Abstract
In Camellia japonica callus culture, the effects of light quality on the accumulation of phenolic compounds were examined. To this end, the calli were cultured under three monochrome [white (W), red (R), and blue (B)] and two mixed [red/blue (RB) and red/green/blue (RGB)] light sources. After 4 weeks of culture, fresh and dry callus weights were determined, and phenolic content was analyzed using high-performance liquid chromatography. We also examined the effects of RGB light on calli derived from four origins (leaf: CL; root: CR; petal: CP; and ovary: CO). Total phenolic and flavonoid content and DPPH radical scavenging activity were highest in calli exposed to RB and RGB. Catechin was detected only in calli exposed to mixed light (RB and RGB). Interestingly, red pigmentation development in CL and CR calli after 2 weeks of culture and organogenic calli in CR and CP samples was observed. Furthermore, expression of the phenylalanine ammonia lyase and chalcone synthase genes was found to be higher in the CR line than in other lines, and total phenolic and flavonoid content was also higher in the CR and CP lines. The findings of the present study revealed that the accumulation of phenolics and flavonoids in callus tissue varies depending on light quality, which stimulates cell division, and may thus affect the proliferation and organogenesis of C. japonica callus. We also established that the effects of light on red pigment expression and the accumulation of bioactive compounds are dependent on the tissue origin of callus material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camellia japonica (Theaceae) is an evergreen tree distributed in East Asia, where it is mainly grown for ornamental purposes (Li et al. 2016). The twigs, leave, and fruits of camellia contain catechin, epicatechin, cyanidin, and other bioactive compounds, and recent studies have demonstrated the antioxidant activity and efficacy of these compounds such as antiatherogenic effect (Lee et al. 2016; Chen et al. 2018; Páscoa et al. 2019). However, under field cultivation, the content of various C. japonica metabolites can differ markedly depending on the season, and the cultivation area is generally limited (Karuppusamy 2009; Li et al. 2016). To date, most of the studies that have examined the in vitro culture of C. japonica have tended to focus on regeneration via somatic embryogenesis (Vieitez et al. 1989; Vieitez and Barciela 1990; Barciela and Vieitez 1993). Different with C. japonica (camellia), many studies have been reported to produce useful bioactive substances in tea tree (C. sinensis) belonging to the same family with camellia (Zagoskina et al. 2003; Zagoskina et al. 2005; Wang et al. 2012). In particular, Zagoskina et al. (2003, 2005) reported that UV stimulates photosystem II (PSII) activity of phototrophic cells to increase photosynthesis-related photochemical activity when UV is irradiated under white light during the callus incubation period of C. sinensis.
Important among the range of strategies that can be employed to enhance the production of useful metabolites is the selection of callus lines containing high amounts of bioactive compounds, which is known to be influenced by variety, genotype, and explant source (Yue et al. 2016). For example, in Tecoma stans and Linum usitatissimum, it has been demonstrated that the content of total polyphenols and flavonoids can vary depending on the source of the explant from which callus is induced, with the highest amounts being detected in the callus induced from the stems of these plants (López-Laredo et al. 2009; Anjum et al. 2017).
Light quality is one of the critical factors affecting plant growth, development, and morphological characteristics (Fukuda et al. 2008), with light of different spectral properties stimulating different morphological and physiological responses (Batista et al. 2018). Although numerous studies have focused on the effect of light on photosynthesis, recent studies have reported that light also has an influence on the content of bioactive compounds (Miao et al. 2016; Llorente et al. 2017) and antioxidant enzyme activities (Carvalho et al. 2016; Qian et al. 2016; Yu et al. 2017) in plants including in vitro cultures (Bello-Bello et al. 2016; Pedroso et al. 2017). Light in the short wavelength region of the spectrum, such as blue and ultraviolet, has been shown to enhance the expression of genes associated with the flavonoid pathway and the content of bioactive compounds in a number of plant species, for example, blue light increased polyphenol contents and the total antioxidant status in lettuce (Johkan et al. 2010; Zoratti et al. 2014), and several phytochemicals and antioxidant enzyme activities in Rehmannia glutinosa (Manivannan et al. 2015). Furthermore, in the in vitro culture of cells and adventitious roots of Eleutherococcus senticosus, illumination with red light (660 nm) has been found to increase the content of bioactive compounds and activity of antioxidant enzymes (Shohael et al. 2006).
Although previous studies have examined the effects of light quality on the bioactive compound content of plants, few studies, with the exception of those on Stevia rebaudiana and Prunella vulgaris (Ahmad et al. 2016; Fazal et al. 2016), have focused on the effects of light quality on callus culture with respect to the production of useful metabolites. In stevia callus culture, blue light enhanced total phenolics and flavonoids; Ahmad et al. (2016) suggested that colored light can be promising to increase antioxidant secondary metabolites in callus cultures. However, to the best of our knowledge, there have been no studies that have investigated the production of secondary metabolites during the in vitro culture of camellia including callus and intact plants.
In this study, we investigated the effect of light quality on callus proliferation and the accumulation of phenolic compounds in camellia callus, and also examined the effect of explant origin on phenolic content and antioxidant activity in calli derived from four different explant sources.
Materials and Methods
Plant Materials
Around 5-year-old C. japonica tree was grown in greenhouse, and for callus induction culture, four explants (leaf, root, petal, and ovary) were dissected from the mother tree. For the surface sterilization, explants were quickly immersed in 70% ethanol for several seconds, rinsed with sterile deionized water, and immersed in 2% (v/v) sodium hypochlorite solution supplemented with three to four drops of Tween-20 for 20 min. After three times rinse in sterile deionized water, explants were cultured on Murashige and Skoog (MS) medium (Murasnige and Skoog 1962) supplemented with 26.85 μM 1-naphthaleneacetic acid (NAA), 0.44 μM 6-benzylaminopurine (BA), 100 mg L−1 casein hydrolysate, 100 mg L−1 citric acid, 100 mg L−1 l-ascorbic acid, 30 g L−1 sucrose, and 2.4 g L−1 gelrite at 24 ± 1°C under dark conditions. The calli were maintained on the same medium for a year and used in this experiment. Masses of callus were plated in petri-dishes (five 250-mg masses per dish), and the four petri-dishes were cultured for the replication in each treatment. After 4 weeks of culture, the callus growth was measured with fresh and dry weight, and the organ (roots and/or shoots) emergence and pigmentation was examined under an SMZ-U stereomicroscope (Nikon, Japan). The organogenic callus was counted that the callus contains root or shoot primordia, and for the organ regeneration, the callus which produced shoots and/or roots longer than 3 mm. Those calli numbers were divided by the total inoculated calli mass per treatment and multiplied by 100 to represent percent data.
The content of phenolic compounds and activities of antioxidant compounds were analyzed. Additionally, we examined the expression of genes related with phenolic compound metabolic pathways in callus.
Light Quality
To investigate the effect of light quality on the stimulation of secondary metabolite synthesis in C. japonica calli, we used LED light sources (PLCC 5450 6pin; Itswell Co., Incheon, Korea). At first, calli were placed under six different light sources: red (R), blue (B), red1/blue1 (RB), red1/green1/blue1 (RGB), white (W), and dark (D) for control. In the second experiment, 4 different origins of calli were placed and cultured under RGB. In both experiments, the wavelength of the light is as follows; R 645–675 nm, blue 440–460 nm, green 530–550 nm, and white 430–640 nm. The cultures maintained under around 65–75 μmol m−2 s−1 light intensity with a 16-h photoperiod at 24 ± 1°C for 4 weeks. Masses of callus were plated in petri-dishes (five 250-mg masses per dish), and the four petri-dishes were cultured for the replication in each treatment.
Flow Cytometry
Cell division and DNA content was analyzed using flow cytometry. Samples used for analysis were prepared from freshly harvested callus (50-mg fresh weight) after 2 weeks of culture. Cell division was analyzed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA) after staining with propidium iodide (PI 05-5022; Sysmex, Lincolnshire, IL). For each cell cycle analysis, measurements were performed on at least three replicates. DNA index and cell division data were determined using the Cytexpert program (Beckman Coulter).
Analysis of Gene Expression Related to Phenolic Biosynthesis
Aliquots of fresh callus (0.1 g) were collected from the culture medium and frozen with liquid nitrogen. Total RNAs from the samples were extracted using a RNeasyⓇ Plant Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions, and treated with RNaseZap (Ambion, Austin, TX) to remove any residual genomic DNA. For cDNA synthesis, total RNAs were reverse transcribed using a ReverTra Ace qPCR RT Master Mix (Toyobo Co., Osaka, Japan). For real-time polymerase chain reaction (qPCR: CFX96 touch™ Real-Time qPCR Detection System; Bio-Rad Laboratories, Inc., Hercules, CA), we used 2 μL cDNA samples in conjunction with the specific primers (Table 1). The PCR conditions used to amplify the cDNA were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 15 s, 55°C for 20 s, and 72°C for 30 s, with a final extension at 72°C for 10 min. For normalization, C. sinensis actin was used as an external standard, and the amplified DNA products were electrophoretically separated on a 1.5% agarose gel for visualization.
Analysis of Phenolic Compounds Extraction
Samples of callus used for analysis of phenolic compounds were dried by freezing-drying in the light quality experiment and oven-dried in the RGB light experiment. The dried material was ground in a sterilized mulberry bowl, and 250-mg aliquots of the resulting powered dry calli were soaked in 20 mL 80% methanol at 24°C for 1 h with sonication (LS-2050-S10; LS-Tech, Gwangju, Korea). The sonicated material was then centrifuged twice at 10,000 rpm for 10 min each, and the collected supernatant was filtered through filter paper (Advantec, 110 mm, Tokyo, Japan; Toyo Roshi Kaisha Ltd., Tokyo, Japan).
Determination of Total Phenolic Compound Content
Total phenolic content was determined using the Folin-Ciocalteu colorimetric method (Folin and Ciocalteu 1927). Methanolic extracts (0.05 mL) were mixed with distilled water (2.55 mL), followed by the addition of Folin-Ciocalteu reagent (0.1 mL, 2 N). After 5 min, the reaction mixture was combined with 2.5 mL Na2CO3 solution (20%) and incubated in the dark at room temperature. The absorbance (change in color) at 760 nm after 30 min was measured using an Optizen POP spectrophotometer (Mecasys Co.). Concentrations were determined from a gallic acid standard curve (Sigma Chemical Co., St. Louis, MO), and total phenolic content was expressed in terms of milligrams of gallic acid equivalent per gram dry callus weight.
Determination of Total Flavonoid Content
Total flavonoid content was determined using the colorimetric method described by Wu et al. (2006). Methanolic root extracts and a (+)-catechin standard (0.25 mL) (Sigma Chemical Co.) were prepared in 1.25 mL distilled water, to which was added 0.075 mL of 5% NaNO2, followed by vigorous shaking. After 6 min, 0.15 mL of AlCl3 solution (10%) was added to the sample, followed by incubation for 5 min at room temperature. Absorbance at 510 nm was measured using an Optizen POP spectrophotometer (Mecasys Co., Daejeon), and the results were expressed as milligrams of (+)-catechin equivalents per gram callus dry weight.
High-Performance Liquid Chromatography Determination of Individual Phenolic Compounds
Samples of powdered callus material (0.25 g) were sonicated (SD-250H, SD‐ULTRASONIC CO., Seoul, Korea) for 1 h in 80% methanol to ensure complete extraction. The extract was filtered through filter paper (Advantec, 110 mm), and the solvent was evaporated. The dried residue was dissolved in 10% methanol and fractionated twice with 10 mL diethyl-ether/ethyl-acetate (1:1) prior to evaporation to dryness under. The residues of both fractions were combined and dissolved in methanol prior to filtration through a membrane filter (0.2-μm pore size; Whatman, Maidstone, UK). A photodiode array (PDA)–equipped HPLC system (2690 Separations Module, Waters Chromatography, Milford, MA) was used to analyze the phenolic compounds. Separation was performed using a Fortis C18 column (5 μL, 150 × 4.6 mm). Acetonitrile (A) and 0.1% aqueous acetic acid (v/v) (B) were used as the mobile phase, with a linear gradient of 8–10% A at 0–2 min, 10–30% A at 2–27 min, 30–90% A at 27–50 min, 90–100% A at 50–51 min, 100% A at 51–60 min, and 100–8% A at 60–70 min. The column was re-equilibrated for 10 min between injections at a 1.0 mL min−1 flow rate, and 20-μL aliquots were injected into the HPLC for each analysis. Calibration plots were obtained by measuring the peak areas. UV absorption spectra and retention times were used as criteria for the identification of individual compounds.
Analysis of Free Radical Scavenging (DPPH Activity)
The antioxidant capacity of callus phenolics was assessed using the 1,1-diphenyl-2-picrylhydrazyl (DPPH: Sigma Chemical Co.) method described by Hatano et al. (1998), with some modification. DPPH radical solution (0.8 mL 200 μM DPPH) was added to 0.2 mL of methanolic sample extract, with a 40% methanol solution serving as a control. The solution thus prepared was incubated for 5 min at room temperature, and absorbance at 517 nm was measured using an Optizen POP spectrophotometer (Mecasys Co.). The scavenging percentage for the antioxidant capacity was calculated as follows:
Statistical Analysis
For the statistical analysis, one-way analysis of variance (ANOVA) was used to determine the significance of differences among the mean of groups. Statistical assessments of the difference between mean values were then performed using Duncan’s multiple range test. A P value of 0.05 was considered to indicate statistical significance, and all data were analyzed using SAS software (SA 9.4; SAS Institute Inc., Cary, NC).
Results and Discussion
Effect of Light Quality on Cell Proliferation and Phenolic Compounds
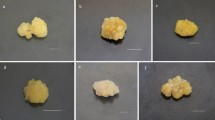
After 4 weeks of callus culture under the five different light treatments, the highest callus fresh weight was obtained in the dark control treatment (D) (Fig. 1). Among the different light treatments, callus fresh weight was higher in material exposed to monochrome R (1.20 g∙mass−1) and mixed RB (1.15 g∙mass−1) illumination than in calli subjected to other light treatments, which represented 2.1- and 2.0-fold increases, respectively, compared with the white light control treatment (W). With the exception of treatment D, dry weight was also highest in callus exposed to red light, followed by RB, RGB, and B. These observations are consistent with the findings of previous studies, which have reported that that red light (610–760 nm) is effective in promoting biomass accumulation, shoot elongation, root development, and organ regeneration in different species (Sivakumar et al. 2006; Daud et al. 2013; Kwon et al. 2015). Crosstalk between red light and cytokinin activity has also been described by To et al. (2004), who demonstrated the involvement of the A-type Arabidopsis response regulator (ARR) 4 gene in this process. Similarly, Osakabe et al. (2002) found that cytokinin-inducible genes were also overexpressed in plants overexpressing the A-type ARR gene in Arabidopsis. Moreover, increased expression of A-type ARR4 has been observed to promote cell division and increase shoot formation (Osakabe et al. 2002; Riefler et al. 2006).
Effects of light quality on callus proliferation in C. japonica. (a) Fresh and dry weight (D: dark, W: white, R: red, B: blue, RB: red1/blue1, RGB: red1/green1/blue). (b) Callus after 4 weeks of culture. (a) W, (b) RGB, and (c) red pigment expression under RGB (white scale bar = 1 cm, black scale bar = 1 mm). Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range test (n = 10)
In the present study, we also detected the initial development of red pigmentation on the surface of callus after 15 d of culture under R, RB, and particularly RGB light (Fig. 1b). Red light has similarly been reported to be involved in red pigment accumulation in strawberries and peas (Wu et al. 2007; Miao et al. 2016), whereas numerous studies have also reported that red pigment is expressed when plants are exposure in blue light (Johkan et al. 2010; Kadomura-Ishikawa et al. 2013; Xu et al. 2014),
The total phenol and flavonoid content of calli was investigated after 4 weeks of photoperiod treatment (Fig. 2a). We found that the total phenolic content was highest in calli subjected to the RB mixed light treatment [9.06 mg g−1 dry weight (DW)], followed by the RGB mixed light treatment (6.03 mg g−1 DW) and control (dark) (5.21 mg g−1 DW). In all calli exposed to the different light treatments, total flavonoid content was higher than in the control calli, with levels being the highest in those exposed to RB (4.87 mg g−1 DW) and RGB (4.45 mg g−1 DW) light.
Effect of light quality on content of total phenolics and flavonoids in C. japonica calli after 4 wk of culture. (a) Content of total phenolics and flavonoids. (b) Free radical scavenging activity determined using DPPH (D: dark, W: white, R: red, B: blue, RB: red1/blue1, RGB: red1/green1/blue). Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range test (n = 4).
Our analysis of the DPPH radical scavenging activity in calli revealed that the highest activity (82.4%) was obtained in calli exposed to RB mixed light (Fig. 2b). The activity was higher under RGB mixed light treatment (58.4%) and red light (34.1%) than that in calli cultured in the dark (30.5%), whereas the lowest DPPH radical scavenging activities were detected in calli cultured under single white (23.2%) and blue (25.0%) lights.
When plants are exposed to light, reactive oxygen species (ROS) are produced via the activity of cytochrome P450, particularly in response to illumination with blue light (Darko et al. 2014; Lobiuc et al. 2017). Furthermore, in a study on cucumber, Wang et al. (2010) detected increases in the levels of H2O2 and salicylic acid and the expression level of phenylalanine ammonia-lyase (PAL) and polyphenoloxidase in response to red light irradiation, and that plants show short- and long-term responses associated with the elimination of ROS, which involves the production of phenolics such as caffeic acid and rosmarinic acid (Lobiuc et al. 2017). Batista et al. (2018) reported that blue light plays a role in regulating microtubule synthesis-related genes and secondary metabolite synthesis-related genes, and that red light and green light are also closely associated to the expression of defense-related genes. Moreover, in lettuce, blue light has been demonstrated to enhance the content of antioxidant polyphenols, anthocyanins, and carotenoids (Li and Kubota 2009; Johkan et al. 2010), whereas Li and Kubota (2009) reported an increase in phenolic compounds in lettuce illuminated with red light.
On the basis of the findings of these studies, we can therefore assume that camellia callus produces larger amounts of phenolics when exposed RB and RGB, as a consequence of defense-related mechanisms.
Characteristics of Calli Cultured from Explants Derived from Multiple Origins Under RGB Light
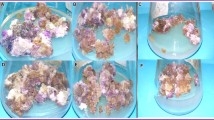
After 4 weeks of culture under RGB light, higher callus fresh weight was obtained in the three lines derived from root, petal, and ovary tissue (CR, CP, and CO), without any significant difference, whereas callus derived from leave material (CL) had a comparably lower fresh weight. Conversely, along with CR, dry weight was found to be higher in CL calli (77.5–78.3 mg∙mass−1) (Fig. 3), and these calli were also observed to be characterized by high organ differentiation. Interestingly, we observed the most pronounced development of red pigmentation (70%) on the surfaces of CL and CR calli with the highest dry weights (Fig. 4). In contrast, CR (66.7%) and CP (93.3%) lines were characterized by predominantly greenish calli.
Effect of RGB (red1/green1/blue1) light on proliferation of C. japonica calli derived from 4 different origins after 4 wk of culture. (a) Fresh and dry weight (n = 10). (b) Percentage of red pigmented calli (n = 4). (c) Percentage of organogenic callus (n = 4). (d) Organ regeneration (CL leaf-induced callus, CR root-induced callus, CP petal-induced callus, CO ovary-induced callus) (n = 4). Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range test.
Given that the levels or responses of endogenous hormones are often organ dependent, the source of explants is considered to be an important factor with respect to callus proliferation and organogenesis (Piovan et al. 2010). In this regard, different levels of endogenous auxin in calli derived from different organs may influence pigment accumulation in callus under different light conditions, and Ji et al. (2015) reported that anthocyanin, one of the red pigments, accumulates via the transcription of anthocyanin biosynthesis-related genes in response to a reduction in auxin content. Moreover, it has been reported that stem-derived callus accumulates larger amounts of anthocyanins than leaf-derived callus in Crataegus sinaica (Maharik et al. 2009). Therefore, we proposed that the observed differences in pigment expression can be attributed to differences in the auxin sensitivity of callus derived from different origins.
In the present study, we observed that of the calli derived from four explants’ different origins, CP calli showed a high percentage of cells in the G2M phase of the cell cycle (Fig. 5a), which indirectly supports our assumption that CP was an organogenic callus, as shown in Fig. 3, as usually organogenic callus contains meristematic cells. We also detected variations in DNA content depending on callus origin (Fig. 5b), with the other three lines showing values ranging between 1.7 and 1.9 when the index value of the CP line was set at 1.0. (data not shown). On the basis of these observations, we suspect that somaclonal variation (SV) may have occurred in these latter three callus cultures, presumably as a consequence of repetitive subcultures. SV is characterized by both genetic and epigenetic variations (Rodríguez López et al. 2010), the former of which influences DNA replication and cell division and can result in altered ploidy, chromosomal rearrangement, and changes in specific sequences (Wang and Wang 2012). Therefore, we assumed that cell division was most active in CP callus, which did not show any evidence of genetic SV.
Effect of RGB (red1/green1/blue) light on cell division in C. japonica calli derived from 4 different tissue origins after 4 wk of culture. (a) Cell cycle of calli (G1-Gap1 stage, S-DNA synthesis, G2 + M-Gap2 and metaphase). (b) Histogram of DNA content in calli of 4 different tissue origins. (a) CL: leaf-derived callus, (b) CR: root-derived callus, (c) CP: petal derived callus, and (d) CO: ovary-derived callus.
Analysis of the expression of PAL and chalcone synthase (CHS), two enzymes involved in the phenylalanine pathway, in the calli derived from four different tissues revealed highest expression levels in the CR line (Fig. 6). The PAL and CHS enzymes play roles in a pathway-associated phenolic and flavonoid biosynthesis (Slatnar et al. 2010), and higher expression levels of PAL and CHS in rose leaves have been reported to result in an increased biosynthesis of phenolics (Shetty et al. 2011). The transcription of PAL and CHS is regulated by endogenous circadian rhythms, which in turn have been shown to be affected by environmental conditions such as light (Thain et al. 2002), and particularly exposure to red and blue light (Feinbaum et al. 1991; Batschauer et al. 1996; Ahn et al. 2015). In this regard, Bordage et al. (2016) reported that the effects of circadian rhythms differ from organ to organ in Arabidopsis, and Thain et al. (2002) found that the expression levels of CHS differ in response to differences in the circadian rhythms associated with the leaves and roots of Arabidopsis. Therefore, we assume that there were differences in the expression of PAL and CHS observed in the present study when calli induced from different organs were cultured under illumination with a mixture of RGB light.
Consistently, we observed that total phenol and flavonoid content was the highest in CR callus (Fig. 7), which was characterized by the highest expression of PAL and CHS. High phenolic content was also detected in CP callus (5.66 mg g−1 DW), which we assumed that it is influenced by the expression of other downstream genes during phenolic biosynthesis. Moreover, along with CO (49.1%), we also found that DPPH radical scavenging activity was higher in CR (51.5%) callus under RGB light compared with that in the other two callus types (Fig. 7b).
Antioxidant activity in C. japonica calli derived from 4 different tissue origins after 4 wk of culture under RGB (red1/green1/blue) light. (a) Content of total phenolics and flavonoids. (b) Free radical scavenging using DPPH (CL leaf-derived callus, CR root-derived callus, CP petal-derived callus, CO ovary-derived callus). Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range test (n = 3).
In total, we detected 22 phenolic compounds, among which high levels of myricetin and physcion were observed in all lines (Fig. 8a). In terms of total phenolic content, CL and CR showed the highest levels (Fig. 8a). Levels of salicylic acid and cinnamic acid were found to be higher CL, CR, and CP calli, whereas (+)-catechin was detected only in CL callus (Fig. 8a). The 22 phenolic compounds we identified can be classified into five groups (hydroxybenzoic acid, hydroxycinamic acid, falvonols, anthraquinones, and other phenolic compounds), among which the content of flavonols and anthraquinones was higher in CL and CR calli (Fig. 8b), whereas no hydroxybenzoic acid was detected in CO callus. In all four callus lines, however, the content of flavonols was found to be higher than those of the phenolics in other groups. Myricetin is one of the major flavonoids found in numerous plant species and is known to have antioxidant, antitumor, anticancer, antiaging, and antiinflammation properties (Jung et al. 2010), whereas physcion is an anthraquinone derivative that plays a role in hepatocyte defense, and has antiinflammatory and antimicrobial activities (Wijesekara et al. 2014). In a comparison of the phenolic content of the roots, leaves, and stems in tea plant, Jiang et al. (2013) observed that the amounts of flavonol derivatives were the highest in the leaves, whereas those of proanthocyanidins were the highest in the roots. Thus, as indicated earlier, differences with respect to the light sensitivity and circadian rhythms of different plant organs may contribute to differences in the types and amounts of phenolics detected in calli derived from these organs.
Phenolic profiling of C. japonica calli derived from 4 different tissue origins after 4 wk of culture under RGB (red1/green1/blue) light. (a) Content of specific compounds. (b) Content of phenolic groups (CL leaf-derived callus, CR root-derived callus, CP petal-derived callus, CO ovary-derived callus). Different letters indicate significant differences at P < 0.05 according to Duncan’s multiple range test (n = 3).
Conclusion
In this study, we investigated the effect of light quality and callus origin on the biosynthesis of phenolics in camellia callus culture. Our observations indicated that calli exposed to mixed light sources (RB and RGB) were characterized by high levels of both total phenolic compounds and DPPH radical scavenging activity. In addition, total phenol and flavonoid content and antioxidant activity were high in root- and petal-derived calli.
References
Ahmad N, Rab A, Ahmad N (2016) Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J Photochem Photobiol B 154:51–56
Ahn SY, Kim SA, Choi SJ, Yun HK (2015) Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic Environ Biotechnol 56:36–43
Anjum S, Abbasi BH, Hano C (2017) Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult 129:73–87
Barciela J, Vieitez AM (1993) Anatomical sequence and morphometric analysis during somatic embryogenesis on cultured cotyledon explants of Camellia japonica L. Ann Bot 71:395–404
Batista DS, Felipe SH, Silva T, de Castro KM, Mamedes-Rodrigues TC, Miranda NA, Torres-Silva G (2018) Light quality in plant tissue culture: does it matter? In Vitro Cell Dev Biol Plant 54:195–215
Batschauer A, Rocholl M, Kaiser T, Nagatani A, Furuya M, Schäfer E (1996) Blue and UV-A light-regulated CHS expression in Arabidopsis independent of phytochrome a and phytochrome B. Plant J 9:63–69
Bello-Bello JJ, Martínez-Estrada E, Caamal-Velázquez JH, Morales-Ramos V (2016) Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr J Biotechnol 15:272–277
Bordage S, Sullivan S, Laird J, Millar AJ, Nimmo HG (2016) Organ specificity in the plant circadian system is explained by different light inputs to the shoot and root clocks. New Phytol 212:136–149
Carvalho SD, Schwieterman ML, Abrahan CE, Colquhoun TA, Folta KM (2016) Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front Plant Sci 7:1328
Chen QR, Li D, Qin XJ, Liu H, Ni W, Yan H, Liu HY (2018) Bioactive triterpenoid glycosides from the twigs and leaves of Camellia reticulata. Phytochem Lett 25:6–11
Darko E, Heydarizadeh P, Schoefs B, Sabzalian MR (2014) Photosynthesis under artificial light: the shift in primary and secondary metabolism. Philos Trans R Soc B 369:1640
Daud N, Faizal A, Geelen D (2013) Adventitious rooting of Jatropha curcas L. is stimulated by phloroglucinol and by red LED light. In Vitro Cell Dev Biol Plant 49:183–190
Fazal H, Abbasi BH, Ahmad N, Ali SS, Akbar F, Kanwal F (2016) Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J Photochem Photobiol B 159:1–7
Feinbaum RL, Storz G, Ausbel FM (1991) High intensity and blue light regulated expression of chimeric chalcone synthase genes in transgenic Arabidopsis thaliana plants. Mol Gen Genet 226:449–456
Folin O, Ciocalteu V (1927) On trysonic and tryptophane determination in protein. J Biol Chem 27:627–650
Fukuda N, Fujita M, Ohta Y, Sase S, Nishimura S, Ezura H (2008) Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci Hortic 115:176–182
Hatano T, Kagawa H, Yasuhara T, Okuda T (1998) Two new flavonoids and other constituents in licorice: their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090–2097
Ji XH, Wang YT, Zhang R, Wu SJ, An MM, Li M, Wang CZ, Chen XL, Zhang YM, Chen XS (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult 120:325–337
Jiang X, Liu Y, Li W, Zhao L, Meng F, Wang Y, Gao L (2013) Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant [Camellia sinensis]. PLoS One 8:e62315
Johkan M, Shoji K, Goto F, Hashida SN, Yoshihara T (2010) Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 45:1809–1814
Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, Lee HJ (2010) Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol 79:1455–1461
Kadomura-Ishikawa Y, Miyawaki K, Noji S, Takahashi A (2013) Phototropin 2 is involved in blue light-induced anthocyanin accumulation in Fragaria x ananassa fruits. J Plant Res 126:847–857
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3:1222–1239
Kwon AR, Cui HY, Lee H, Shin H, Kang KS, Park SY (2015) Light quality affects shoot regeneration, cell division, and wood formation in elite clones of Populus euramericana. Acta Physiol Plant 37:65
Lee HH, Paudel KR, Jeong J, Wi AJ, Park WS, Kim DW, Oak MH (2016) Antiatherogenic effect of Camellia japonica fruit extract in high fat diet-fed rats. Evid Based Complement Alternat Med 2016:9679867
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67:59–64
Li Q, Lei S, Du K, Li L, Pang X, Wang Z, Xu L (2016) RNA-seq based transcriptomic analysis uncovers α-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci Rep 6:36463
Llorente B, Martinez-Garcia JF, Stange C, Rodriguez-Concepcion M (2017) Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light. Curr Opin Plant Biol 37:49–55
Lobiuc A, Vasilache V, Oroian M, Stoleru T, Burducea M, Pintilie O, Zamfirache MM (2017) Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens. Molecules 22:2111
López-Laredo AR, Ramírez-Flores FD, Sepúlveda-Jiménez G, Trejo-Tapia G (2009) Comparison of metabolite levels in callus of Tecoma stans (L.) Juss. Ex Kunth. Cultured in photoperiod and darkness. In Vitro Cell Dev Biol Plant 45:550–558
Maharik N, Elgengaihi S, Taha H (2009) Anthocyanin production in callus cultures of Crataegus sinaica Boiss. Int J Acad 1:30–34
Manivannan A, Soundararajan P, Halimah N, Ko CH, Jeong BR (2015) Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic Environ Biotechnol 56:105–113
Miao L, Zhang Y, Yang X, Xiao J, Zhang H, Zhang Z, Jiang G (2016) Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria× ananassa) fruit. Food Chem 207:93–100
Murasnige T, Skoog M (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Osakabe Y, Miyata S, Urao T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2002) Overexpression of Arabidopsis response regulators, ARR4/ATRR1/IBC7 and ARR8/ATRR3, alters cytokinin responses differentially in the shoot and in callus formation. Biochem Biophys Res Commun 293:806–815
Páscoa RN, Teixeira AM, Sousa C (2019) Antioxidant capacity of Camellia japonica cultivars assessed by near-and mid-infrared spectroscopy. Planta 249:1053–1062
Pedroso RCN, Branquinho NAA, Hara AC, Costa AC, Silva FG, Pimenta LP, Januario AH (2017) Impact of light quality on flavonoid production and growth of Hyptis marrubioides seedlings cultivated in vitro. Rev Bras 27:466–470
Piovan A, Caniato R, Cappelletti EM, Filippini R (2010) Organogenesis from shoot segments and via callus of endangered Kosteletzkya pentacarpos (L.) Ledeb. Plant Cell Tissue Organ Cult 100:309–315
Qian H, Liu T, Deng M, Miao H, Cai C, Shen W, Wang Q (2016) Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem 196:1232–1238
Riefler M, Novak O, Strnad M, Schmülling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
Rodríguez López CM, Wetten AC, Wilkinson MJ (2010) Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol 186:856–868
Shetty R, Fretté X, Jensen B, Shetty NP, Jensen JD, Jørgensen HJL, Christensen LP (2011) Silicon-induced changes in antifungal phenolic acids, flavonoids and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol 157:111
Shohael AM, Ali MB, Yu KW, Hahn EJ, Islam R, Paek KY (2006) Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem 41:1179–1185
Sivakumar G, Heo JW, Kozai T, Peak KY (2006) Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J Hortic Sci Biotechnol 81:546–548
Slatnar A, Mikulic Petkovsek M, Halbwirth H, Stampar F, Stich K, Veberic R (2010) Enzyme activity of the phenylpropanoid pathway as a response to apple scab infection. Ann Appl Biol 156:449–456
Thain SC, Murtas G, Lynn JR, McGrath RB, Millar AJ (2002) The circadian clock that controls gene expression in Arabidopsis is tissue specific. Plant Physiol 130:102–110
To JP, Haberer G, Ferreira FJ, Deruere J, Mason MG, Schaller GE, Alonso JM, Ecker JP, Kieber JJ (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16:658–671
Vieitez AM, Barciela J (1990) Somatic embryogenesis and plant regeneration from embryonic tissues of Camellia japonica L. Plant Cell Tissue Organ Cult 21:267–274
Vieitez AM, Carmen San-Jose M, Ballester A (1989) Progress towards clonal propagation of Camellia japonica cv. Alba Plena by tissue culture techniques. J Hortic Sci 64:605–610
Wang H, Jiang YP, Yu HJ, Xia XJ, Shi K, Zhou YH, Yu JQ (2010) Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur J Plant Pathol 127:125–135
Wang QM, Wang L (2012) An evolutionary view of plant tissue culture: somaclonal variation and selection. Plant Cell Rep 31:1535–1547
Wang YS, Gao LP, Wang ZR, Liu YJ, Sun ML, Yang DQ, Wei CL, Shan Y, Xia T (2012) Light-induced expression of genes involved in phenylpropanoid biosynthetic pathways in callus of tea (Camellia sinensis (L.) O. Kuntze). Sci Hortic 133:72–83
Wijesekara I, Zhang C, Van Ta Q, Vo TS, Li YX, Kim SK (2014) Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol Res 169:255–261
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193–199
Wu MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, Chang HM (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101:1753–1758
Xu F, Cao S, Shi L, Chen W, Su X, Yang Z (2014) Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J Agric Food Chem 62:4778–4783
Yu W, Liu Y, Song L, Jacobs DF, Du X, Ying Y, Wu J (2017) Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. J Plant Growth Regul 36:148–160
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2016) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 36:215–232
Zagoskina NV, Dubravina GA, Alyavina AK, Goncharuk EA (2003) Effect of ultraviolet (UV-B) radiation on the formation and localization of phenolic compounds in tea plant callus cultures. Russ J Plant Physiol 50:270–275
Zagoskina NV, Alyavina AK, Gladyshko TO, Lapshin PV, Egorova EA, Bukhov NG (2005) Ultraviolet rays promote development of photosystem II photochemical activity and accumulation of phenolic compounds in the tea callus culture (Camellia sinensis). Russ J Plant Physiol 52:731–739
Zoratti L, Karppinen K, Luengo Escobar A, Häggman H, Jaakola L (2014) Light-controlled flavonoid biosynthesis in fruits. Front Plant Sci 5:53
Funding
This work was supported by the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Advanced Production Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number 315013-4), and the authors were partially supported by BK 21 of Chungbuk National University.
Author information
Authors and Affiliations
Contributions
EB Jang contributed to the data acquisition and wrote the manuscript. TT Ho participated in the experiment and sample analysis. S-Y Park made substantial contributions to data interpretation, revising of the manuscript, the conception, and design of the study.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Yong Eui Choi
Rights and permissions
About this article
Cite this article
Jang, E.B., Ho, TT. & Park, SY. Effect of light quality and tissue origin on phenolic compound accumulation and antioxidant activity in Camellia japonica calli. In Vitro Cell.Dev.Biol.-Plant 56, 567–577 (2020). https://doi.org/10.1007/s11627-020-10121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10121-9