Abstract
Basal respiration is one of the key indicators of soil C mineralization. Temperature sensitivity (Q10) of basal respiration is important for predicting changes in C mineralization due to warming. A modified methodology of Q10 determination is proposed. Soil samples were incubated at 25°C with periodic short-term (2 h) decline of temperature to 15°C and high-frequency measurements of CO2 production rates. The temperature sensitivity is estimated as the average rate of CO2 production at 25°C (before and after temperature decline) divided by the rate of CO2 production at 15°C. With this method we demonstrated that glucose addition most strongly affects the Q10 values at low temperature ranges (20–10°C), while temperature range affects Q10 stronger than the glucose additions. The negative effect of soil moisture on Q10 of basal respiration was demonstrated: the Q10 values decreased with increasing soil moisture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
An increase in the activity of soil organic matter mineralization and СО2 emission from soil into the atmosphere as a result of warming is the key mechanism of positive feedback of ecosystems to global climate change [8–14]. It is called positive because warming results in the increasing emission of СО2—the main greenhouse gas—which additionally accelerates warming [17–19]. Most models of global carbon cycle take into account this relationship, taking that the rate of carbon mineralization increases twofold with the temperature rise by every 10°С (Q10 = 2) [12, 14]. Because soils are the main source of СО2 in the atmosphere [24, 26, 27], high variability of Q10 values in different soil types can be one of the causes of ambiguities in models of global carbon cycle.
In spite of a great number of works on temperature sensitivity, the mechanisms of response and the factors that control it remain insufficiently studied [20, 21]. Most researchers measure the rate of СО2 emission under the temperatures, which do not allow direct calculation of Q10. The obtained data are approximated by different models (Arrhenius [7], van’t Hoff [29], Lloyd–Taylor [22], and others), on the basis of which Q10 and other parameters of temperature sensitivity are calculated. Inequality of models and mathematical errors are the source of high variation in Q10 values obtained by different authors [11, 12, 14, 18, 28, 31, 32].
In this study, we suggest an improved method of estimating Q10 of basal respiration—one of the main indicators of carbon mineralization in soils—by means of automatic regular measurements of temperature [16]. The modification consists of in additional automatic variation in the temperature of soil samples with regular short-term (for 2 h) temperature decrease by 10°С. The short time of temperature decrease is important in order to prevent changes in the species composition of microbial community. On the contrary, many researchers apply temperature rise for determining Q10. In this case, the rate of mineralization increases, and this results in a quick depletion of substrate available for microorganisms [12, 18]. The substrate deficiency decreases the rate of СО2 emission and, hence, the Q10 of soil organic matter mineralization.
Our work was aimed to testing the modified method of assessing the temperature sensitivity of С mineralization and to determining the influence of (1) added of easily decomposable organic substrate (glucose) in a wide range of concentrations, (2) temperature range, and (3) soil moisture (and a combination of these factors) on Q10 of С mineralization in the gray forest soil.
Object of Study
The samples of the upper (0–10 cm) mineral horizon of gray forest soil (А horizon) were taken under three herbaceous birch groves in the area of Akademgorodok (city of Krasnoyarsk). The 80-year-old birch groves are typical ecosystems of Krasnoyarsk forest-steppe. The samples taken under different birch groves were combined into one sample. The obtained sample was sieved through a 1 mm sieve to remove coarse roots and homogenize the soil mass. The time from soil sampling to the beginning of measuring СО2 flux was less than 2 h, and this allows us to consider the dynamics of mineralization of fresh organic matter.
Measurement of the Rate of СО2 Production, Calculation of Q10, and Variants of Treatment
The 100-g portions of soil were placed into 16 glass vessels of 0.5 L in volume, and 5 mL of water solution of glucose in concentrations of 0 (control), 25, 125, 250, 500, 1250, 2500, and 5000 μg С/g were added into every two vessels. The vessels were placed into an incubator with a Peltier cooler and programmed temperature regime (Memmert, Germany). Every vessel was connected via the 16-port multiplexer Li-Cor 8150 with an IR СО2 analyzer Li-Cor 8100 (Li-Cor Incorporated, Lincoln, Nebraska, USA). The multiplexer closed by turn each of 16 vessels into a hermetical chain for 1.5 min, and the rate of СО2 production by the soil sample was calculated from the rate of СО2 accumulation in a vessel. While the measurement was carried out in one vessel, other vessels were vented with room air in order to prevent the accumulation of СО2, the excessive concentration of which limits the rate of СО2 production.
Overall, three cycles of measurements were performed, and each cycle continued for three days. Incubation was carried out at 25°С with regular (every 8 h) short-term (2 h) lowering of temperature to 15°С in cycles 1 (low moisture) and 3 (increased moisture). The diagram of temperature regime during incubation is presented in Fig. 1a. In cycle 3, the soil water content was increased from 30 to 100% of the field capacity along with the added glucose. In cycle 2, the range of temperatures (20–10°C) was 5°C lower than in cycles 1 and 3. The rate of СО2 production was measured every 2 h; four measurements were performed at temperatures 20 or 25°C, and one measurement characterized low-temperature (10 or 15°C) periods (Fig. 1c). The Q10 coefficient was calculated for every cycle of lowering/rising temperature according to the following equation: Q10 = (F1 + F2)/(2F3), where F1 and F2 are the activities of СО2 emission at 25°С before and after temperature lowering, and F3 is the activity of СО2 emission at 15°С.
Statistical Analysis of Data
The rate of СО2 production and calculated Q10 values were tested for normality of distribution (Kolmogorov–Smirnov test) and uniformity of the sample (Levin test). Then, the two-way ANOVA was performed for repeated measurements of the rate of СО2 production and Q10 values. The two major factors were the concentration of glucose (eight levels, including the control) and the cycle (three levels, cycles 1–3). Forty time points for the rate of СО2 production and eight points for Q10 were the repeated measurements. The analysis demonstrated the absence of the influence of time on Q10, i.e., its values did not change with time (Fig. 1c). So, average values were calculated for every variant, and the two-way ANOVA was performed again as described above, but without repeated measurements. All effects were significant at Р < 0.050.
RESULTS AND DISCUSSION
The temperature coefficient Q10 for the rate of reaction was determined as a measure of its change with an increase in temperature by 10°С [7, 29]. The temperature sensitivity (Q10) of carbon mineralization and basal respiration has been widely studied all over the world [1, 5, 31]. The method suggested by us has certain advantages: (1) it is based on regular short-time lowering of temperature, which allows one to take into account limitation of the rate of reaction by the substrate; (2) the calculation of Q10 is performed without fitting of exponential models, and this excludes mathematical errors and reduces labor expanses; (3) automation increases the accuracy and efficiency of measurements and ensures standardization of the time under particular temperature [16]. Automation of measurements and their high efficiency make it possible to test many hypotheses about the impact of particular substrates or environmental factors on the rate of the CO2 production. While automated measurements was previously reported [16], we advanced the methodology by automated temperature variation and the way of Q10 estimation accounting for a decline of CO2 respiration rates with time. In this study, we tested the influence of glucose added in different concentrations, soil water content, and soil temperature range on Q10 of basal respiration. The temperature regime during incubation in cycles 1 and 3 is shown in Fig. 1a; it was characterized by periodical lowering of temperature from 25 to 15°С. The rate of СО2 production gradually decreased in the course of the entire incubation period and during each cycle of temperature lowering (Fig. 1b). This is explained by the loss of easily available carbon and gradual drying of the sample because of ventilation of the vessels [16]. However, calculated values of Q10 were about 2 and did not change with time (Fig. 1c); they were close in different replicates of the experiment, i.e., the applied method ensured stable results.
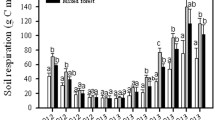
As expected, the rate of СО2 emission was lower at 20°С than at 25°С (Fig. 2). A sharp increase in the rate of СО2 emission was observed, when the samples were moistened; the initial fresh samples were relatively dry, limiting the activity of microorganisms. An increase in the rate of СО2 production was also observed with the rise in the concentration of glucose, because the rate of the reaction depends on the amount of the substrate.
The temperature range had a maximum effect on Q10, i.e., the Q10 value was significantly greater (Fig. 3) under low temperatures (10–20°С) than under higher temperatures (15–25°С). The decrease in temperature sensitivity with an increase in temperatures was noted by Arrhenius [7, 12]. The reason for this phenomenon is that the reaction rate increases with the rise in temperature, but, at the same time, the availability (concentration) of the substrate decreases, and this decrease is more pronounced.
The effect of concentration of added С was negative and was only observed in the low temperature range (cycle 2). The addition of glucose in different concentrations had no influence on Q10 of basal respiration in the range of higher temperatures both under low and high moisture content values.
Two concepts were suggested earlier to explain the influence of temperature on the rate of carbon mineralization in relation to the quality and availability of organic matter [12]. The concept of quality (the kinetic theory of Arrhenius [7]) stipulated that soil organic matter is a mixture of different substances, each of which has its own energy of activation, i.e., their mineralization requires some minimal temperature. Most molecules of organic matter are stable, i.e., their energy of activation is high, so that they begin to decompose under high temperatures [12]. On the contrary, glucose is characterized by the low energy of activation and can be mineralized under lower temperatures, and this is the cause of much quicker decomposition of added glucose than soil organic matter by microorganisms [12, 14]. Thus, the emission of СО2 and temperature sensitivity of C mineralization in the case of added glucose are mainly determined by glucose mineralization [10, 12, 20, 25, 28]. Hence, the addition of glucose introduction should decrease Q10 of С mineralization. The availability of soil organic matter for microorganisms has an opposite effect on Q10. In fact, organic substrate should be available in loci of enzyme activity. In other words, there may a lot of organic matter in the soil sample, but its concentration in the reaction loci may be low. In this case, the diffusion transport that depends both on moisture and temperature takes place. In general, the decrease in concentration of available substrate results in the underestimation of Q10, and this explains the decrease of Q10 in the range of higher temperatures. The kinetic theory of Arrhenius was confirmed experimentally in our study, and the increase on Q10 values was observed in the range of lower temperatures (cycle 3) in comparison with the range of higher temperatures (Fig. 3). However, neither the kinetic theory of Arrhenius nor the concept of availability explain the absence of the effects of glucose and its concentrations on Q10 of basal respiration in cycles 2 and 3. Recent studies suggest the significant influence of priming effect—short-term change in the rate of C mineralization in the case of carbon or nitrogen addition—on Q10 of С mineralization [2, 32]. We suggest that the decrease in Q10 of С mineralization in response to an increase in glucose concentration, which was observed in the range of lower temperatures, could take place because of priming effect. It is likely that priming effect also existed in the range of higher temperatures. However, the value of Q10 of basal respiration became higher under lower temperatures, and this made the participation of new fractions of organic matter in mineralization processes more pronounced according to Q10 values.
If the Q10 index of glucose mineralization differed significantly from Q10 of soil organic matter mineralization, then the effect of added glucose adding would also be observed in the range of higher temperatures. According to the kinetic theory of Arrhenius, the fraction of mineralizable organic matter decreases in the range of lower temperatures. At the same time, Q10 of С mineralization increased significantly (Fig. 3). We suppose that the addition of glucose caused limitation of the development of microorganisms by nitrogen. In order to get nitrogen, microorganisms began to mineralize soil organic matter, and this process took place at higher temperatures and stopped at lower temperatures. Mineralization of this fraction of organic matter under the influence of priming effect in the range of lower temperatures brought Q10 to the values observed in range of higher temperatures. This assumption is confirmed by the fact that Q10 of basal respiration decreased, when concentrations of added glucose were in the range of 25–1250 μg С/g, under which the maximum priming effect is observed [15].
It is also important that we confirmed the decrease in Q10 values with an increase in soil moisture (Fig. 3). The most plausible explanation of this is the increase in the rate of diffusion of organic substances to the loci of enzymatic reactions [3, 4, 6, 11, 23, 30]. This confirms the influence of organic matter availability on Q10 of carbon mineralization.
Thus, environmental factors have different effects on Q10 of soil organic matter mineralization. Prevalent theories—the kinetic theory by Arrhenius and the theory of organic matter availability—do not explain the entire diversity of effects. However, the addition of the theory of priming effect and its temperature sensitivity can improve significantly our understanding of the behavior of Q10 of С mineralization under changing environmental factors.
CONCLUSIONS
(1) It was experimentally confirmed that Q10 of basal soil respiration is higher at the lower temperature range and decreases at the higher temperatures range, which is in agreement with the Arrhenius kinetic theory.
(2) The addition of glucose led to a decrease of Q10, and this effect was more pronounced in the range of lower temperatures. We argue that priming effect is responsible for the influence of added glucose on Q10 of С mineralization at lower temperatures.
(3) The influence of soil moisture on Q10 was confirmed: with the rise in the soil water content, the Q10 values decreased because of an increase in the diffusion of organic matter to the loci of enzymatic reactions thus confirming the concept about limitation of C mineralization by the availability of the substrate.
REFERENCES
I. V. Yevdokimov, I. A. Yusupov, A. A. Larionova, S. S. Bykhovets, M. V. Glagolev, and S. A. Shavnin, “Thermal impact of gas flares on the biological activity of soils,” Eurasian Soil Sci. 50, 1455–1462 (2017).
A. I. Zhuravleva, V. M. Alifanov, and E. V. Blagodatskaya, “Effect of contrasting trophic conditions on the priming effect in gray forest soils,” Eurasian Soil Sci. 51, 204–210 (2018).
A. V. Ivanov, M. Braun, and V. A. Tataurov, “Seasonal and daily dynamics of the CO2 emission from soils of Pinus koraiensis forests in the south of the Sikhote-Alin Range,” Eurasian Soil Sci. 51, 290–295 (2018).
I. N. Kurganova, V. O. Lopes de Gerenyu, J. F. Gallardo Lancho, and C. T. Oehm, “Evaluation of the rates of soil organic matter mineralization in forest ecosystems of temperate continental, Mediterranean, and tropical monsoon climates,” Eurasian Soil Sci. 45, 68–79 (2012).
A. A. Larionova, A. K. Kvitkina, I. V. Yevdokimov, S. S. Bykhovets, and A. F. Stulin, “Effect of temperature on the decomposition rate of labile and stable organic matter in an agrochernozem,” Eurasian Soil Sci. 47, 416–424 (2013).
A. A. Larionova, A. N. Maltseva, V. O. Lopes de Gerenyu, A. K. Kvitkina, S. S. Bykhovets, B. N. Zolotareva, and V. N. Kudeyarov, “Effect of temperature and moisture on the mineralization and humification of leaf litter in a model incubation experiment,” Eurasian Soil Sci. 50, 422–431 (2017).
S. Arrhenius, “Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren,” Z. Phys. Chem. 4, 226–248 (1889).
S. F. Chapin III, J. McFarland, D. A. McGuire, E. S. Euskirchen, R. W. Ruess, and K. Kielland, “The changing global C cycle: linking plant-soil C dynamics to global consequences,” J. Ecol. 97, 840–850 (2009).
P. M. Cox, R. A. Betts, C. D. Jones, S. A. Spall, and I. J. Totterdell, “Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model,” Nature 408, 184–187 (2000).
S. S. Dai, L. J. Li, R. Ye, X. Zhu-Barker, and W. R. Horwath, “The temperature sensitivity of organic carbon mineralization is affected by exogenous carbon inputs and soil organic carbon content,” Eur. J. Soil Biol. 81, 69–75 (2017).
E. A. Davidson, E. Belk, and R. D. Boone, “Soil water content and temperature as independent or confound factors controlling soil respiration in a temperate mixed hardwood forest,” Global Change Biol. 4, 217–227 (1998).
E. A. Davidson and I. A. Janssens, “Temperature sensitivity of soil carbon decomposition and feedbacks to climate change,” Nature 440, 166–169 (2006).
E. A. Davidson, I. A. Janssens, and Y. Luo, “On the variability of respiration in terrestrial ecosystems: moving beyond Q10,” Global Change Biol. 12, 154–155 (2006).
E. A. Davidson, S. E. Trumbore, and R. Amundson, “Soil warming and organic carbon content,” Nature 408, 789–790 (2000).
M. de Graaff, A. T. Classen, H. F. Castro, and C. W. Schadt, “Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates,” New Phytol. 188, 1055–1064 (2010).
H. Heinemeyer, O. Insam, E. A. Kaiser, and G. Walenzik, “Soil microbial biomass and respiration measurements: An automated technique based on infra-red gas analysis,” Plant Soil 116 (2), 191–195 (1989).
R. A. Houghton, E. A. Davidson, and G. M. Woodwell, “Missing sinks, feedbacks, and understanding the role of terrestrial ecosystems in the global carbon balance,” Global Biogeochem. Cycles 12, 25–34 (1998).
K. Karhu, M. D. Auffret, J. A. Dungait, D. W. Hopkins, J. I. Prosser, B. K. Singh, J. A. Subke, P. A. Wookey, G. I. Agren, M. T. Sebastià, et al., “Temperature sensitivity of soil respiration rates enhanced by microbial community response,” Nature 513 (7516), 81–84 (2014).
M. U. F. Kirschbaum, “The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage,” Soil Biol. Biochem. 27, 753–760 (1995).
A. A. Larionova, I. V. Yevdokimov, and S. S. Bykhovets, “Temperature response of soil respiration is dependent on concentration of readily decomposable C,” Biogeosciences 4, 1073–1081 (2007).
J. Liski, H. Ilvesniemi, A. Makela, and K. J. Westman, “CO2 emissions from soil in response to climatic warming are overestimated-The decomposition of old soil organic matter is tolerant of temperature,” Ambio 28, 171–174 (1999).
J. Lloyd and J. A. Taylor, “On the temperature dependence of soil respiration,” Funct. Ecol. 8, 315–323 (1994).
V. O. Lopes de Gerenyu, I. N. Kurganova, L. N. Rozanova, and V. N. Kudeyarov, “Effect of soil temperature and moisture on CO2 evolution rate of cultivated phaeozem: analysis of a long-term field experiment,” Plant Soil Environ. 51 (5), 213–219 (2005).
Y. Luo, “Terrestrial carbon cycle feedback to climate warming,” Annu. Rev. Ecol. Evol. Syst. 38, 683–712 (2007).
K. Min, C. A. Lehmeier, and S. A. Billings, “Carbon availability modifies temperature responses of heterotrophic microbial respiration, carbon uptake affinity, and stable carbon isotope discrimination,” Front. Microbiol. 7, 2083 (2016).
J. W. Raich and W. H. Schlesinger, “The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate,” Tellus B 44, 81–99 (1992).
D. S. Schimel, B. H. Braswell, E. A. Holland, R. McKeown, D. S. Ojima, T. H. Painter, W. J. Parton, and A. R. Townsend, “Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils,” Global Biogeochem. Cycles 8, 279–293 (1994).
M. Song, J. Jiang, G. Cao, and X. Xu, “Effects of temperature, glucose and inorganic nitrogen inputs on carbon mineralization in a Tibetan alpine meadow soil,” Eur. J. Soil Biol. 46 (6), 375–380 (2010).
J. H. Van’t Hoff, Lectures on Theoretical and Physical Chemistry, Part 1: Chemical Dynamics (Edward Arnold, London, 1898).
Z. M. Zheng, G. R. Yu, Y. L. Fu, Y. S. Wang, X. M. Sun, and Y. H. Wang, “Temperature sensitivity of soil respiration is affected by prevailing climatic conditions and soil organic carbon content: a trans-China based case study,” Soil Biol. Biochem. 41 (7), 1531–1540 (2009).
T. Zhou, P. Shi, D. Hui, and Y. Luo, “Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback,” J. Geophys. Res.: Biogeosci. 114, 1–2 (2009).
B. Zhu and W. Cheng, “Rhizosphere priming effect increases the temperature sensitivity of soil organic matter decomposition,” Global Change Biol. 17, 2172–2183 (2011).
Funding
This work was supported by the Russian Foundation for Basic Research, project nos. 17-04-01776 and 18-54-52005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by T. Chicheva
Rights and permissions
About this article
Cite this article
Gromova, M.S., Matvienko, A.I., Makarov, M.I. et al. Temperature Sensitivity (Q10) of Soil Basal Respiration as a Function of Available Carbon Substrate, Temperature, and Moisture. Eurasian Soil Sc. 53, 377–382 (2020). https://doi.org/10.1134/S1064229320020052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229320020052