Abstract
The phase transitions of sulfide minerals—pentlandite and chalcopyrite—are tested in roasting with mixture with ammonium sulfate. The behavior of the mixture during roasting is assessed via the synchronous thermal and X-ray phase analyses, and by scanning electron microscopy. The basic optimal technology parameters for the efficient processing of sulfide-bearing copper–nickel ore are selected to be: the roasting temperature of 400 °C, the ore: ammonium sulfate ratio of 1:10, the particle size of –40 \(\mu\)m and the roasting time of 240 min. An important condition is joint milling of ore and ammonium sulfate down to the specified size. Given these parameters, the later on water leaching of clinker provided extraction of 94.8% of copper, 91.5% of nickel and 82.3% of cobalt. The research findings are of essential practical interest owing to the high recovery of the target minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, more than 400 copper-nickel ore deposits have been discovered in the world. Information on copper and nickel reserves is updated annually. According to the US Geological Survey, copper reserves are estimated at 1.6 billion t, nickel reserves—89 million t [1]. The deposits of sulfide copper-nickel ores account for \(\sim\)65% of the global production of nickel, which is more than 2 million t/year. From these ores, about 700 thousand t/year of copper, 150 thousand t/year of cobalt, as well as platinum group metals are produced [2].
No new large sulfide deposits have been discovered in recent years, and some deposits that have been mined for decades have a limited remaining life. Therefore, ores sent for concentration are increasingly characterized by the presence of oxidized forms and a significant amount of fine sulfide dissemination. The flow charts and reagent regimes do not often provide the required concentration efficiency, the loss of nickel with tailings is up to 27% of the content in the ore. Significant losses are associated with sulfide accretions that do not disclose at the accepted grinding size or are represented by undisclosable intergrowths with silicates. Losses due to the incorporation of nickel into the composition of crystal lattices of different rock-forming minerals reach 45% of the total losses [3–5].
The predominance of extensive mining and processing operations results in the incomplete mining of already discovered deposits and significant waste accumulation. At the same time, in hypergenesis conditions, the mineral composition of sulfide ores is changed significantly, which causes ore dilution. During the oxidation of sulfides, heavy metals are converted into water-soluble salts, which causes further ingress of heavy metals into surface and subsurface waters and, as a result, environmental pollution [6–8]. This impact is especially negative in the climatic conditions of Extreme North, since natural ecosystems are highly vulnerable to external influences. Under the current conditions of intensive mining activity, there have been significant changes in the hydrochemical regime of surface waters, chemical composition of bottom sediments, structural and functional organization of biotic communities in the Murmansk region [9].
Decline in the reserves of commercial copper-nickel minerals determines the search for alternative dressing technologies; low-temperature roasting of copper-nickel ore with ammonium sulfate seems promising. The creation of technology requires fundamental research into the changes that sulfide minerals undergo during roasting in a mixture with ammonium sulfate. To expand the resource base of the mining and processing industry and minimize environmental damage, it is necessary to search for an economically and environmentally justified method to extract nonferrous metals from rebellious ores. One of the promising and environmentally safe methods is the low-temperature roasting of copper-nickel ore with ammonium sulfate \(\mathrm{ (NH_4)_2SO_4}\). This reagent is found to be effective from a technological point of view, since nonferrous metal sulfides enter into reaction with the formation of water-soluble sulfates [10–15].
This paper aims at determining the change in phase composition of copper and nickel sulfides during low-temperature roasting, as well as the selection of effective process parameters for roasting copper-nickel sulfide ore in mixture with ammonium sulfate.
1. MATERIALS AND METHODS
To determine the phase transformations that sulfide minerals undergo during low-temperature roasting, nickel sulfides—pentlandite (\(\mathrm{ Fe_{4.5}Ni_{4.5}S_8}\)) and copper sulfides—chalcopyrite (CuFeS2) were synthesized by the Kullerud method. The effective parameters for low-temperature roasting were selected using massive sulfide copper-nickel ore from the Allarechensky technogenic deposit (hereinafter-TD) with a nickel content of 6.42% and copper content of 5.12%. The deposit is a rock dump formed in the process of mining the primary Allarechensky deposit, the ore is characterized by pyrrhotite, less often pyrite-pyrrhotite sulfide association.
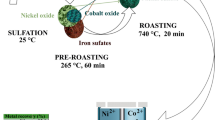
Copper-nickel ore and synthesized minerals were mixed with ammonium sulfate (chemically pure, GOST 3769-78). The resulting mixture was ground in a BMU-100 ball mill (HT Machinery Co., Ltd., China) and roasted for 240 min at different temperatures in a SNOL 3/11 muffle furnace (LLC NPF Termiks, Russia). Ammonium sulfate decomposes at 218°C, melting point is 235°C [16–22]. The roasting temperature was varied from 300 to 500°C in increments of 50°C. Heating up to the desired temperature 60 min. After roasting, the clinker was cooled in open air for 60 min. Then, it was leached in distilled water heated to \(\sim 80^\cdot\)C for 40 min with constant stirring at an intensity of 230 min\(^{-1}\) using an MV-6 overhead stirrer (LLC NV-LAB, Russia).
To diagnose the mineral composition of sulfide minerals, original ore, clinker and sediment after ore leaching, the method of powder X-ray diffraction was used. X-ray diffraction patterns were taken on a DRON-2.0 device (Burevestnik Research-and-Production Co., Russia), CuK\(_{\alpha}\)-radiation—in the laboratory of physical methods for the analysis of mineral matter at the Institute of Mineralogy, Ural Branch, RAS. The surface morphology of mineral particles was determined using a SEM Leo-420 scanning electron microscope (Carl Zeiss Ltd., Germany).
When roasting sulfide minerals in mixture with ammonium sulfate, temperature effects were determined by the method of simultaneous thermal analysis (STA) on a TG/DSC analyzer NETZSCH STA 409 (Netzsch Geraetebau GmbH, Germany). Samples of pentlandite, chalcopyrite and sulfide ore from the Allarechensky TD were heated in alumina crucibles from 30 to 1000 °C at a rate of 10 °C/min in air atmosphere.
After leaching, concentrations of nonferrous metal ions in solutions were determined by atomic absorption spectroscopy on a Shimadzu-AA7000G device (Shimadzu Corp., Japan) with electrothermal atomization (PND F 14.1:2:4.140-98). Experimental results were processed in the Microsoft Excel 2010 software using statistical methods.
2. RESULTS AND DISCUSSION
The behavior of pentlandite when heated was studied in [23–27]. When pentlandite is heated, magnetite (Fe3O4) is formed as a result of iron atoms migration from the core of a mineral grain to the surface and their subsequent oxidation. Nickel-enriched pyrrhotite (Fe\(_{1 - x}\)S) is formed in areas with atmospheric oxygen deficiency. A further increase in temperature leads to an increase in the proportion of nickel sulfide and magnetite transformation into hematite (Fe2O3). Thus, a mineral grain is formed with a core of nickel sulfide (crowningshieldite—NiS) and a shell of iron oxides (magnetite and hematite) as a result of pentlandite grain oxidation.
Sulfur also actively interacts with atmospheric oxygen forming dioxide SO2. The results of thermal analysis of synthesized pentlandite obtained in the course of study are consistent with the reference data. The oxidation processes recorded during heating are shown on DSC curve by exothermic peaks at 450.3, 661.1 and 759.3°C (Fig. 1a). Predominant oxidation of iron and sulfur compared to nickel is probably due to the different redox potentials of these elements. When ammonium sulfate is added to pentlandite, TG/DSC curves significantly change their shape. The sample starts loosing its weight at a temperature of 300 °C, which is due to ammonium sulfate decomposition (Fig. 1b). Taking into account the results of [22], it can be concluded that ammonium sulfate decomposes with the formation of ammonium hydrosulfate (NH4HSO4), which under certain conditions can form hydrosulfate of a different composition \(\mathrm{ (NH_4)_3H(SO_4)_2}\), sulfamic acid (\(\mathrm{ NH_2SO_2OH}\)) and ammonium pyrosulfate (\(\mathrm{ (NH_4)_2S_2O_7}\)). Therefore, when the temperature of 400 °C is reached, the mineral interacts with several compounds at once.
After pentlandite roasting, in addition to the original mineral, X-ray diffraction analysis detected reflexes of pyrrhotite, pyrite (FeS2), crowningshieldite, and magnetite in the sample. After roasting a mixture of pentlandite with ammonium sulfate (a natural analog is mineral mascagnite) at a ratio of 1:2, reflexes of the following minerals were recorded: sabieite (\(\mathrm{ NH_4Fe(SO_4)_2}\)), polymidite (\(\mathrm{ Ni_3S_4}\)), crowningshieldite, magnetite. The reflexes of nickel sulfate NiSO4 were recorded. Note that mascagnite reflexes are not recorded in clinker. At this ratio of pentlandite and ammonium sulfate, the reagent have probably entered into interaction completely.
Reflexes of the mentioned sulfides still appear in the sediment after clinker leaching, violarite (FeNi2S4), ammoniojarosite (\(\mathrm{ (NH_4)(Fe^{3 + })_3(SO_4)_2(OH)_6}\)) and magnetite were also found among sulfides. A significant number of sulfide reflexes in the sediment indicates the need to increase the consumption of ammonium sulfate.
When the ratio of pentlandite to ammonium sulfate increases to 1:7, the reflexive intensity of sulfate minerals pyracmonite (\(\mathrm{(NH_4)_3Fe(SO_4)_3}\)) and nickel sulfate grows in clinker. As with a lower consumption of ammonium sulfate, reflexes of violarite and crowningshildite are manifested in the sediment. Pentlandite and pyrite reflexes at a given ratio are not detected in the sediment. The composition of natural pentlandites varies widely and differs from the stoichiometric ratio of metals and sulfur; therefore, characteristic features of natural minerals must be taken into account when selecting the most effective roasting parameters.
The behavior of chalcopyrite during low-temperature roasting requires special study. As the main copper concentrator in ores, this mineral is quite inert when in contact with different reagents. The behavior of chalcopyrite during roasting is the subject of [28–32]. Based on the results of these works, it follows that no visible changes occur when chalcopyrite is heated to 350 °C. In the temperature range of 350–450 °C, chalcopyrite is oxidized with the formation of iron sulfates (FeSO4) and copper sulfates (chalcocyanite—CuSO4), as well as hematite (Fe2O3). In the same temperature range, in areas with complicated oxygen diffusion, chalcopyrite decomposes to form a more stable mineral—bornite (Cu5FeS4). Upon reaching a temperature of 450 °C, bornite decomposes with the formation of pyrite and pyrrhotite [28]. Chalcocyanite is stable at this temperature and starts oxidizing at \(>600~^\cdot\)C to oxide (CuO). As the temperature rises, copper oxide enters into reaction with hematite to form ferrite CuFe2O4[28, 29]. These oxidative processes are confirmed by DTA results, the peak at 481.8 °C is especially distinguished among exothermic peaks (Fig. 2a). On DSC curve of chalcopyrite and ammonium sulfate mixture at a mass ratio of 1:2, an endothermic effect appears at 357.5 °C (Fig. 2b) due to ammonium sulfate decomposition.
As in the experiment with pentlandite, chalcopyrite was heated in an air atmosphere, so oxygen flew freely to the surface of mineral particles. During roasting at 400°C, the XRF analysis detected the formation of both minerals characteristic of the oxidizing environment—melanterite (\(\mathrm{ FeSO_4\times 7H_2O}\)), and phases formed in areas with limited oxygen access—bornite (Cu5FeS4). In addition to these two minerals, proper reflexes of chalcopyrite were recorded after roasting. After roasting a mixture of chalcopyrite with ammonium sulfate, reflexes of melanterite were recorded in clinker, a hydroxide-iron phase—goethite (FeO(OH)) appeared and original chalcopyrite was found.
Reflexes of chalcopyrite, goethite, pyrite and covellite were manifested in the sediment after leaching of the mixture. As in the case of pentlandite, detection of sulfides in the sediment and absence of copper sulfate reflexes indicate the need to increase the reagent consumption.
An increase in the consumption of ammonium sulfate to a ratio of 1:7 led to the fact that no reflexes of sulfide minerals were recorded in clinker. The detected phases belong to pyracmonite, morite, goethite and copper sulfate—chalcocyanite. After clinker leaching at a given consumption of ammonium sulfate, reflexes of other sulfides—fukuchilite (Cu3FeS8) and nukundamite (\(\mathrm{ Cu_{3.4}Fe_{0.6}S_8}\)) were detected in the sediment, chalcopyrite was present in an insignificant amount.
Experiments with synthesized minerals showed that addition of ammonium sulfate causes the formation of nonferrous metal sulfates already at a temperature of 400 °C. To ensure the completeness of interaction between copper and nickel sulfide minerals, roasting is advisable in the range of ore : ammonium sulfate ratios of 1:7–1:10. Subsequent leaching of sulfates in heated water leads to their dissolution. Mostly hydroxides and a small part of unreacted sulfides remain in the sediment. Further studies were carried out with an ore sample from the Allarechensky technogenic deposit, which was roasted at the optimum temperature of 400 °C.
The following phases were noted in the original ore sample from the Allarechensky TD: chalcopyrite, pyrrhotite, pentlandite, cristobalite (SiO2), awaruite (Ni2Fe). After roasting a mixture of ore and ammonium sulfate in a ratio of 1:7 at a temperature of 400° C, the diffraction pattern showed reflexes of copper and nickel sulfates, sabieite, pyracmonite, as well as a few reflexes of sulfides—pentlandite, pyrrhotite and chalcopyrite in the sample. Mascagnite is completely consumed, and no reflexes of this mineral were recorded in the sample. After water leaching of clinker, predominance of ammoniojarosite, presence of magnetite, as well as some reflexes of sulfide minerals—pyrite, chalcopyrite, marcasite and fukuchilite was noted in the sediment.
Figure 3 shows the external shape of particles of the original ore from the Allarechensky TD, a mixture with ammonium sulfate, clinker and sediment after leaching. After grinding, ore particles are characterized by a chipped shape, which provides the best contact with the reagent during mixing and subsequent roasting (Fig. 3a). Images of mineral grain surface mixed with ammonium sulfate are shown in Fig. 3b. The selected method of grinding allows disclosing the grains and mineral accretions to provide their contact with the reagent. The particle surface after roasting the ore with ammonium sulfate at a ratio of 1:7 is shown in Fig. 3c. Analysis of clinker micrographs suggests a high intensity of interaction between ore particles and ammonium sulfate, since almost all original particles are covered with a sulfate crust after roasting. There is no sulfate crust on sediment particles after leaching. The surface of sediment particles after leaching is characterized by fracturing (Fig. 3d).
Results of leaching of nonferrous metals from ore depending on the ratio of ore:ammonium sulfate and particle size of the mixture are shown in At original ore size of 100 \(\mu\)m and a small consumption of ammonium sulfate (1:2), 24.6% of copper and 27.5% of nickel were extracted during water leaching from ore into solution after roasting at 400 °C (Fig. 4a).
Given the low recovery of metals by the end of the experiment, it is advisable to increase the consumption of ammonium sulfate. At a ratio of 1:7 that is determined as optimum during the roasting of synthesized minerals, 42.4% of copper and 45.3% of nickel were extracted into solution by way of leaching. An increase in the consumption to a ratio of 1:10 contributed to the maximum recovery of metals at a given size: 50.4% of copper and 50.6% of nickel. A further increase in the reagent consumption did not lead to a significant increase in metal recovery. On this basis, finer joint grinding of minerals to a size of –40 \(\mu\)m was expedient. Thus, at a ratio of 1:10 and size of –40 \(\mu\)m of the mixture sent for roasting, 94.8% of copper and 91.5% of nickel were extracted from ore by the end of the experiment (Fig. 4b).
Another valuable metal contained in the ore is cobalt (0.1%). The calculated recovery of this metal under optimum roasting parameters was 82.3%.
CONCLUSIONS
Laboratory studies of sulfide copper-nickel ore processing by low-temperature roasting in mixture with ammonium sulfate showed sustainability of this approach. The optimal technology parameters of roasting were determined: the ore : ammonium sulfate ratio of 1:10, the particle size of –40 \(\mu\)m, the roasting temperature of 400 °C and the roasting time of 240 min. Given these parameters, the later on water leaching of clinker provided extraction of 94.8% of copper, 91.5% of nickel and 82.3% of cobalt. The results of simultaneous thermal analysis and micrographs of particle surface after roasting lead to the conclusion that increasing the temperature is impractical in the technology under study.
Note that roasting temperature of 400 °C is much lower than the temperature in conventional pyrometallurgy. Water, sulfur dioxide and trioxide, sulfuric acid, hydrogen and nitrogen are released into the gas phase during roasting. The composition of waste gases indicates the possibility of their capture and regeneration of ammonium sulfate at the roasting stage, which can optimize ore processing by reducing the reagent costs and increase the environmental attractivenes due to reduced emissions into the atmosphere. Given the process optimization, low-temperature roasting of sulfide minerals in mixture with ammonium sulfate can also be considered promising for processing low-grade ores due to high selectivity, relatively low energy consumption and low cost.
ACKNOWLEDGMENTS
The authors are grateful to Krasavtseva E. A., Researcher of the Institute of North Industrial Ecology Problems, Kola Science Center, RAS for synthesizing sulfide minerals, as well as to Zenovich E. D. and Parshina N. V., Researchers of the Institute of Mineralogy, South Urals Federal Research of Mineralogy and Geoecology, Ural Branch, RAS for carrying out X-ray phase analysis.
FUNDING
This study was carried out as per R&D plan, project nos. 1021051803680-5, 0226-2019-0011, and under State Assignment, project no. AAAA-A-21-121011990025-5.
REFERENCES
Mineral Commodity Summaries, U. S. Geological Survey, 2019.
Information and Technical Guide on the Best Available Technologies ITS 12-2016 Nickel and Cobalt Production, Moscow: Byuro NDT, 2019.
Dyachenko, A.N., Kraidenko, R.I., Chegrintsev, S.N., and Poryvai, E.B., Opening of Copper-Smelting Slags with Ammonium Chloride, Tsvet. Metallurgiya, 2015, no. 5, pp. 9–12.
Likhacheva, S.V., and Neradovskiy, Yu.N., Reduction of Nickel Losses with Flotation Tailings of Copper-Nickel Ores in Pechenga, Tsvet. Metally, 2013, no. 10, pp. 37–40.
Chernousenko, E. V., Neradovsky, Yu.N., Kameneva, Yu.S., Vishnyakova, I.N., and Mitrofanova, G.V., Increasing Efficiency of Pechenga Rebellious Copper-Nickel Sulphide Ore Flotation, J. Min. Sci., 2018, vol. 54, no. 6, pp. 1035–1040.
Chanturia, V.A., Makarov, V.N., Makarov, D.V., Vasil’eva, T.N., Pavlov, V.V., and Trofimenko, T.A., Influence Exerted by Storage Conditions on the Change in Properties of Copper-Nickel Technogenic Products, J. Min. Sci., 2002, vol. 38, no. 6, pp. 612–617.
Masloboev, V.A., Seleznev, S.G., Makarov, D.V., and Svetlov, A.V., Assessment of Eco-Hazard of Copper-Nickel Ore Mining and Processing Waste, J. Min. Sci., 2014, vol. 50, no. 3, pp. 559–572.
Svetlov, A.V., Pripachkin, P.V., Masloboev, V.A., and Makarov, D.V., Classification of Low-Grade Copper-Nickel Ore and Mining Waste by Ecological Hazard and Hydrometallurgical Processability, J. Min. Sci., 2020, vol. 56, no. 2, pp. 275–282.
Dauval’ter, V.A. and Kashulin, N.A., Ecological and Economic Assessment of the Need to Extract Bottom Sediments from Lake Nyudyavr, Monchegorsk District, Murmansk Region, Vestn. MGTU, 2011, vol. 14, no. 4, pp. 884–891.
Yang, Z.P., Jin, X.Z., and Zhu, G.C., Process of Techniques Researches on Low-Grade Manganese Ore by Roasting with Ammonium Salt, China Manganese Ind., 2006, vol. 24, no. 3, p. 12.
Zhu, G.C., Li, F.P., and Xiao, M.G., Process of Enriching and Recovering Mn by Roasting the Low-Grade Manganese Carbonate Ore with Ammonium Sulfate, J. Guilin Univ. Technol., 2005, vol. 25, no. 4, p. 534.
Li, D.F., Wang, C.Y., Yin, F., Chen, Y.Q., Jie, X.W., Yang, Y.Q., and Wang, J., Leaching of Valuable Metals from Roasted Residue of Spent Lithium–Ion Batteries with Ammonium Sulfate, Chin. J. Process Eng., 2009, vol. 9, no. 2, p. 264.
Sukla, L.B., Panda, S.C., and Jena, P.K., Recovery of Cobalt, Nickel and Copper from Converter Slag through Roasting with Ammonium Sulphate and Sulphuric Acid, Hydrometallurgy, 1986, vol. 16, no. 2, pp. 153–165.
Mu, W., Cui, F., Huang, Z., Zhai, Y., Xu, Q., and Luo, S., Synchronous Extraction of Nickel and Copper from a Mixed Oxide-Sulfide Nickel Ore in a Low-Temperature Roasting System, J. Clean. Prod., 2018, vol. 177, pp. 371–377.
Li, G., Xiong, X., Wang, L., Che, L., Wei, L., Cheng, H., Zou, X., Xu, Q., Zhou, Z., and Li, S., Sulfation Roasting of Nickel Oxide-Sulfide Mixed Ore Concentrate in the Presence of Ammonium Sulfate: Experimental and DFT Studies, Metals, 2019, vol. 9, p. 1256.
Goryachev, A.A., Chernousenko, E.V., Potapov, S.S., Tsvetov, N.S., and Makarov, D.V., A Study of the Feasibility of Using Ammonium Sulfate in Copper–Nickel Ore Processing, Metals 2021, vol. 11, p. 422.
Dixon, P., Formation of Sulphamic Acid during the Thermal Decomposition of Ammonium Sulphate, Nature, 1944, vol. 154, p. 706.
Halstead, W.D., Thermal Decomposition of Ammonium Sulphate, J. Chemic. Technol. Biotechnol., 1970, vol. 20, no. 4, pp. 129–132.
Kiyoura, R. and Urano, K., Mechanism, Kinetics, and Equilibrium of Thermal Decomposition of Ammonium Sulfate, Industrial and Eng. Chemistry Process Design and Development, 1970, vol. 9, no. 4, pp. 489–494.
Thege, I.K., DSC Studies of Binary Inorganic Ammonium Compound Systems, J. Thermal Analysis, 1983, vol. 27, no. 2, pp. 275–286.
Jariwala, M., Crawford, J., and LeCaptain, D.J., In Situ Raman Spectroscopic Analysis of the Regeneration of Ammonium Hydrogen Sulfate from Ammonium Sulfate, Industrial Eng. Chemistry Res., 2007, vol. 46, no. 14, pp. 4900–4905.
Kosova, D.A., Thermodynamic Properties of Individual Substances and Phase Equilibria in Systems Based on Sulfur-Containing Ammonium Salts, Cand. Phys. Math. Sci. Thesis, Moscow, 2017.
Imideev, V.A., Study and Development of a Combined Method for Processing Nickel Sulfide Concentrates with the Production of Nickel Hydroxide, Cand. Tech. Sci. Thesis, Moscow, 2015.
Huihui, Z., Chen, J., and Deng, J., Oxidation Behavior and Mechanism of Pentlandite at 973K in Air, Metallurgical Materials Transactions, 2012, vol. 43B, pp. 494–502.
Dunn, J.G. and Kelly, C.E., A TG/MS and DTA Study of the Oxidation of Pentlandite, J. Therm Anal. Calorim., 1980, vol. 18, pp. 147–154.
Warner, T.E., Rice, N.M., and Taylor, N., An Electrochemical Study of the Oxidative Dissolution of Synthetic Pentlandite in Aqueous Media, Hydrometallurgy, 1992, vol. 31, pp. 55–90.
Lergand, D.L., Bancroft, G.M., and Nesbitt, H.W., Oxidation/Alteration of Pentlandite and Pyrrhotite Surfaces at Ph 9.3. Part 1. Assignment of XPS Spectra and Chemical Trends, Am. Mineral, 2005, vol. 90, pp. 1042–1054.
Aneesuddin, M., Char, P.N., Hussain, M.R., and Saxena, E.R., Studies on Thermal Oxidation of Chalcopyrite from Chitradurga, Karnataka State, India, J. Thermal Analysis, 1983, vol. 26, no. 2, pp. 205–215.
Prasad, S. and Pandey, B.D., Alternative Processes for Treatment of Chalcopyrite—A Review, Minerals Eng., 1998, vol. 11, no. 8, pp. 763–781.
Habashi, F., Chalcopyrite: Its Chemistry and Metallurgy, New York, McGraw-Hill, 1978.
Ponomarev, V.D. and Margulis, E.V., On the Problem of Chalcopyrite Behavior during Oxidative Roasting, Vestn. AN KazSSR, 1959, no. 11 (176), pp. 47–52.
Sahyoun, C., Kingman, S.W., and Rowson, N.A., The Effect of Heat Treatment on Chalcopyrite, Phys. Sep. Sci. Eng., 2003, vol. 12, no. 1, pp. 23–30.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Fiziko-Tekhnicheskie Problemy Razrabotki Poleznykh Iskopaemykh, 2022, No. 3, pp. 116-125. https://doi.org/10.15372/FTPRPI20220312.
Rights and permissions
About this article
Cite this article
Goryachev, A.A., Belyaevsky, A.T., Makarov, D.V. et al. Copper–Nickel Ore Processing by Low-Temperature Roasting in Mixture with Ammonium Sulfate. J Min Sci 58, 447–455 (2022). https://doi.org/10.1134/S1062739122030127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062739122030127