Abstract—
Intraracial differences in the Carpathian honeybee race Apis mellifera carpatica from Russia and Tajikistan with the race standard (Vuchkovski line from Transcarpathian oblast of Ukraine) are compared by morphometric and molecular-genetic study. The most informative features, the “cubital index” and the “discal displacement,” are shown to deviate significantly from the standard. However, the calculated genetic distance between datasets from different regions indicates the uniformity of the Carpathian race across its distribution range. The nucleotide composition of the mtDNA COI gene (1535 bp) reveals two groups of haplotypes associated with the apiary foundation time and differing by the amino acid composition of the COI protein (group I, asparagine; group II, serine). In addition to honeybee samples from different regions, group I includes honeybees from an isolated apiary in the mountain-forest zone of Crimea, which originated from the honeybee line 77 from the Transcarpathian. Group II includes honeybee samples of the modern “Vuchkovski” standard line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The honeybee Apis mellifera L. is characterized by intraspecific differentiation that allows us to distinguish from 25 (Ruttner, 1992) to 28 (Engel, 1999) geographic honeybee races, adapted to local conditions (Ruttner, 1988). The Carpathian honeybee race Apis mellifera carpatica (Avetisyan, Gubin, Davidenco, 1966) (Gaidar and Levchenko, 2003) has recently become widespread in Eastern Europe. At the beginning of the 20th century, some researchers drew attention to the fact that, based on the complex of biological and morphological features, the Carpathian bees are a special group, significantly different from Apis mellifera carnica from Austria (Gaini, 1923). However, to date there are different views on the degree of difference between the Carpathian honeybee race and the A. m. carnica branch, up to isolating an independent subspecies (Zinovieva et al., 2013). Gubin (1972) separated A. m. carpatica into a special population of A. m. carnica that was formed as a result of dispersal of honeybees in the valleys of Danube River and then the Tisza River, entering the Eastern Beskids. From this point of view, the Carpathian race is an ecotype of A. m. carnica (Mannapov et al., 2015). Some authors considered the Carpathian honeybees an intermediate form between the A. m. carnica and Apis mellifera sossimai (Ukrainian) honeybee races (Sanduljak, 1965) or an eastern population of the Ukrainian super breed A. m. carnica Pollm. (Ufimtseva and Osintseva, 2009). These hypotheses are based on morphological and ecological approaches, using genetic comparison methods.
Initially, the genetic method of studying honeybee races was based on comparative analysis of the length and nucleotide variability of the COI–COII intergenic mtDNA region and on analysis of some microsatellites of the nuclear chromosomes. For example, it has been shown that the Romanian populaiton of A. m. carpatica honeybees is homogeneous in the COI–COII locus (Cauia et al., 2008). Other studies of A. m. carpatica populations, also based on analysis of the intergenic locus and microsatellites, noted the differences in populations inhabiting different regions (Adygea–Transcarpathian) and showed the Carpathian race to be distant from A. m. carnica (Kalashnikov, 2013). However, a contemporary comparative analysis of microsatellite populations (Zinoviev et al., 2013) showed that the degree of intrapopulation differentiation in A. m. carpatica and A. m. carnica is sometimes higher than between populations of A. m. carpatica and A. m. carnica. These data, according to the authors, may indicate their common origin and existence of A. m. carpatica as a population of A. m. carnica. Thus, the choice of the COI–COII intergenic region and microsatellites for analysis of interpopulation differences gives contradictory results. Recently, there have been studies indicating the value of the mtDNA COI gene for analysis of the racial and population diversity of A. mellifera (Martimianakis et al., 2011; Ozdil and Ilhan, 2012; Bykova et al., 2016).
In morphological studies of honeybees, it is assumed that the external features (proboscis length, length and width of the anterior wing, length of the third and fourth tergites, cubital index, discal displacement, tergite coloration, shape of the posterior edge of the wax plate on the fifth sternite) are the most stable and can be used for race identification (Mannapov et al., 2015).
The prevailing current understanding is that only an integrated approach allows us to describe a honeybee breed fully. The study of morphogenetic differences is relevant due to the great economic value of honey bees. “Over a relatively short period of time, the Carpathian honey bees … have begun to be used massively in apiaries in almost all the republics of the former Soviet Union. In addition to this, in the last 15 years, beekeepers have begun to breed Carpathians profitably in Hungary, the Czech Republic, Slovakia, and especially Poland, where Krajina bees have been the most popular” (Gaidar and Levchenko, 2003). It is hypothesized that the superiority of A. m. carpatica over A. m. carnica is due to the peculiarities of their ethological characters (Morev et al., 2013). However, there is growing evidence in the modern literature that the intensive breeding of honeybee breeds occurs as a result of human activity. Cross-breeding with other races leads to changes in the ecological plasticity of the hybrids with loss of useful features of the original bee races (Morev et al., 2013). “The loss of breed diversity is not only the loss of unique and invaluable genetic diversity, but also narrowing of the genetic potential that fundamentally limits the possibilities of the selective breeding process in the present and future” (Stolpovskii, 2010). Thus, preservation of the gene pools of various breeds of animals, including honeybees, and optimization of their use depend directly on the degree of development of methods for controlling the level of their biodiversity and population genetic structure. To date, there is not enough literature data estimating the limits of the intraracial variability of honeybees based on a complex of features.
This work was aimed at studying the intraracial diversity of A. m. carpatica honeybees based on analysis of morphological and molecular data.
MATERIALS AND METHODS
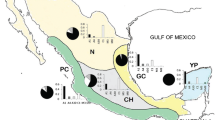
Worker honeybee specimens from the apiaries located in the Republic of Crimea: “Krasnye Peschery” (K) and the settlement of Ukromnoe (U) in Simferopol region, the town of Feodosia (F); in the Republic of Tajikistan: the village of Khistevarz in Bobodzhan Gafurov district, Sughd region (T1 and T2); in Moscow oblast, the town of Gzhel (MO); in Lipetsk oblast (L); on the territory of Skryabin State Academy of Veterinary Medicine and Biotechnology, Moscow (M); in Transcarpathian oblast, Ukraine (TO) were analyzed (Fig. 1). Honeybee colonies were brought to the apiary K from Central Asia and were bred in the mountain-forest zone of Crimea without importation of queens of known races for 30 years. The specimens from Transcarpathia were of the Vuchkovski line of the Carpathian race.
Locations of collected specimens of the Carpathian honeybee race Apis mellifera carpatica. The letter designations in Figs. 1–3 are collection sites: K, “Krasnye Peschery”; U, settlement of Ukromnoe; F, town of Feodosia (Republic of Crimea); T1 and T2, settlement of Khistevarz in Bobodzhon Gafurov district of Sughd region (Republic of Tajikistan); MO, town of Gzhel (Moscow oblast); L, Lipetsk oblast; M, territory of Skryabin State Academy of Veterinary Medicine and Biotechnology, Moscow; TO, Transcarpathian oblast, Ukraine.
Analysis of morphometric characters. The working hypothesis on the possible variability of features is based on the prolonged isolation or prolonged breeding without importation of queens of known races. Therefore, the analysis used datasets of honeybees from apiaries of the southern part of the Carpathian race distribution range: Crimea and Tajikistan. The control was a dataset of the Vuchkovski line of honeybees. The following morphometric features of the honeybee race identification (Mannapov et al., 2015) were analyzed: proboscis length, cubital index, discal displacement, and length and width of the right anterior wing. Table 1 is based on comparison of our own and known published data on the Carpathian race of honeybees; therefore, the “cubital index” features is given in the table both as a percentage (ratio of the smaller vein length to the larger vein length ×100) and a proportion (inverse ratio). Measurements were conducted using the MBS (LOMO, Russia) and VHX-1000E (Keyence, Japan) microscopes. Statistical analysis was conducted using Statistica 10.0 software. We used single-factor ANOVA with an unequal number of replications to identify the differences between populations in the values of the morphometric characters. The Fisher’s LSD post hoc test with a 95% significance level was used for multiple comparison of values.
Molecular genetic analysis. The total DNA was extracted from the legs of adult honeybees using a Diatom 200 kit (Izogen, Moscow). Amplification was conducted using a 5xMasterMix PCR kit (Dialat, Moscow). The analyzed fragment of the mtDNA COI gene was 1535 base pairs. Three pairs of primers were used for the amplification of this locus. PCR was conducted with the LCO1490 and HCO2198 primers for the first portion of the COI gene in accordance with the protocol (Folmer et al., 1994). To amplify the remaining region of the gene, we used two pairs of primers we had experimentally chosen: F2029 (TTCTTCACCTTCAGTAGATTTTG) and R3248 (TGAATTAAGTGGGGAAATTTTA); Fint2587 (AGCAACTTATCATGGTTCAAAA) and Rint2734 (GAACATAATGAAAATGTCCAACA).
The reaction was under the following conditions: 3 min at 95°C (1 cycle); 45 s at 95°C, 1 min at 50°C, 2 min at 72°C (35 cycles); 5 min at 72°C (1 cycle). The amplification product was purified by precipitation in a solution of ethyl alcohol with the addition of 5 M sodium acetate. Electrophoresis and reading of the nucleotide sequences of the amplification product were conducted on an ABI PRISM 3130 automatic sequencer (Applied Biosystems, United States) using the BigDye Terminatot kit 3.1 (Applied Biosystems) in the Laboratory of the molecular diagnostic methods of the Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences.
The data was processed using the MEGA 5 software packages (Tamura et al., 2011), Network ver. 4.6.1.1 (Bandelt et al., 1999), ARLEQUIN ver. 3.5 (Excoffier and Lischer, 2011), and MrBayes ver. 3.0 (Ronquist and Huelsenbeck, 2003). A total of 26 sequences were analyzed. The resulting sequences of the mtDNA COI gene from the A. m. carpatica specimens, represented by individual haplotypes or samples of identical haplotypes from different collection sites, were deposited in GenBank (htpp:// www.ncbi.nlm.nih.gov/) under accession numbers MF100910–MF100926. A total of 17 samples were registered in GenBank. For comparative analysis, we used the sequences of the homologous locus in the Russian Apis mellifera mellifera (KJ396188.1) and Italian Apis mellifera ligustica (LO6178.1) races from GenBank.
RESULTS
Morphometric analysis. The results of the analysis are presented in Table 1. The length of the upper wing ranges from 8.97 to 9.54 mm. Only honeybees from the T2 apiary do not differ from the control (Vuchkovski line, TO) by the wing length. The width of the wing in all datasets is smaller than in the control dataset; wings of the honeybees from apiary T2 are particularly narrow. The “discal displacement” feature has mainly a positive and neutral value. Some specimens from the apiaries U, T1, and especially K have a negative value of this feature. The “cubital index” feature varies from 2.25 (45.2%) in T2 honeybees to 2.71 (38.8%) in the Vuchkovski line (TO). The apiary T2 has distinct percentage and proportion values. The proboscis length varies from 5.53 to 6.42 mm. Significant differences were only found between the honeybees of the Vuchkovski line and the colonies from apiaries of Tajikistan.
Molecular genetic analysis. When aligning nucleotide sequences of the mtDNA COI gene of the honeybee specimens, we found nine variable sites, five of which are parsimony-informative. The total number of substitutions is eight transitions, one transversion. The nucleotide composition is 13.1% cytosine, 41.45% thymine, 34.42% adenine, and 11.03% guanine. The nucleotide variability of the total dataset is 0.1% (SE = 0). Nine haplotypes were described, of which four were unique, five were found in several samples with a frequency of 2–9. Dendrograms constructed by the MrBayes, Neighbor-Joining, and Maximim Parsimony methods based on nucleotide sequences and amino acids coincide in key nodes, indicating two groups.
In both groups there are specimens collected from all apiaries analyzed. However, nucleotide sequences of honeybee specimens belonging to group I have seven variable sites, of which three are parsimony-informative. It contains specimens from the apiary K. Group II consists of specimens the nucleotide sequences of which have only one variable site. In this group, there are specimens of the Vuchkovski line.
The dendrogram (Fig. 2) based on amino acids confirms the fundamental separation of the samples into two groups, despite low support. The substitution of guanosine with adenosine in the second codon position (site 110) leads to the substitution of serine with asparagine. Asparagine is typical for group I, which includes samples from the apiary K; serine is typical for group II, which includes samples from theapiary TO (Vuchkovski standard).
Dendrogram of the relationship between samples of the Carpathian honeybee race Apis mellifera carpatica from different regions, based on the amino acid sequence of protein for the mtDNA COI gene using the Neighbor-Joining method and p-distance model. The scale is the genetic distance between haplotypes. H1–H7, individual haplotypes; for Figs. 2 and 3. Amino acids: I, asparagine; II, serine. Specimens of the Vuchkovski line and from apiary K are in bold.
The relationship between the haplotypes is also illustrated by the median network (Fig. 3). The central place in it is occupied by haplotype 2 (H2), from which all other haplotypes branch. One of the haplotypes of this group gives rise to H1, which includes the Vuchkovski line as well as some specimens from other populations. H2 originates through a hypothetical ancestor from the European (Central Russian) dark bee, and H1 is closer to the Italian breed.
Median haplotype network of samples of the Carpathian honeybee race Apis mellifera carpatica from different regions based on the nucleotide sequence of the mtDNA COI gene. Circles, individual haplotypes; the diameter corresponds to the number of samples. The black dot marks the ancestral haplotype. Numbers on branches, numbers of nucleotide substitutions; absence of a number between haplotypes, one substitution. In H1: black, Vuchkovski line specimens; gray, specimens from apiary K.
DISCUSSION
It is known that the Carpathian honeybee race is characterized by sufficiently stable complex of morphoecological features (Gaidar, 2004; Papp et al., 2013; Mannapov et al., 2015). Gubin (1976), summarizing the studies on the morphological features of the honeybees local to the Ukrainian Carpathians, proposed a morphoetological standard for the Carpathian bees. He drew attention to the fact that the proposed standard is not the average character value for the bees of Transcarpathia, but the standard of the purebred honeybee of the Carpathian population. However, there is little published evidence of how these characters would make it possible to identify the honeybees from a given apiary as a studied race. This issue is relevant due to the fact that, since the 1970s, Carpathian race honeybees have widely spread not only in Eastern Europe but also in Central Asia, Tajikistan in particular (Gaidar and Levchenko, 2003).
Deviations from the standard length and width of the wing and the proboscis length that were revealed in the analysis of the material can be determined not only by the contribution of the drones of local races with free cross-breeding in apiaries, but also by honeybee adaptations to local conditions and flora of a particular region. This is confirmed by morphometric analysis of honeybees from Tajikistan and Crimea, especially honeybees from the apiary K. For example, the length of the upper wings of the “Vuchkovski” standard corresponds only to the character values of the studied TO Vuchkovski line (Table 1). However, the width of the wing of the TO honeybees is greater than the Vuchkovski line standard, while in the other studied datasets, it is smaller (Table 1). The shortened proboscis in the honeybees from apiaries of Tajikistan should be noted.
According to our data, the “discal displacement” and “cubital index” can be considered the most informative morphological features for determining the purity of a honeybee breed.
It is known that the Carpathian race has 95 to 100% positive displacement, and up to 5% neutral displacement (Gaidar, 2004; Papp et al., 2013; Mannapov et al., 2015). The TO honeybees have character values close to the “Vuchkovski” standard. The colonies from apiaries of Tajikistan and Crimea, especially the apiary K, have specimens with not only positive but also neutral or negative discal displacement of the wing. Apparently, this feature can be used to estimate the degree of pure-breed quality of a race in a given hive.
The “cubital index” feature, which varies in honeybees not only from different apiaries but also from different hives, is not associated with the presence of the “negative discal displacement” feature in the datasets (Table 1). It is known that with a standard of 33–43% (Gaidar, 2004), the value of this featurecan increase up to 45% in honeybees from other regions (Morev et al., 2013). It is also known that the Central Russian (European dark) and a number of southern honeybee breeds have a high cubital index (55 to 65%), while A. m. sossimai and A. m. carnica have a low cubital index (30–45%) (Bykova et al., 2016). The high cubital index in honeybees from our datasets (Table 1: T1, T2, and K) may also indicate the contribution of local drones of other races to the decrease in the breed purity of the apiary.
The first analysis of the nucleotide sequence variability of the complete mtDNA COI gene in our study allowed us to describe another direction in the diversity of the Carpathian race honeybees: diversity of honeybee queens.
The minimum genetic distance (0.001) between the datasets from different regions indicates the unity of the Carpathian breed across its distribution range. However, honeybee samples from different regions are characterized by high haplotype diversity and low nucleotide diversity (Table 2). This may indicate a rapidly increasing number of Carpathian race honeybee colonies from an initially small number of founders in a given apiary if sufficient time has passed for the haplotype variability level to be restored due to mutations, but not enough for significant differences between nucleotide sequences to accumulate. The vast majority of mutations affect the third base in codons and do not lead to a change in the amino acid sequence, with the exception of substitution of guanosine with adenosine in the second position of the codon, which leads to the substitution of serine with asparagine.
The separation of honeybee samples into two groups on the phylogenetic tree may be associated with the apiary foundation time. It is known that the type of the Carpathian bee had primarily been formed on the basis of line 77 of Transcarpathian bees, which was subsequently lost (Papp et al., 2013). Our hypothesis is that the honeybees from the isolated apiary in the mountain forest zone of Crimea (K), which were brought from Tajikistan and included in group I, are descendants of the line 77 honeybees. The long breeding time of honeybees from the apiary of this line is confirmed by the greater variability of the COI gene. The “Vuchkovski” standard was created on the basis of the restored apiary. It can be considered an analogue but not direct descendant of the mass breeding original line of this race of honeybees. The recent breeding of queen bees from this line is confirmed by the low nucleotide variability. The differences in the nucleotide sequences of the mtDNA COI gene provide information on the honeybee specimens that were founders of the apiaries. It is assumed that pure lines and amateur apiaries differ in a number of morphological features (Kalashnikov, 2013). Differences between the lines derived from the Carpathian race might be associated with both the adaptive capabilities of honeybee colonies and the artificial or uncontrolled breeding of queen bees (Papp, 2013; Mannapov et al., 2015). According to our studies, the amino acid composition of the protein of the mtDNA COI gene can most reliably indicate the original line of honeybees in the apiary.
An integrated approach to studies of the diversity of Carpathian race honeybees by region allows us to estimate both the time of breeding, i.e., the relative time of obtaining the queen bees from which an apiary originates, and the degree of breed purity of honeybees in the apiary with a long process of breeding, based on the deviation from the standard of selected morphological characters.
REFERENCES
Bandelt, H.J., Foster, P., and Röhl, A., Median-joining networks for inferring intraspecific phylogenies, Mol. Biol. Evol., 1999, vol. 16, no. 1, pp. 37–48.
Bykova, T.O., Triseleva, T.A., Ivashov, A.V., and Safonkin, A.F., Morphogenetic diversity of the honeybee Apis mellifera L. from the mountain-forest zone of Crimea, Biol. Bull. (Moscow), 2016, vol. 43, no. 6, pp. 541–546.
Cauia, E., Usurelu, D., Magdalena, L.M., Cimponeriu, D., Apostol, P., Siceanu, A., Holban, A., and Gavrila, L., Preliminary researches regarding the genetic and morphometric characterization of honeybees (A. mellifera L.) from Romania, Lucrări ştiinţifice Zootehnie şi Biotehnologii, Timişoara, 2008, vol. 41, no. 2, pp. 278–286.
Engel, M.S., The taxonomy of recent and fossil honey bees (Hymenoptera: Apidae, Apis), J. Hym. Res., 1999, vol. 8, pp. 165–196.
Excoffier, L. and Lischer, H.E.L., Arlequin suite ver. 3.5: a new series of programs to perform population genetics analyses under Linux and Windows, Mol. Ecol. Res., 2011, vol. 10, pp. 564–567.
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R., DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates, Mol. Mar. Biol. Biotech., 1994, vol. 3, no. 5, pp. 294–299.
Gaidar, V.A., Morphoetological standard of Carpathian bees, Pchelovodstvo, 2004, no. 6, pp. 14–15.
Gaidar, V.A. and Levchenko, I.A., Comparative evaluation of the Carpathian and Krajina bees, Pchelovodstvo, 2003, no. 8, pp. 18–20.
Gaini, M., Beekeeping in Carpathian Rus, Uzhhorod, 1923. http://bdjilka.in.ua/index.php/ru/component/content/ article/79-pchely/90-istoriya-vozniknoveniya-karpatskoj- porody-na-zakarpate.
Gubin, V.A., Carpathian population of carnica, Pchelovodstvo, 1972, no. 5, pp. 28–31.
Gubin, V.A., On the morphological breed standard, Pchelovodstvo, 1976, no. 2, pp. 11–12.
Kalashnikov, A.E., Study of differentiation of domestic populations of the honey bee Apis mellifera and their infection with RNA-containing viruses using molecular genetic techniques, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Dubrovitsy: GNU VIZh Rossel’khozakademii, 2013.
Mannapov, A.G., Khoruzhii, L.I., Simoganov, N.A., and Red’kova, L.A., Tekhnologiya proizvodstva produktsii pchelovodstva po zakonam prirodnogo standarta (Beekeeping Production Technology according to Natural Standard Laws), Moscow: Prospekt, 2015, pp. 24–30.
Martimianakis, S., Klossa-Kilia, E., Bouga, M., and Kilias, G., Phylogenetic relationships of Greek Apis mellifera subspecies based on sequencing of mtDNA segments (COI and ND5), J. Agric. Res., 2011, vol. 50, no. 1, pp. 42–50.
Morev, I.A., Moreva, L.Ya., and Kozub, M.A., Environmental plasticity of bee populations in southern Russia, Vestn. Tambov. Univ., 2013, vol. 18, no. 3, pp. 890–895.
Ozdil, F. and Ilhan, F., Phylogenetic relationship of Turkish Apis mellifera subspecies based on sequencing of mitochondrial cytochrome c oxidase I region, Genet. Mol. Res., 2012, vol. 11, no. 2, pp. 1130–1141.
Papp, V.V., Dynamics of some traits of autochthonous Carpathian bees under the influence of directional selection, Aktual. Probl. Intens. Zhivotnovod., 2013, no. 16, pt. 2, pp. 201–206.
Papp, V.V., Kerek, S.S., and Keil’, E.I., In-depth study of some morphological features of Carpathian bees, their breed characteristics, and differentiation with the aid of the Beemorph software, Sil’s’kii Gospodar, 2013, nos. 11–12, pp. 25–31.
Ronquist, F. and Huelsenbeck, J.P., MRBAYES 3: Bayesian phylogenetic inference under mixed models, Bioinformatics, 2003, vol. 19, pp. 1572–1574.
Ruttner, F., Biogeography and Taxonomy of Honeybees, Berlin: Springer Verlag, 1988.
Ruttner, F., Naturgeschichte der Honigbienen, Munich: Ehrenwirth Verlag, Grenoble, Bukarest: Apimondia, 1992, pp. 380−383.
Sendulyak, E., Contributii la studiul genofenotipic al albinelor Apis mellifera carpatica, in Cercet. Genet. Lucr. 1 Simpos. Nat. Genet., Bucuresti, 1965.
Stolpovskii, Yu.A., Population-genetic basis of the conservation of resources of gene pools of domesticated animal species, Extended Abstract of Doctoral (Biol.) Dissertation, Moscow: Inst. Obshch. Genet., 2010.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S., MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods, Mol. Biol. Evol., 2011, vol. 28, pp. 2731–2739.
Ufimtseva, N.S. and Osintseva, L.A., Porody i metody razvedeniya medonosnoi pchely, Apis mellifera L. (Breeds and Breeding Techniques of the Honey Bee Apis mellifera L.), Novosibirsk: Biol.-Tekhn. Inst., 2009.
Zinov’eva, N.A., Fornara, M.S., Borodachev, A.V., Gladyr’, E.A., Lebedev, V.I., Akimova, S.N., Krivtsov, N.I., and Ernst, L.K., Differentiation of the Carpathian and Krajina bees using microsatellites, Pchelovodstvo, 2013, no. 1, pp. 14–17.
ACKNOWLEDGMENTS
We are grateful to A.V. Ivashov (Crimean Federal University) for valuable research ideas, I.G. Meshcherskii (Severtov Institute of Ecology and Evolution, Russian Academy of Sciences) for assistance in primer selection, A.A. Varshavskii (Severtov Institute of Ecology and Evolution, Russian Academy of Sciences) for designing the map of material collection; and A.B. Makhmudov (Hystevarz), E.A. Pivovarov (Gzhel), and A.V. Korolev (Moscow) for the materials used in the analysis.
Funding
This study was supported by the Presidium of the Russian Academy of Sciences, program no. 41 “Biodiversity of Natural Systems and Biological Resources of Russia.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by A. Lisenkova
Rights and permissions
About this article
Cite this article
Safonkin, A.F., Triseleva, T.A. & Bykova, T.O. Intraracial Diversity of the Carpathian Race of Honeybees Apis mellifera carpatica. Biol Bull Russ Acad Sci 46, 492–499 (2019). https://doi.org/10.1134/S1062359019050091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019050091