Ca2+ is present in the solution. The depression of phosphate is possibly due to formation of aqueous CaHPO4. The selective flotation of carbonates from phosphate in acidic media can be enhanced by minimizing free Ca2+ in solution (Mohammadkhani et al. 2011; Snow and Zhang 2002).
Flotation of dolomite and apatite under various conditions of pH and different concentrations of fatty acid emulsions is illustrated in Figs. 7 and 8. The flotation tests of these minerals was carried out at 50, 100, 150 mg/L concentrations of fatty acids and constant pH of 4.8–5.2 adjusted by phosphoric acid. As seen in Fig. 7, while apatite floated at negligible amounts, dolomite floated well with increasing the concentration of fatty acid. The concentration of 150 mg/L was selected as an optimum collector concentration and the effect of pH was further investigated at this particular fatty acid concentration.
The flotation of dolomite and apatite minerals against the pH range of pH 4.8–9.5 is presented in Fig. 8 at 150 mg/L of collector concentration in the presence of H3PO4 and CaO to both adjust the pH and depress apatite. Evidently, there is a considerable difference between the floatability of the two minerals at acidic and neutral pH but decreased at alkaline pH. Accordingly the acid medium of pH 4.8–5.2 was selected to achieve apatite–dolomite separation using fatty acids (Fig. 8).
Therefore, it is established that the selectivity of separation by flotation of dolomite from apatite is induced with the use of phosphoric acid and fatty acids as collector in acidic medium created part of the depressing action of apatite.
Flotation of quartz and apatite using amines
Flotation of quartz and apatite was carried out with the use of amine collector. The amines were used with the addition of phosphoric acid and CaO to make acidic basic medium, respectively. The flotation results presented in Fig. 9 show that quartz in acidic medium is relatively less floatable and reaches its peak value at pH 8.5 and then decrease upon increasing pH to 10.5. Interestingly, the pH range of 7.8–8.5 appears to be the best for flotation of quartz and beyond pH 9 the hydrolysis of amine resulted in the depression of quartz. On the other hand, the floatability of apatite in Fig. 9 steadily decreased with increasing pH from 4.5 up to 7.8 and then remained almost constant all the way in alkaline medium. The concentration of the mixture of amines at 30 mg/L represents an optimum to reach a window of selectivity between quartz and apatite at pH 7.8–8.5. Further experiments shown in Fig. 10 as a function of amine concentration reveal that indeed the selected concentration of 30 mg/L provides indeed the best selectivity between quartz and apatite minerals.
Closed circuit of phosphate ore flotation
The difficulty of enriching carbonated phosphate ores, having a complex mineralogical composition, lies in the high dispersion of apatite; the existence of calcium and magnesium carbonates with similar floatability to that of apatite with a difficulty of separation of dolomite. The studied phosphate ore contains some oxides that not only decrease the difference in floatability of minerals but are found in the phosphate concentrate and reduce its quality. They also contain clay impurities which give rise to a large quantity of slimes having a great influence on the flotation process.
Figure 11 shows the results obtained in presence of 5 kg/ton H3PO4 at pH 4.8–5.2 at first flotation stage using fatty acids 300, 400, 500 g/ton. Best results were obtained when 5 kg/ton of H3PO4, 500 g/ton fatty acids were used with feed size fraction—160 + 20 μm.
Figure 12 shows the results obtained using amines at 500, 600, and 750 g/ton in the presence of CaO as regulator at pH 7.8–8.5 at second flotation stage. The increase in the consumption of amines causes a decrease in recovery of P2O5 in phosphate concentrate and an increase in float products of quartz and carbonate. The cationic stage (flotation of silicate minerals—quartz) using amine collector was then carried out and final phosphate concentrate obtained.
The results in closed flotation circuit are displayed in the Table 6 and Fig. 13 shows that one can obtain a phosphate concentrate with a high content of P2O5 up to 30% with a relatively low MgO content of about 0.8–1.05% and insoluble residue of 11.7%.
Conclusion
The flotation method proposed give satisfactory experimental results for the flotation of a carbonate and silicates rich phosphate ore. Flotation in this case is carried in two stage operation. Anionic–cationic double reverse flotation was found to be an appropriate method to upgrade grade phosphate rocks. The two steps process is to float the carbonates at pH = 4.8–5.2; the second stage includes flotation of the quartz at pH = 7.8–8.5. Using fatty acids (0.5 kg/ton) in the first flotation stage and mixed amines (0.5 kg/ton) in the flotation second stage gave good results, concentrate assayed 30.1 P2O5% at a recovery of 94%.
References
Abdel-Khalek NA (2000) Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonates gangues. J Miner Eng 13:789–793
Abouzeid A-ZM, Negm AT, Elgillani DA (2009) Upgrading of calcareous phosphate ores by flotation: effect of ore characteristics. Int J Miner Process 90:81–89
Abu-Eishah SI, El Djallad IS, Muthaker M, Touqan M, Sadeddine W (1991) Beneficiation of calcareous phosphate rocks using dilute acetic acid solutions. Int J Miner Process 31:115–126
Al-Fariss TF, Ozbelge HO, Abdulrazik AM (1991) Flotation of carbonate rich sedimentary phosphate rock. J Fertilizer research 29:203–208
Al-Fariss TF, Abd El Aleem FA, El-Nagdy KA (2013) Benefication of Saudi phosphate ores by column flotation technology. J King Saud University - Eng Sci 25:113–117
Bogdanov OS, Maximov II (1990) Theory and technology of ores flotation. Nedra, Moscow
Brophy GP, Nash JT (1968) Compositional, infrared, and X ray analysis of fossil bone. Am Mineral 53:445–454
Deghani A, Azizi A, Mojtahedzadeh SH, Ghribi K (2012) Optimizing rougher flotation parameters of the Esfordi phosphate ore. Miner Process Extr Metall Rev 33:260–268
Dos Santos MA, Ricardo CS, Fabiano C, Carlos HA, Marcos ASB (2012) Influence of the water composition on the selectivity of apatite flotation. Sep Sci Technol 47:606–612
Doudenkov SV, Choubov LY (1969) Principles of theory and practice of use of flotation reagents. Nedra, Moscow
Drzymala J (1991) Characterization of materials by Hallimond tube. Part 1: maximum size of entrained particles. Int J Miner Process 42:139–152
Eigels MA (1967) Principles of flotation of non-sulphide minerals. Moscow
Feng D, Aldrich C (2004) Influence of operating parameters on the flotation of apatite. Miner Eng 17:453–455
Hendricks SB, Jefferson V, Mosley Z (1932) Kristallogr., Kristallgeom., Kristallphys., Kristallchem 81:325-369
Hounslow AW, Chao GY (1970) Monoclinic chlorapatite from Ontario. Can Mineral 10:252–259
Howie RA, Broadhurst FM (1958) X-ray data for dolomite and ankerite. Am Mineral 43:1210–1216
Kouachi S, Bouhenguel M, Amirech A, Bouchemma A (2010) Yoon-Luttrell collision and attachment models analysis in flotation and their application on general flotation kinetic model. Desalination 264:228–235
Lima OA, Deglon DA, Leal Filho LS (2009) A comparison of the critical impeller speed for solids suspension in a bench-scale and a pilot-scale mechanical flotation cell. Miner Eng 22:1147–1153
Liu WG, Wei DZ, Cui BY (2011) Collecting of N-dodecylethylene-diamine and its adsorption mechanism on mineral surface. Trans Nonferrous Metals Soc China 21:1155–1160
Miller JD, Xuming W, Minhua LA (2002) Selective collector for phosphate flotation. Univ. Of Utah, Florida Institute of Phosphate Research
Mohammadkhani M, Noaparast M, Shafae SZ, Amini A, Amini E, Abdollahi H (2011) Double reverse flotation of a very low grade sedimentary phosphate rock, rich in carbonate and silicate. Int J Miner Process 100:157–165
Pyke B, Fornasiero D, Ralston J (2003) Bubble particle heterocoagulation under turbulent conditions. J Colloid Interface Sci 265:141–151
Ralston J, Dukhin SS (1999) The interaction between particles and bubbles. Colloids Surf A Physicochem Eng Asp 151:3–14
Ralston J, Laskowski JS (1992) Ed. Chapter in colloid chemistry in minerals processing. Elsevier
Reay D, Ratcliff GA (1973) Removal of fine particles from water by dispersed air flotation: effects of bubble size and particle size on collection efficiency. Can J Chem Eng 51:178–185
Shuvalova NK, Ratobilskaya LD (1984) Study of flotation properties after treatment with sulfuric acid under phosphorite enrichment conditions. J Colloid Chemistry Russia 1693–1698
Snow R, Zhang P (2002) Surface modification for improved phosphate flotation. J Colloid Interface Sci 256(1):132–136
Unkelbach KH, Wasmuth HD (1991) A High intensity drum-equipped magnetic separator with superconducting magnet. Industrial Minerals Supplement 48–54
Wills BA, Nappier-Munn T (2006) Wills’ mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery, Butterworth-Heinemann, Elsevier
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amirech, A., Bouhenguel, M. & Kouachi, S. Two-stage reverse flotation process for removal of carbonates and silicates from phosphate ore using anionic and cationic collectors. Arab J Geosci 11, 593 (2018). https://doi.org/10.1007/s12517-018-3951-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-3951-2














 ) carbonate product, (
) carbonate product, ( ) quartz product
) quartz product
 ) carbonate product, (
) carbonate product, ( ) quartz product
) quartz product