Abstract
The effect of different intensity of light exposure (5000 and 10 000 lux) on the growth of two strains (1 and 2) of the tea plant (Camellia sinensis L.) callus cultures and the accumulation of flavanols in them, including oligomeric forms—proanthocyanidins. The composition and content of monomeric flavanols—(+)-catechin and (–)-epicatechin in methanol extracts of cultures were studied by high-performance liquid chromatography (HPLC). Data on the morphology of calluses are presented and the greening of the strain 1 is noted, especially at high light intensity (10 000 lux). Its high ability to accumulate flavanols was established, which was 12–35 times higher than that of the strain 2. These differences depended on the age of the cultures and the intensity of light exposure. By HPLC method, monomeric flavanols—(+)-catechin and (–)-epicatechin were identified in methanol extracts of the callus strain 1, the amount of which increased at the end of the passage. Calluses of the low-producing strain 2 are characterized by the formation of only (–)-epicatechin, the amount of which did not change during their growth and did not depend on the intensity of light exposure. All this indicates significant differences in the accumulation of flavanols in the two strains of tea callus cultures and their response to light of different intensities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Light is one of the important environmental factors whose intensity and duration of exposure affects the growth and development of plants (Ruban, 2015; Paradiso and Proietti, 2022). Light activates the processes of photomorphogenesis, leading to significant changes in the structural organization of plant cells and tissues, their metabolism and functional activity (Kusnetsov et al., 2020; Teixeira, 2020). The intensity and duration of its exposure, as well as the photoperiod, are important for their metabolomics, including secondary compounds, which are the key components of the plants’ “interaction” with the environment (Salam et al., 2023).
Phenolic compounds (PCs) are one of the most common secondary metabolites in plants. They are present in almost all their organs, but in different amounts and composition (Ku et al., 2020). The most widespread and extremely structurally diverse PCs are flavonoids, the number of which exceeds 6000 (Shen et al., 2022). In aboveground organs, their accumulation is higher, and the composition is more diverse. To a certain extent, this may be a consequence of the light effect on plants. Under these conditions, the formation of primary metabolites (carbohydrates, amino acids and other compounds) necessary for the biosynthesis of PCs increases (Landi et al., 2020). There is an increase in the activity of some enzymes of phenolic metabolism, as well as the formation of chloroplasts—the main sites of their biosynthesis in the cells of green plants (Zagoskina et al., 2023).
The great interest in the study of plant PCs, including flavonoids, is due to their characteristic high antioxidant activity. It may exceed that of known antioxidants—tocopherol and ascorbic acid (Belščak-Cvitanović et al., 2018). This property of PCs is important both for maintaining the viability of plants and humans, where they enter through food chains. Currently, these compounds of secondary metabolism are considered as promising nutraceuticals and pharmacologically valuable plant metabolites that are successfully used in the treatment of many diseases (Duda-Chodak and Tarko, 2022).
Tea (Camellia sinensis L.) is among the unique plants with a high ability to accumulate PCs. The main components of its young shoots phenolic complex are catechins belonging to the class of flavonoids and exhibiting P-vitamin capillary-strengthening activity (Teixeira and Sousa, 2021). These compounds largely determine the quality of the industrial products obtained from the plant (green and black tea), which are widely used all over the world.
The ecology changing on the planet, including due to man-made impacts, as well as the potential reduction in the availability of plant raw material requires new approaches not only to preserve plant biodiversity, but also to obtain pharmacologically valuable natural metabolites. And in this case, the use of biotechnology methods, including plant cell and tissue cultures, is very promising (Wu et al., 2021). They are grown in vitro, under strictly controlled conditions and in most cases retain the ability to form specialized metabolites characteristic of an intact plant. This is also noted for callus cultures of the tea plant. They accumulated PCs, including catechins, although at a lower level compared to the initial explants (Zagoskina et al., 2003). With long-term passage of tea calluses under light conditions, the amount of these substances increased, which correlated with the formation of chloroplasts in the cells (Zagoskina et al., 2000). However, all these data reflected only the effect of light on the total content of these biologically active substances, without taking into account the balance of their individual representatives.
The aim of the work was to study the effect of different light intensities (5000 and 10 000 lux) on two strains of tea callus cultures with different PCs accumulation. This approach is important for elucidating the regulation mechanisms of the catechins accumulation at different levels in plant cells, as well as for the development/testing of light exposure conditions for obtaining pharmacologically valuable plant metabolites with P-vitamin capillary-strengthening activity by biotechnology.
MATERIALS AND METHODS
The object of the study were two strains (1 and 2) of the tea plant callus cultures (Camellia sinensis L.) of stem origin. They were grown on a Heller nutrient medium containing 2,4-dichlorophenoxyacetic acid (5 mg/L) and glucose (25 g/L) (Zagoskina et al., 2020). The duration of the passage was 45 days at 24°C, in dark conditions (the phytotron chambers of the IPR RAS). During the experiments, the callus was grown for 40 days under conditions of a 16 h photoperiod at different light intensity (5000 and 10 000 lux). The material was fixed with liquid nitrogen on the 10th and 40th days of growth and stored at –70°C (for biochemical determinations) or subjected to lyophilic drying (for PCs analysis by high-performance liquid chromatography).
Cultures morphometric parameters (appearance, color, density) were evaluated.
The water content was determined after drying the callus at 70°C to a constant weight. The calculation of this indicator was carried out according to the standard methodology (Shuyskaya et al., 2023).
To determine PCs, frozen plant material (50 mg sample) was ground in 1.5 mL of 96% ethanol (Nikolaeva et al., 2022). The homogenate was centrifuged (13 500 g, 10 min), the supernatant was separated and used for further determinations. The amount of flavanols was determined using a vanillin reagent, and the amount of proanthocyanidins was determined with a butanol reagent (Zubova et al., 2020). The flavanols content was expressed in mg-eq. epicatechin/g of dry weight, and the content of proanthocyanidins was expressed in mg–eq. cyanidine/g of dry weight.
Obtaining extracts from callus cultures and analyzing the composition of their phenolic profile by high-performance liquid chromatography (HPLC) was based on a previously approved methodological approach (Kazantseva et al., 2022). Lyophilic dried plant material (35–50 mg) was subjected to extraction of 1 mL of 80% aqueous methanol in an encapsulated glass vial at 4°C for 7 days. After that, vials with methanol extracts were placed in an ultrasonic bath for 30 min, the infusion liquid was separated and used for analysis on an Agilent Technologies chromatograph (model 1100). Chromatograph was equipped with a flow vacuum degasser (G1379A), a 4-channel low pressure gradient pump (G13111A), an automatic injector (G1313A), a column thermostat (G13116A) and a diode matrix detector (G1316A). The analysis was performed on a chromatographic column “ZORBAX” SB-C18 (2.1 × 150 mm) filled with octadecylsilyl sorbent (grain size 3.5 microns). The composition of the eluent: solution A—0.1% aqueous solution of H3PO4, solution B—90% acetonitrile. The feed rate of the mobile phase is 0.30 mL/min; gradient chromatography mode. The temperature of the column thermostat is 40°C; the sample volume is 1 µL. The parameters of the removal of the spectrum are 190–400 nm, for catechins—273 nm. The compounds were identified by comparing their spectral characteristics and retention time with similar characteristics of the standards. The content was calculated using calibration graphs of the peak area dependence on the substance concentration.
All determinations were carried out in three biological and three analytical replicates. The obtained data were statistically processed using Microsoft Excel 2010 14.0 (Redmond, WA, USA) and SigmaPlot 12.2 (Technology Networks, Sudbury, UK) software. The figures and tables show the arithmetic means ± standard deviations (±SDs). Statistical analyses of data were performed using one-way analysis of variance (ANOVA). Mean separation was performed using Normality Test (Shapiro–Wilk) and all Pairwise Multiple Comparison Procedures (Holm-Sidak method). The significant differences at p < 0.05 are denoted by different Latin letters.
RESULTS AND DISCUSSION
Morphophysiological Parameters of Tea Callus Cultures
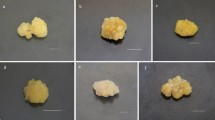
The two strains of the tea callus cultures of stem origin differed significantly in morphological characteristics. The callus of the strain 1 at the beginning of the passage (the 10th day) was compact, had a dense structure, light beige color, which by the end of the passage (the 40th day) acquired a light green color (Figs. 1a, 1b). The strain 2 was formed by large cell conglomerates. At the beginning of the passage, it had a white-yellow color, which acquired a beige toneby the end of the passage (Figs. 1d, 1e).
Consequently, when growing the strain 1 in light conditions, apparently, the formation of chloroplasts in cells took place and as a result, this was reflected in the greening of calluses. The presence of these organelles was also noted when growing Stevia rebaudiana suspension in the light (Bondarev et al., 2019). The strain 2 showed no pronounced changes in the color of the callus, which indirectly indicates the “loss” of their ability to form chloroplasts. Such a reaction may be a consequence of genetic and epigenetic changes in this strain, which was also noted for other in vitro cultures (Bednarek and Orłowska, 2020).
At the same time, the morphology of the calluses of the strains 1 and 2 grown at different intensities of light exposure was almost the same at the end of the passage (Figs. 1b, 1c, 1e, 1f). Perhaps this is due to a short period of its exposure (one passage).
The next stage of our work was to compare the growth of these strains (Table 1). In both cases, it had fairly similar values. At the same time, in calluses grown at 10 000 lux, the increase in callus mass (by raw weight) slightly, but significantly, exceeded that at 5000 lux. Consequently, increased doses of light exposure, although they did not cause changes in the morphology of callus, contributed to their better growth. The positive effect of increased light exposure doses was also noted for the growth of Stevia rebaudiana cell cultures (Bondarev et al., 2019).
The water content determination of the callus cultures showed higher values in the strain 2 relative to the strain 1 (Table 1). This is also consistent with the data on their morphology, when the strain 2 had a lower density of callus (see Fig. 1). At the same time, the water content in the callus of each strain was constant at different stages of their growth and under light influences. This indicates the stability of this physiological parameter in in vitro tea cultures, which is important for maintaining their viability (Adil et al., 2019).
Flavanol Content in Tea Callus Cultures
The main components of the tea plants’ phenolic complex, as well as of the in vitro cultures initiated from them, are flavanols (Zubova et al., 2020; Xiang et al., 2021). The determination of their content showed that in the strain 1 it exceeded that of the strain 2 by 12–35 times, depending on the age of the culture and the intensity of light exposure (Fig. 2). For the strain 2, the formation of these PCs is also characteristic, but at a very low level. Perhaps these differences are a consequence of the well-proliferating cells selection at the initial stages of its passivation. This led to significant changes in their metabolism, as is typical for many in vitro cultured cells and tissues (Bednarek and Orłowska, 2020).
The study of the content of flavanols in the tea callus strain 1, grown at different light intensity, showed its increase with growth (Fig. 2a). So, at the beginning of the passage (the 10th day) this indicator was significantly higher in callus grown at 5000 lux, exceeding by 15% that at 10 000 lux. By the end of the passage (the 40th day), the flavanols content in the callus increased significantly: almost 2 times at 5000 lux and 3 times at 10 000 lux. Consequently, high-intensity light had a more pronounced effect on the accumulation of these secondary metabolites in calluses of the strain 1 at the end of the passaging cycle. As for the strain 2, the amount of flavanols in all variants was almost equal. This indicates that this one has no response to light exposure in relation to their biosynthesis. Perhaps this is due to its low activity, and is also a consequence of weak cell differentiation, including the absence of chloroplasts—one of the centers of PCs biosynthesis in plant cells (Zaprometov and Nikolaeva, 2003; Zago-skina et al., 2023).
Composition and Content of the Flavanols in Tea Callus Cultures
Determination of the flavanols content in tea callus culture does not allow to characterize the balance of their individual representatives. These include the so-called simple flavanols—(+)-catechin and (–)-epicatechin. We have previously shown that these metabolites dominate in tea callus cultures (Ossipov et al., 2022). The formation of (+)-catechin and (–)-epicatechin is also characteristic of intact plants (Zhang et al., 2020). It is known that these monomers of a flavanol nature are formed at the final stages of polyphenol biosynthesis and serve as precursors of their oligo- and polymer forms—proanthocyanidins (Dixon and Sarnala, 2020). In this regard, the assessment of the simple catechins “balance” in in vitro tea cultures is of great interest. This possibility is provided by the use of the HPLC method. Its application allows not only to study the composition of these PCs, but also to assess their content, which was the subject of our further research.
According to the HPLC chromatography data, (+)-catechin and (–)-epicatechin were present in methanol extracts of tea calluses. At the same time, the peaks corresponding to these compounds in strain 1 were well expressed in comparison with those in the strain 2 (Fig. 3).
The HPLC chromatograms (Base Peak Chromatogram, 273.8 nm) of methanol extracts of the tea callus cultures strain 1 (a) and strain 2 (b), grown for 40 days at a light exposure intensity of 5000 lux. The abscissa axis—the retention time, min; the ordinate axis—the detector signal, units of optical density. Identified compounds: (–)-epicatechin (1), (+)-catechin (2) and gallic acid (3).
In calluses of the strain 1, the amount of (–)-epicatechin significantly exceeded the amount of (+)-catechin (Fig. 4). The highest accumulation of these monomers was observed on the 40th day of the culture growth at 5000 lux. In this case, the content of (+)-catechin and (–)-epicatechin exceeded that of the earlier growth period (the 10th day) by 2.5 and 3.5 times, respectively. This indicates a significant activation of the (–)-epicatechin biosynthesis as the callus grows at an illumination intensity of 5000 lux. As for the growth of this strain at a higher light intensity (10 000 lux), in most cases the flavanol monomers content was almost equal to that in callus grown at 5000 lux. The exception concerned (–)-epicatechin, the accumulation of which increased 2.5 times on the 40th day of growth, that is, less than in conditions of lower light intensity.
It is possible that the cultivation of the strain 1 at high intensity of light exposure, when their greening and the formation of chloroplasts in cells were noted, led to changes in the PCs biosynthesis. In this case, a decrease in the flavanol monomers content may be a consequence of the formation (new formation) of other compounds of a phenolic nature, namely flavonols. These may include derivatives of kaempferol and quercetin, which biosynthesis is associated with the functioning of chloroplasts in cells (Zaprometov and Nikolaeva, 2003). This is also confirmed by HPLC data indicating the kemferol-3-O-rutinoside formation in the strain 1 (Fig. 5). Its amount per 10 days of the culture growth at 5000 and 10 000 lux was 0.03 and 0.05 g/kg dry weight, respectively, and by the 40th day it increased in 3 times and reached 0.11 and 0.14 g/kg of dry weight. These changes in the PCs composition are to some extent consistent with a decrease in the flavanol monomers accumulation in callus culture of the strain 1.
The HPLC chromatograms (Base Peak Chromatogram, 350 nm) of methanol extracts of the tea callus cultures strain 1 grown for 40 days at a light exposure intensity of 5000 lux. The abscissa axis—the retention time, min; the ordinate axis—the detector signal, units of optical density.Identified compound: kaempferol-3-O-rutinoside.
As noted above, the low-productive strain 2 with respect to the flavanols formation had no changes in their accumulation under the action of light of varying intensity. The formation of flavonols is also not typical for this culture (data are not provided). All this indicates significant differences in the biosynthesis of PCs in the studied strains of the tea callus cultures. These differences may be the result of distinctions in their epigenetic and genetic characteristics, as well as the intracellular level of their differentiation, which is important for metabolomics (Bednarek and Orłowska, 2020; Wu et al., 2021).
Content of Proanthocyanidins in Tea Callus Cultures
The oligo- and polymer forms of PCs include proanthocyanidins (Dixon and Sarnala, 2020). Their formation is characteristic not only for tea plants, but also for callus cultures initiated from them (Ossipov et al., 2022). Despite significant advances in the study of PCs biosynthesis, very little is still known about the proanthocyanidins formation and the regulation of this process. We have analyzed the accumulation of these PCs in two strains of the tea callus cultures grown at different light intensity (Fig. 6). Their level was quite high in the strain 1, unlike the strain 2, where they were not detected. The absence of these phenolic polymers in the cells of the strain 2 correlated with a low accumulation of catechins which are necessary for the formation of flavan nature polymers in them (Lu et al., 2022).
As for the strain 1, for 10 days of growth, theproanthocyanidins content in callus grown at 5000 lux was 35% higher than that in callus grown at 10 000 lux. Based on these data, it can be assumed that at high intensity of light exposure, the polymerization processes of flavanols in tea callus occur at a slower rate compared to those at lower illumination. This is consistent with the thesis of other authors about the “flexibility” of the proanthocyanidin-specific branch of flavonoid biosynthesis, including the chemical condensation of these metabolites (Lu et al., 2022).
As the strain 1 continued to grow under light conditions, the accumulation of proanthocyanidins on the 40th day exceeded that on the 10th day by 40% at 5000 lux and by 1.5 times at 10 000 lux. The consequence of these changes is the greatest number of these metabolites at the end of the passage. Based on these data, it can be assumed that prolonged exposure to high-intensity light activates the biosynthesis of proanthocyanidins. Probably, under these conditions, the polymerization process of monomeric flavanols (catechins) increases, which leads to the accumulation of their oligo- and polymer forms. Another trend can also be considered, namely, a significant activation of PCs biosynthesis under these conditions, the high content of which can have a negative effect on the vital functions of plant cells and even cause their death (Singh et al., 2021). The reduction of their toxic effect can be achieved by the formation of PCs oligo- and polymers, in particular proanthocyanidins, which are transported to the vacuole. So, in this case, they do not have a negative effect on the metabolism of plant cells, and herewith theones can be used in the future as “reserve” energy-valuable metabolites (Lu et al., 2022).
CONCLUSIONS
Light is an important and necessary factor for the growth of plant cells and their functional activity. This also concerns the accumulation of various secondary metabolites. These include PCs, which have high antioxidant activity and are successfully used in medicine. Changing the ecology of our planet, reducing plant biodiversity and limiting available plant sources, contributes to the production of in vitro cultured cells and tissues as potential producers of valuable metabolites. Tea plants (Camellia sinensis L.) are characterized by a unique ability to accumulate such PCs as flavanols for which high P-vitamin capillary-strengthening activity is noted. The callus cultures obtained from them retained the ability to form these metabolites, although at a lower level. Our studies have shown the possibility of regulating the accumulation of both monomeric and oligomeric forms of flavanols in tea calluses grown at different light intensity (5000 and 10 000 lux). Morphophysiological characteristics of calluses, including the differentiation level, as well as the initial content of PCs, are important in their response. Thus, the low intracellular differentiation characteristic of one of the studied strains correlated with a small amount of flavanols in it, the absence of their oligomeric forms—proanthocyanidins and an insignificant reaction to light exposure. A different trend is characteristic of thetea callus high-productive strain, in which the formation of chloroplasts was noted when exposed to light and this process was more pronounced at its high intensity (10 000 lux). Using different intensity and duration of light exposure to tea calluses (up to 40 days), it is possible to regulate directly the accumulation of various compounds of flavanol nature in them (monomers, oligo- and polymers). Such studies are important for solving fundamental issues on the regulation of PCs biosynthesis, as well as the practical production of these unique plant metabolites with antioxidant properties by biotechnology methods.
REFERENCES
Adil, M., Ren, X., and Jeong, B.R., Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino, J. Photochem. Photobiol. B: Biol., 2019, vol. 196, p. 111509.
Bednarek, P.T. and Orłowska, R., Plant tissue culture environment as a switch‑key of (epi)genetic changes, Plant Cell, Tissue Organ Cult., 2020, vol. 140, pp. 245–257.
Belšcak-Cvitanovic, A., Durgo, K., Hudek, A., Bacun-Družina, V., and Komes, D., Overview of polyphenols and their properties, in Polyphenols: Properties, Recovery, and Applications, Galanakis, C.M., Ed., Amsterdam: Elsevier, 2018, pp. 3–44.
Bondarev, N., Reshetnyak, O., Bondareva, T., Il’in, M., and Nosov, A., Impact of cultivation factors in vitro on the growth and the biosynthesis of steviol glycosides in Stevia rebaudiana cell cultures, Physiol. Mol. Biol. Plants, 2019, vol. 25, pp. 1091–1096.
Dixon, R.A. and Sarnala, S., Proanthocyanidin biosynthesis—a matter of protection, Plant Physiol., 2020, vol. 184, pp. 579–591.
Duda-Chodak, A. and Tarko, T., Possible side effects of polyphenols and their interactions with medicines, Molecules, 2023, vol. 28, p. 2536.
Kazantseva, V.V., Goncharuk, E.A., Zagoskina, N.V., Zaitsev, G.P., and Klykov, A.G., Plant ploidy level and the presence of cadmium in the growing environment changes the content of the main components of the phenolic complex in buckwheat sprouts, Dokl. Biochem. Biophys., 2022, vol. 502, pp. 10–14.
Ku, Y.S., Ng, M.S., Cheng, S.S., Lo, A.W., Xiao, Z., Shin, T.S., Chung, G., and Lam, H.M., Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption, Nutrients, 2020, vol. 12, p. 1717.
Kusnetsov, V.V., Doroshenko, A.S., Kudryakova, N.V., and Danilova, M.N., Role of phytohormones and light in de-etiolation, Russ. J. Plant Physiol., 2020, vol. 67, pp. 971–984.
Landi, M., Zivcak, M., Sytar, O., Brestic, M., and Allakhverdiev, S.I., Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: a review, Biochim. Biophys. Acta, Bioenerg., 2020, vol. 861, no. 2, p. 148131.
Lu, N., Jun, J.H., Liu, C., and Dixon, R.A., The flexibility of proanthocyanidin biosynthesis in plants, Plant Physiol., 2022, vol. 190, no. 1, pp. 202–205.
Ossipov, V., Zubova, M., Nechaeva, T., Zagoskina, N., and Salminen, J.P., The regulating effect of light on the content of flavan-3-ols and derivatives of hydroxybenzoic acids in the callus culture of the tea plant, Camellia sinensis L., Biochem. Syst. Ecol., 2022, vol. 101, p. 104383.
Paradiso, R. and Proietti, S., Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: the state of the art and the opportunities of modern LED systems, J. Plant Growth Regul., 2022, vol. 41, no. 2, pp. 742–780.
Salam, U., Ullah, S., Tang, Z.H., Elateeq, A.A., Khan, Y., Khan, J., Khan, A., and Ali, S., Plant metabolomics: An overview of the role of primary and secondary metabolites against different environmental stress factors, Life, 2023, vol. 13, p. 706.
Shen, N., Wang, T., Gan, Q., Liu, S., Wang, L., and Jin, B., Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity, Food Chem., 2022, vol. 383, p. 132531.
Shuyskaya, E., Rakhmankulova, Z., Prokofieva, M., Kazantseva, V., and Lunkova, N., Impact of salinity, elevated temperature, and their interaction with the photosynthetic efficiency of halophyte crop Chenopodium quinoa Willd., Agriculture, 2023, vol. 13, no. 6, p. 1198.
Singh, S., Kaur, I., and Kariyat, R., The multifunctional roles of polyphenols in plant-herbivore interactions, Int. J. Mol. Sci., 2021, vol. 22, no. 3, p. 1442.
Teixeira, R.T., Distinct responses to light in plants, Plants, 2020, vol. 9, vol. 7, p. 894.
Teixeira, A.M. and Sousa, C.A., Review on the biological activity of Camellia species, Molecules, 2021, vol. 26, p. 2178.
Wu, T., Kerbler, S.M., Fernie, A.R., and Zhang, Y., Plant cell cultures as heterologous bio-factories for secondary metabolite production, Plant Comm., 2021, vol. 2, p. 100235.
Xiang, P., Zhu, Q., Tukhvatshin, M., Cheng, B., Tan, M., Liu, J., Wang, X., Huang, J., Gao, S., Lin, D., Zhang, Y., Wu, L., and Lin, J., Light control of catechin accumulation is mediated by photosynthetic capacity in tea plant (Camellia sinensis), BMC Plant Biol., 2021, vol. 21, no. 1, pp. 1–13.
Zagoskina, N.V., Dubravina, G.A., and Zaprometov, M.N., The specific features of chloroplasts and phenolic compound accumulation in photomixotrophic tea callus cultures, Russ. J. Plant Physiol., 2000, vol. 47, no. 4, pp. 468–473.
Zagoskina, N.V., Zubova, M.Y., Nechaeva, T.L., Kazantseva, V.V., Goncharuk, E.A., Katanskaya, V.M., Baranova, E.N., and Aksenova, M.A., Polyphenols in plants: structure, biosynthesis, abiotic stress regulation, and practical applications (review), Int. J. Mol. Sci., 2023, vol. 24, p. 13874.
Zaprometov, M.N. and Nikolaeva, T.N., Chloroplasts isolated from kidney bean leaves are capable of phenolic compound biosynthesis, Russ. J. Plant Physiol., 2003, vol. 50, pp. 623–626.
Zhang, Q., Li, T., Wang, Q., LeCompte, J., Harkess, R.L., and Bi, G., Screening tea cultivars for novel climates: Plant growth and leaf quality of Camellia sinensis cultivars grown in Mississippi, United States, Front. Plant Sci., 2020, vol. 11, p. 280.
Zubova, M.Y., Nechaeva, T.L., Kartashov, A.V., and Zagoskina, N.V., Regulation of the phenolic compounds accumulation in the tea-plant callus culture with a separate and combined effect of light and cadmium ions, Biol. Bull. (Moscow), 2020, vol. 47, pp. 593–604.
Funding
This research was supported by Russian Science Foundation: “Conducting Basic Scientific Research and exploratory scientific research by small individual scientific groups,” grant no. 23-24-00359.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This article does not contain any studies involving animals or human participants performed by any of the authors.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zagoskina, N.V., Zubova, M.Y., Aksenova, M.A. et al. The Effect of the Light Intensity on the Growth and Accumulation of Monomeric and Oligomeric Flavanols in Callus Cultures of Camellia sinensis L.. Biol Bull Russ Acad Sci 50 (Suppl 3), S373–S381 (2023). https://doi.org/10.1134/S1062359023604901
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359023604901