Abstract
The cold hardiness of soil invertebrates (37 species of insects and 27 species of other taxa) was studied in the continental areas of Northeast Asia, a region with extreme winter temperatures. Insects overwinter mostly (34 species) in a supercooled state surviving within the temperature range of –12 to –35°C. Thirteen species of invertebrates (including insects, centipedes, slugs, earthworms, and amphipods) can withstand temperatures within the range of –5 to –45°C in a frozen state. The eggs of slugs, cocoons of earthworms, and larvae of some species of elaterids use cryoprotective dehydration, which allows them to survive at temperatures from –20 to –40°C, down to the record minimum of –196°C. Most of the organisms studied can tolerate temperatures of –25 to –30°C, which correspond to the average minimal temperatures in the upper soil horizons in most habitats of the continental regions of Northeast Asia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The Holarctic is inhabited by thousands of species of invertebrates adapted to surviving at temperatures below zero. In Russia, the proportion of territories with above-zero winter temperatures is negligible and permafrost covers almost 60% of the entire area of the country. The situation is similar in the northern part of North America: in Canada and Alaska.
However, to date, only the most general characteristics of cryoresistance of about 450 species of insects and less than 100 species of other invertebrates have been studied. On the contrary, dozens of publications have been devoted to detailed study of the mechanisms of maintaining cold hardiness in several model objects at the physicochemical, biochemical, and genetic levels. In particular, series of papers in this field have been devoted to the Antarctic and high-latitude North American animals. The fullest reviews of literature on the physiology and biochemistry of the cold hardiness of insects can be found in two monographs: Insects at Low Temperatures (Insects…, 1991) and Low Temperature Biology of Insects (2010).
However, the general notion of the places, temperature conditions, phenology, and the success of overwintering depending on the physiological state of the animals of various taxa and environmental factors remains quite insufficiently developed. Therefore, further progress in understanding the various components of the strategies of cold adaptations will be significantly determined by the ecological approaches and analysis of the adaptability of individual animal species to their habitats.
Most articles with an ecological outlook consider the resistance of individual species, rarely groups of species, which overwinter above the level of the snow cover: in wood, under the tree bark, in galls, or in the air (like the pupae of butterflies, which are attached to the branches and trunks) (Danks, 2005). An important exception is a publication on the winter ecology of 51 insect species (Merivee, 1978) studied simultaneously in one place: in the environs of Tartu (Estonia) in the 1970s. However, this work, as the majority of other such works, was performed in a region with a temperate climate. It mainly focused on the openly overwintering forms, for which the limits of physiological capacities in extremely cold years were estimated.
Research on the existence of invertebrates in regions with a very cold winter is almost absent. In this respect, the continental areas of northeastern Asia are of special interest. This region, recognized as the Pole of Cold of the Northern Hemisphere, is characterized by a long winter with an extremely low average monthly and minimal air temperatures; the seasonal temperature amplitudes are rather high in this region, and the microclimatic differentiation is quite distinct. The invertebrate population, including the soil-dwelling ones, is considerably diminished compared with the more southern areas. Nevertheless, the adaptive potential of the organisms manifests itself to its fullest in these extreme conditions of the ultracontinental climate.
The above information has determined the overall goal of our long-term (since 1983) studies. It consists in revealing the role of cold hardiness in the survival of soil-dwelling invertebrates in the conditions of the ultracontinental climate of northeastern Asia. As a possible evaluation criterion, the ratio of the animal’s maximum tolerated temperatures and the overwintering temperature conditions called a “reserve of cold hardiness” by Balashov (1998) was used.
Study of soil-dwelling animals involves a standardized approach, if possible, including the following sequence of tasks.
(1) The seasonal dynamics of resistance to negative temperatures was determined.
(2) The mechanisms ensuring cold hardiness were identified.
(3) The physiological capabilities of invertebrates were compared with winter temperature conditions in the overwintering sites (i.e., the “cold hardiness reserve” mentioned above was determined).
(4) The role of cold hardiness in limiting the landscape distribution and geographical distribution was assessed.
Based on the results of the research, several works on individual species and groups of invertebrate animals have been published, references to which are given in the text. The main purpose of this article is a brief synthesis of the results obtained for the first two cycles of problems. The second part is devoted to analysis of the results for points 3 and 4.
THE STUDY AREA, MATERIALS, AND METHODS
The natural conditions of the Upper Kolyma basin have been described many times (Gornye tundry…, 1980; Poyas…, 1985; Alfimov, 1985; Berman et al., 2010). We will give only a brief physical and geographical description of the area and the overwintering conditions of invertebrate animals.
In the territory under consideration, larch forests are prevalent and well-drained areas of relief are occupied by the Siberian dwarf pine. The landscape background has small spots of alder (Duschekia fruticosa), aspen (Populus tremula), birch (Betula platyphilla), various meadows, including xerophytic ones, and relict steppes. The period with subzero average daily temperatures lasts about seven months (from October to early May) both in the air and in the upper 20 cm layer of soil. Permafrost is ubiquitous throughout the region, during the warm season the thawing of soils varies from 0.3 to 2.5 m (Alfimov, 1984, 1985). At an average monthly air temperature of –32.6°C in January and an average absolute minimum of –51°C, the minimum temperatures in the upper 20 cm layer vary from –10 to –40°C depending on the different combinations of factors that form the winter thermal regime of soil horizons (air temperature dynamics, accumulation and density of snow, quantitative characteristics of the vegetation cover, etc.).
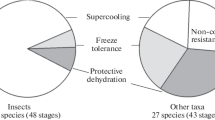
The cold hardiness of the following mass invertebrate species in northeastern Asia was investigated: ants (Hymenoptera: Formicidae), 12; click beetles (Coleoptera: Elateridae), 13; orthopterans (Orthoptera: Acrididae, Tetrigidae), 11; gastropods (Geophila: Agriolimacidae, Euconulidae, Discidae, Pupillidae, Oxychilidae, Vertiginidae), 9; centipedes (Chilopoda: Geophilidae, Lithobiidae), 3; harvestmans (Opiliones: Phalangiidae), 2; amphipods (Amphipoda: Talitridae), 1; and earthworms (Oligochaeta: Lumbricidae, Moniligastridae), 12. An indispensable criterion for selecting objects was the possibility of a lifetime determination of their species affiliation. The cold hardiness of some species from other regions was studied for comparison. A total of 37 insect species (48 overwintering stages) and 27 species (43 stages) of other invertebrates from five groups were studied.
At present, three mechanisms of cold hardiness are known: supercooling, the ability to tolerate freezing and protective dehydration (Low…, 2010). Supercooling, as the possibility to remain unfrozen in the physical sense (i.e., without the formation of ice in the body) at temperatures below 0°C, is achieved due to lower temperatures of freezing and supercooling of body fluids as a result of the accumulation of antifreeze. In the animals that tolerate freezing, crystallization begins, as a rule, at moderate negative temperatures in the presence of cryoprotectants of various nature, providing protection to the cellular structures and macromolecules. Resistance to low temperatures in the organisms that overwinter in the state of protective dehydration is associated with the loss of almost all osmotically active water, which leads to an increase in the osmotic concentration of liquids in tissues and a decrease in their freezing point (Holmstrup and Zachariassen, 1996), while maintaining a small ability to supercooling.
The methodological approaches to working with the animals that use these strategies differ. For those that overwinter in a supercooled state, the maximum supercooling temperature (supercooling point, SCP) is lethal, the animals can withstand it only for a short time, whereas freezing leads to death. SCP is easy to measure using a thermocouple, which registers the evolution of heat accompanying crystallization. In addition to SCP, the freezing temperature (freezing point, FP) was also registered, which is inevitably underestimated, but if the cooling conditions are constant, it can be used to compare series of animals of the same species or individuals of different species but similar in size (Berman et al., 2010). SCP below –20°C with FP of about –10°C unambiguously indicates that the supercooling mechanism is at work; the insects that are resistant to freezing have a SCP of up to –7°C and FP about –3°C (cases of resistance to freezing at very low SCP—below –50°C—have not been found among our objects). The animals that survive winter in the state of protective dehydration can be characterized by a similarly low SCP as in the first case, but their supercooling does not exceed 1–2°C.
However, the mortality of invertebrates at constant low temperatures can also depend on the time of exposure. Therefore, in relation to each group of organisms, the ratio of lethal temperature (at which half of the individuals (LT50%) or the entire sample (LT100%) die after prolonged (more than a day) exposure) and mean SCP was determined. In this way, the possibility of using SCP as a sufficient characteristic for assessing the cold hardiness for a particular species was elucidated.
For animals that survive winter in a frozen state, SCP is not informative. For them, as for the animals with protective dehydration, the main indicator is LT100% or—which is the same—the critical thermal minimum (CTmin).
The parameters of cold hardiness are very labile; they depend on the regime of acclimation and the duration and storing conditions of animals before the experiments. If possible, all these factors were taken into account; in order to clarify the effect of some of them on the characteristics being determined, special experiments were carried out (Berman et al., 2010, 2010a; Leirikh et al., 2005).
Detailed descriptions of the thermocouples, their calibration methods, thermostatic equipment, the validation of the acclimation regimes and cooling rates, and the methods for the analysis of chemical substances were given in our publications (Berman et al., 1984, 1989, 2002, 2007, 2010; Leirikh and Meshcheryakova, 2015). Let us dwell briefly on the main scheme of the works.
Most invertebrates were collected in the basin of the upper Kolyma, in the vicinity of the Aborigen station of the Institute of Biological Problems of the North, Far East Branch, Russian Academy of Sciences (62° N, 150° E); part of the slugs, harvestmens, and earthworms were collected in the vicinity of Magadan, Khabarovsk, and in the Moscow suburbs. To obtain eggs, the animals were kept in a laboratory. Winter studies were performed on the invertebrates collected in the late fall; they were immediately divided into series, acclimated in accordance with the temperatures in their natural habitats and kept under “soft wintering” conditions (–10… –15°C). Ants were the only exception; they underwent acclimation in natural conditions in the nests marked from fall, from which they were removed in winter and delivered to the laboratory. The series for determining SCP consisted of 30–60 individuals depending on the availability of the material. When the thresholds of tolerable temperatures were ascertained, samples of 20–100 specimens were exposed at 3–8 temperature values in 3–5°C increments or placed in late fall into the soil at collection sites along with minimal thermometers (“natural experiment”).
The water content was calculated after the sample was dried to constant weight at a temperature of 60°C. Glucose and polyols were extracted with 70% ethanol; the amount was determined using the spectrophotometric method (Pryce, 1967; Vaskovsky and Isay, 1969). Glycogen was extracted with a 30% solution of potassium hydroxide; the amount was calculated from the glucose formed after acid hydrolysis with 2 N sulfuric acid (Mosin and Petrova, 1980). The total lipids were isolated with a mixture of hexane and acetone (Hara and Radin, 1978), and the quantitative analysis was performed using the nephelometric method (Canal et al., 1972).
RESULTS AND DISCUSSION
Ants. The 12 ant species studied by us in northeastern Asia hibernate at the stage of imago (genus Formica) or adults and larvae of older ages (Leptothorax, Camponotus, Myrmica) in the supercooled state. The most cold-resistant species are L. muscorum (Nylander 1846), L. acervorum (Fabricius 1793), and C. herculeanus (Linnaeus 1758) (the mean values of SCP from different nests by species was –37…–44, –38…–43, and –37…–40°C, respectively); the species of the genus Myrmica (–27…–32°C) and F. gagatoides Ruzsky 1904 (–27…–30°C) proved to be less resistant; the most sensitive to cold were F. candida F. Smith 1878 (–24…–25°C), F. lemani Bondroit 1917 (–20…–24°C), F. exsecta Nylander 1846 (–19…–22°C), and F. sanguinea Latreille 1798 (–17…–18°C) (Fig. 1). In terms of the magnitude of seasonal changes in cold hardiness and the mechanisms ensuring it, two groups are distinguished. In the first group (C. herculeanus, all species of the genera Myrmica and Leptothorax), SCP values vary from summer to winter by a maximum of 30°C, mainly due to a decrease in the freezing temperature; in the second group (the genus Formica), it changes by 6–12°C, to a large extent because of the increase in the ability to supercool; FP varies only by 1–6°C.
The winter temperatures tolerated for long periods of time by ants exceed the average SCP by 3–5°C. The conservation of the liquid state of the supercooled solution at a temperature below FP is unstable. Staying in a supercooled state for months indicates the existence of special mechanisms for maintaining it.
Typical cold-protective substances for all Formica are sugars (3–6%). The change in SCP in the ants of the first group is determined by the accumulation of significant amounts of polyols (10–20%) performing the functions of antifreeze. In the second group, they are found in winter (up to 2%) only in the most cold-resistant species, F. gagatoides. The increase in the osmotic concentration of body fluids is also facilitated by a decrease in the water content in overwintering insects compared with summer ones: from 8% in the representatives of the genus Myrmica to 12% in Formica (Berman et al., 2010).
Click beetles. Three mechanisms of adaptation to low winter temperatures (Berman et al., 2013) have been identified in 13 species of click beetles. Most species of these beetles overwinter in a supercooled state. These are the larvae and imagoes of Orithales serraticornis (Paykull 1800), Selatosomus melancholicus (Fabricius 1798), Sericus brunneus (Linnaeus 1758), Ampedus sp., Limonius koltzei Reitter 1895, Prosternon sericeum (Gebler 1824), Selatosomus gloriosus (Kishii 1955), and Selatosomus impressus (Fabricius 1792). In summer, the average SCP of the larvae of all these species are within –4…–11°C and FP is about –3°C. In winter, SCP of O. serraticornis imagoes decreases to –27.5°C and that of S. brunneus to –28.7°C; in the larvae of most species, it is from –26.5°C to –31.2°C (Fig. 2) at FP –10…–13°C. The thresholds of tolerable temperatures were determined only in the larvae of O. serraticornis and L. koltzei: half of the individuals die within 24 hours at –25°C. In the hibernating larvae of this group of species, specific cold-protective substances are few: from 0.2% of polyols in S. brunneus to 0.9% in P. sericeum, glucose: 0.1–0.3%. The water content in winter decreases in O. serraticornis by 4–8%, in P. sericeum by 10%, and in other species it was not determined.
The second group includes the freezing larvae of Denticollis varians (Germar 1846) and the imago of Hypnoidus hyperboreus (Gyllenhal 1827), in which SCP is lower in winter (–9°C) than in summer (–4…–7°C). After the overwintering that lasted four months with a gradually decreasing temperature down to –15°C, 80% of H. hyperboreus individuals survived.
The stability of D. varians to cold is very high: at temperatures of down to –35°C all animals survive, at –40°C, 30–40% of the animals die. In the overwintering larvae of D. varians, no accumulation of polyols and glucose was noted, but the amount of lipids was high (11.2%); the water content remains about 58% all year round.
The third group includes larvae of the genus Oedostethus and H. hyperboreus, hibernating in a protective dehydration state (Berman et al., 2013). Their winter SCP values are close to the values of the first group of species, but they are achieved not by increasing the ability to supercool, but due to a very strong decrease in FP: up to –20°C in Oe. kolymensis Dolin et Bessolitzina 1990 and –25…–26°C in the rest (including H. hyperboreus). The magnitude of supercooling from 5–7°C in summer decreases to 1–2°C in winter. The large amount of reserve lipids (18–25%) is combined with a slightly elevated glucose level (0.45%) and accumulation of polyols (3–5%). The main role in increasing the osmotic concentration of body fluids and lowering the freezing temperature is performed by significant dehydration: from 60–65 in summer to 43–44% in winter.
Resistance to prolonged exposure to low temperatures was determined in H. hyperboreus, Oe. mediocris (Gurjeva 1972), and Oe. nubilus (Bessolitzina 1974). As in the species of the first group, the death of half the sample after exposure for 24 hours was recorded at –23…–25°C, but around 10% of the larvae survive at –31°C.
Thus, in spite of the different mechanisms ensuring cold hardiness, the winter SCP values of the majority of larvae of the click beetle species studied in detail are about –26…–28°C, and insects are capable of enduring –22…–25°С for long periods of time. Only D. varians, hibernating in a frozen state, can withstand temperatures below –40°C.
Orthoptera. Of the 15 mass species of orthopterans in the upper Kolyma basin (Berman et al., 1983), the cold hardiness of eggs was determined in ten species of true locusts and the postembryonic stages of one species of groundhoppers. Among them are Aeropedellus variegatus (Fischer von Waldheim 1846), Aeropus sibiricus (Linnaeus 1767), Chorthippus biguttulus (Linnaeus 1758), Ch. fallax (Zubovski 1900), Ch. montanus (Charpentier 1825), Stethophyma grossum (Linnaeus 1758), Melanoplus frigidus (Boheman 1846), Podismopsis gelida (Miram 1931), Primnoa polaris (Miram 1928), Bryodema tuberculatum (Fabricius 1775) (Berman and Leirikh, 2006), and Tetrix fuliginosa (Zetterstedt 1828).
The eggs of locusts, as in all the insects studied, hibernate in a supercooled state. The highest SCP values at the end of the summer were found in Ch. biguttulus: an average of –27.7°C. Lower values are obtained for Ae. sibiricus: the average SCP was –30.8°C. In B. tuberculatumSCP reached –32.2°C, but the average values for individual egg capsules also varied considerably (‒25…–35°C). Similar cold hardiness (–32.5°C) was shown by Pr. polaris and A. variegatus. SCP of the eggs of the remaining five species proved to be slightly lower and distributed in a narrow interval: –34.4°C in M. frigidus, –34.8°C in Ch. fallax, –34.7°C in P. gelida, –35.0°C in Ch. montanus, and ‒35.5°C in S. grossum (Fig. 3).
By the end of the first overwintering, the cold hardiness of eggs of C. biguttulus increased the most (by 4.5°C), that of Pr. polaris, B. tuberculatum, and A. variegatus increased slightly less (by 2–3°C); in the other species, the changes did not exceed 1°C compared to the summer values. The eggs Ae. sibiricus (–30.0…–31.8°C), Ch. biguttulus (–32.1°C), and Pr. polaris (–32.9°C) were found to be the least cold-resistant. The SCP values of the remaining species were in the range from ‒33.7 to –35.6°C. As shown for Ch. fallax (Hao and Kang, 2004), LT50% of the overwintering eggs coincides with the mean value of SCP, which gives reason to expect the absence of mortality up to –25°C in Ae. sibiricus and up to –29…–31°C in the group of the most cold-resistant species.
It should be noted that the eggs of all the acridid species under discussion have a superdiapause, and SCP of the species that hibernate for the second and third times increases. A certain decrease in cold hardiness is associated with the deterioration in their condition and an increase in the proportion of nonviable ones, rather than with the degree of development. The revealed cold hardiness ensures a favorable overwintering of the eggs of the species studied in the majority of the biotopes of the region.
The chemical composition of the eggs was determined once during their first overwintering. In all species, a high content of lipids was found, the amount of which varied insignificantly in different samples of one species and ranged from 7.5 (A. variegatus) to 10–15% (A. sibiricus and M. frigidus, respectively). Small reserves of glycogen were also registered: from 0.4 to 1.9%. Unlike Locusta migratoria Linnaeus 1758 (Zambin, 1939), no glucose was found in any of the species studied. The content of polyols in the eggs of the surveyed species was small, 0.8% at maximum (A. variegatus).
The dark groundhopper Tetrix fuliginosa differs fundamentally in its life cycle from the members of the Acrididae family. No eggs could be found either in late fall or early spring (probably, they do not overwinter), but in summer they possess the capability to resist significant supercooling (the mean SCP is –18.0 ± 1.2°С, n = 20). The larvae of different ages and imagos are the ones that overwinter. Their summer cold hardiness is not high: SCP was –5.0…–6.5°С at FP ‒1.3…–1.6°С, and freezing resulted in death. In winter, both characteristics decreased only by 1°C, but the insects developed the ability to tolerate freezing.
During the overwintering in “soft” conditions, the death of groundhoppers varied from 6 to 35% (on average, 20%) in different samples, whereas in the conditions close to natural (with a minimum temperature of –24°C), it was about 8% (Fig. 4). The death of only 18–24% of individuals after exposure at –43…–44°C indicates that the threshold of 100% mortality is, apparently, much lower.
The accumulation of cryoprotective substances in groundhoppers in winter was not detected: the content of polyols and glucose was not higher than 0.3–0.4%, that of lipids was practically invariable (about 8%), and glycogen decreased from 2.4 to 0.7%. A slight decrease in the proportion of water (to 69.6 ± 0.7% vs. 72.9 ± 0.8% in summer) cannot contribute to survival in a frozen state at such low temperatures either (Berman et al., 1989). Thus, T. fuliginosa is resistant to freezing in the absence of significant amounts of low-molecular cryoprotectants.
Harvestmans. In the Northeast, two species of harvestmans are found: Mitopus morio (Fabricius 1799) and Homolophus arcticus Banks 1893. Both species are the northernmost in the order. Near Magadan they are almost ubiquitous and common in the grass associations of the lower part of the mountains. In the upper reaches of the Kolyma River, M. morio occurs exclusively along rivers valleys and streams, while H. arcticus is extremely rare. These harvestmans have a one-year development cycle; only their eggs hibernate (Leirikh et al., 2009).
In fall, SCP of the eggs of M. morio is –25.5°C and that of H. arcticus is –29.6°C, decreasing in the specimens acclimated at –10°C to –28.2 and –30.1°C, respectively. The yield from the acclimated series in different years varied between 53–58% (M. morio) and 55–69% (H. arcticus). Further stepwise cooling to ‒27°C did not affect the survival of M. morio embryos, but they did not survive –28°C (Fig. 5). The success rate of eggs of H. arcticus decreased as the exposure temperature decreased to 20% at –32.5°C; LT100% was not obtained, but one can expect (Fig. 5) its coincidence with the lowest SCP in the entire sample (‒34°C). The proximity of their values indicates a high stability of the supercooled state.
Centipedes. The centipedes inhabiting the upper Kolyma proved to be the only group of invertebrate, in which the species were able to withstand freezing both in summer and in winter. After one day of storage at ‒6°C, 9% of the geophilids Escaryus japonicus Attems 1927 survived, although SCP did not exceed –3.2 ± 0.2°С (n = 56). In winter, SCP of this species dropped to –7.7 ± 0.2°С (n = 47), and LT100% was recorded at –35°С. The water content in the organism of centipedes in winter decreases to 64% compared with 73% in summer.
The two lithobiid species studied were even more cold-resistant. The average SCP of Lithobius steingeri Bollman 1893 in summer was –3.2 ± 0.2°С (n = 56), and in Dakrobius krivolutskyi Zalesskaja 1975, it was ‒5.6 ± 0.1°С (n = 30). Some specimens of L. steingeri showed a two-stage freezing: the second peak of crystallization was observed a few degrees below the first. The death threshold of 50% of L. steingeri is between –5 and –9°C, and for D. krivolutskyi it is between –4.5 and –6°C. Thus, in summer, L. steingeri tolerates temperatures slightly below SCP, while D. krivolutskyi perishes during freezing. In winter, SCP of L. steingeri decreases to –7.1 ± 0.2°С (n = 47), and SCP of D. krivolutskyi remains the same as in summer: –5.7 ± 0.3°С (n = 43). In winter, LT50% of the studied species is significantly lower than SCP: –31…–34°C for L. steingeri and –31°C for D. krivolutskyi.
The diplopod Angarozonium amurense (Gerstfeldt 1859) is the only species of millipedes from more than a hundred registered in Siberia and the Far East that penetrates into the territories with continuous permafrost, including the Arctic Circle (Mikhaljova and Marusik, 2004). About 82% of the specimens survived cooling to –27°C, which is 1°C below the minimum SCP for the sample; at a temperature of –31°C, all millipedes died (Berman et al., 2015). By the mechanism of cryoresistance, this species can be attributed to moderately resistant to freezing, withstanding temperatures slightly below SCP (Sinclair, 1999).
The amphipodTraskorchestia ditmari (Derzhavin 1923) is one of the northernmost species of the Talitridae family, marking the northern boundary of its distribution on the western coast of the Pacific Ocean. This detritophage occurs in the Northeast exclusively along the coastal grassy slopes, along the fringes of the larch and the stone birch forests. It is a superdominant in these areas (in terms of abundance and biomass) of a peculiar type of soil mesofauna. The biotope distribution and temperature conditions of the overwintering of T. ditmari were studied in the northern part of Gertner Bay (Tauiskaya Guba), 20 km from Magadan. The crustaceans overwinter at a depth of a first ten of centimeters from the surface of the soil. After freezing at an average SCP of –5.9°C, they tolerate temperatures to –25°C without damage (Fig. 6). The death of the entire sample after a 24-hour exposure was noted at a temperature of –42°C (Berman et al., 1991).
Gastropod terrestrial shell mollusks. With varying degrees of detail, we investigated five species of small terrestrial mollusks: Euconulus cf. fulvus, Discus ruderatus (Férussac 1821), Pupilla muscorum (Linneus 1758), Perpolita petronella (L. Pfeiffer 1853), and Vertigo modesta (Say 1824) (definitions of Ya.I. Starobogatov).
In summer, the average SCP of all these species ranges from –4.8 ± 0.3 to –7.2 ± 0.6°С; their FP is not lower than –1…–2°С. Preparation for overwintering is manifested in a significant increase in the ability to supercool with a slight change in the freezing temperatures. In winter, the average SCP of E. cf. fulvus, P. petronella, and D. ruderatus decreases to –20°C. The minimum values reach –25…–26°C. The corresponding characteristics of V. modesta and P. muscorum are approximately 5°C lower. The mortality of E. cf. fulvus, P. petronella, and D. ruderatus is close to 100% at a temperature of –20…–22°C, V. modesta at ‒25°C. All these mollusks do not tolerate freezing.
Slugs of the genusDeroceras. In the northeast of Asia, in addition to the marsh slug Deroceras laeve (Müller 1774), which is common throughout the cold regions, the Altai slug D. altaicum (Simroth 1886) is found (Prozorova and Kavun, 2006). In addition, the reticulated D. reticulatum (Müller 1774) and the field slug D. agreste (Linnaeus 1758) were found in the vicinity of Magadan (Berman et al., 2011).
In fall, for all species of slugs, SCP is about –5°C, but their resistance to low temperatures is different. The slugs D. reticulatum and D. altaicum died already at –1°C. In contrast, 100% (n = 50) of D. laeve slugs endured four months at a temperature of –10°C. The death threshold of 50% of individuals ranges from –18 to –24°С; the lowest temperature that individuals of this species can withstand is –28°C (Fig. 7).
The cold hardiness of the eggs is fundamentally different than that of slugs. Their overwintering takes place in a state of protective dehydration. The D. reticulatum embryos tolerate the lowest temperatures. Almost half of them survived after exposure at ‒12…‒15°С, and 8–10% retained viability at temperatures of –30…–35°С. The proportion of water decreases from 82% in the eggs kept at room temperature to 61% in those cooled to –3°C and to 47% after exposure at ‒20°C. The eggs of D. altaicum and D. agreste with the same loss of water are less cold-resistant (Berman et al., 2011).
The D. leave embryo, unlike those of the previous species, are not resistant to low temperatures. In the control (18°C) all individuals (n = 30) developed, after a daily cooling to 0°C the proportion of eggs that successfully completed the development decreased to 20% (n = 20), and after –1°C to 5% (n = 20). Cooling below –1°C was lethal.
Thus, slugs have three life cycle schemes: overwintering at low temperatures in the embryonic stage (D. reticulatum, D. altaicum), in the postembryonic stage (D. laeve), and in both phases of development (D. agreste).
Earthworms can hibernate at the stage of eggs and/or worms of different ages. The average SCP values of the 12 species studied and the two subspecies of these animals vary from –1 to –3.6°C in fall (Meshcheryakova and Berman, 2014). However, their ability to supercool does not reflect the real cold hardiness. Only five taxa of earthworms withstand negative temperatures: from –3°C in Aporrectodea caliginosa (Savigny 1826) to –35°C in Eisenia nordenskioldi (Eisen 1879), more precisely, E. nordenskioldi nordenskioldi, belonging to the 9th genetic line (Shekhovtsov et al., 2015) (Fig. 8). The mechanism of endurance of negative temperatures by the named species is the same: resistance to freezing.
The condition of the tissues of frozen worms was studied using the E. nordenskioldi line. The covers of the frozen worms retained elasticity; the water “frozen” from cells and tissues crystallized in the body cavity, forming ice shells around the organs. From the glycogen accumulated in the fall, glucose and glycerol are synthesized, serving as cryoprotectants (Berman and Leirikh, 1985).
The cold hardiness of hibernating cocoons varies drastically in the species studied and does not correlate with the resistance of worms. The viability of E. fetida cocoons (Savigny 1826) is lost already in the process of long-term storage at low positive temperatures. The embryos of A. rosea (Savigny 1826) and Lumbricus terrestris Linnaeus 1758 die at temperatures of 0…–3°C. The remaining eight species studied showed significant resistance of the embryonic stage to the cold. The cocoons of A. caliginosa, Drawida ghilarovi Gates 1969, and Octolasion lacteum (Orley 1885) can withstand temperatures down to –15°C, while in other species (Dendrobaena octaedra (Savigny 1826), Dendrodrilus rubidus tenuis (Eisen 1874), E. nordenskioldi, L. castaneus (Savigny 1826), L. rubellus Hoffmeister 1843) withstand –35°C and lower (Meshcheryakova and Berman, 2014), down to –196°C (D. rubidus tenuis).
The resistance of embryos to negative temperatures is provided by another mechanism, protective dehydration (Holmstrup and Westh, 1994; Meshcheryakova and Berman, 2014). As the temperature in the negative region decreases, the cocoons of most of these species lose about half of their mass due to dehydration (Holmstrup and Zachariassen, 1996; Berman et al., 2002). An additional contribution to cold protection is made by sorbitol (Holmstrup, 1995).
CONCLUSIONS
In northeastern Asia, in soil invertebrates, all three known cold-resistance strategies have been identified: supercooling, extracellular freezing of liquids, and protective dehydration.
The average supercooling temperatures of the insects with the first strategy range from –17 to –42°C, and the thresholds of the temperatures endured are 3–7°C higher. The organisms that withstand freezing have high SCP (up to –3…–10°C), but survive after staying at temperatures of –25…–45°С. Protective dehydration ensures the viability of organisms cooled to the temperatures of –20 to –40°C at a record value of –196°C. This method of cold protection is characteristic not only of the cocoons of earthworms, in which it was originally described, but also for the eggs of slugs, which also have permeable shells, and, more importantly, for the larvae of click beetles with chitinous coverings. The latter circumstance suggests that the mechanism of protective dehydration may appear to be more widespread in different groups of invertebrates than is now known.
The adaptive potential of various strategies of cold hardiness of soil invertebrates is similar: the most resistant species tolerate temperatures of about –40°C, regardless of the mechanism used (supercooling ants Leptothorax acervorum, freezing larvae of Tetrix fuliginosa, cocoons of the earthworm of the 9th line of the nominative subspecies Eisenia nordenskioldi dehydrated in the process of supercooling).
Among the insects studied, the most common is the mechanism of supercooling: 34 out of 37 studied species; only three overwinter in a frozen state (the groundhopper Tetrix fuliginosa, click beetle Hypnoidus hyperboreus, and click beetle larva Denticollis varians). In other taxa, resistance to freezing prevails: out of the 27 species tested, only nine survive the cold season in the supercooled state (the eggs of harvestmans and terrestrial mollusks). The rest either freeze (earthworms, slugs, centipedes, and amphipods) or are dehydrated (slug eggs and cocoons of earthworms).
Based on resistance to low temperatures, the species surveyed can be divided into four groups. The first group includes seven species that can withstand temperatures down to –35°C: ants Camponotus herculeanus, Leptothorax acervorum, and L. muscorum, the earthworm Eisenia nordenskioldi (9th line), the groundhopper Tetrix fuliginosa, the sandhopper Traskorchestia ditmari, and the beetle larva Denticollis varians. The second, most numerous, group is formed by the ants Formica gagatoides and species of the genus Myrmica, the majority of larvae of click beetles, the eggs of locusts and harvestmans, the slugs Deroceras laeve, and the scale insect Arctorthezia cataphracta (Shaw 1794), which overwinter at temperatures from –25 to –30°C (Leirikh, 2015). The third group includes species with a cold hardiness within –15… ‒20°С: four of five species of ants of the genus Formica, the majority of gastropod land shell mollusks, the earthworm Drawida ghilarovi, and the eggs of slugs. Representatives of the fourth group (the earthworm Dendrobaena octaedra and the eggs of the slugs Deroceras altaicum and D. agreste) withstand temperatures of at least –10…–12°C.
In general, the cold hardiness of soil invertebrates inhabiting the coldest regions of the Holarctic varies within –25…–30°C. This temperature range is characteristic of the upper part of the soil profile in most habitats of the surveyed territory of the continental part of the Northeast (Alfimov, 1985; Berman et al., 2010). It is known that, in the northern regions, the root zone occupies only the uppermost part of the profile (10–15 cm), in which the soil organisms are concentrated.
We should note that the cold hardiness of invertebrates that hibernate above the snow level even in the milder climate of the vicinities of Fairbanks (Alaska) proved to be more significant. There, the average SCP of the supercooling species reaches –56°C (the minimum temperatures are up to –63°C) and the species resistant to freezing endure –70°C (Miller, 1982).
A similar result can be predicted for invertebrate animals in the continental regions of the Northeast. If the above is confirmed, it will be possible to discuss the existence of a “tuning” of the level of cold hardiness of background invertebrates (which is what we worked with) relative to the temperatures of the upper horizons of the soil.
REFERENCES
Alfimov, A.V., The thermal regime of the upper layers of soil in the main ecosystems of the sparse forest belt of the Upper Kolyma basin, in Poyas redkolesii verkhovii Kolymy (raion stroitel’stva Kolymskoi GES) (The Sparse Forest Belt of the Upper Reaches of the Kolyma River (the Kolyma HPP Construction Area)), Vladivostok: Dal’nevost. Nauch. Tsentr Akad. Nauk SSSR, 1985, pp. 9–29.
Alfimov, A.V., Thermal regime of mountain tundras, in Pochvennyi yarus ekosistem gornykh tundr khrebta Bol’shoi Annachag (verkhov’e Kolymy) (The Soil Layer of Mountain Tundra Ecosystems of the Bol’shoi Annachag Ridge), Vladivostok: Dal’nevost. Nauch. Tsentr Akad. Nauk SSSR, 1984, pp. 6–40.
Balashov, Yu.S., Iksodovye kleshchi – parazity i perenoschiki infektsii (Ixodid Mites—Parasites and Vectors of Diseases), St. Petersburg: Nauka, 1998.
Berman, D.I. and Leirikh, A.N., The ability of the earthworm Eisenia nordenskioldi (Eisen) (Lumbricidae, Oligochaeta) to endure subfreezing temperatures // Doklady Biological Sciences, Proceedings of the Academy of Sciences of the USSR. Biol. Sci. Sections, 1985, vol. 285, no. 1–6, pp. 845–848.
Berman, D.I. and Leirikh, A.N., Factors affecting the population dynamics of Acridoidea (Orthoptera: Insecta) of North-East Asia, in Populyatsionnaya ekologiya zhivotnykh. Materialy Mezhdunar. konf. “Problemy populyatsionnoi ekologii zhivotnykh” (Population Ecology of Animals: Proc. Int. Conf. “The Problems of Population Ecology of Animals”), Tomsk: Tomsk. Gos. Univ., 2006, pp. 275–277.
Berman, D.I., Budarin, A.M., and Kritskaya, I.G., The fauna and spatial distribution of orthopterans of the continental regions of the North-East of the USSR, in Biologicheskie problemy Severa. Tez. X Vsesoyuz. Simpoz. (Biological Problems of the North: Proc. X All-Union Symp.), Magadan, 1983, part 2, pp. 345–346.
Berman, D.I., Zhigul’skaya, Z.A., and Leirikh, A.N., Biotopic distribution and cold hardiness of Formica exsecta (Formicidae) in the northeastern boundary of the range (upper reaches of the Kolyma River), Byull. Mosk. Obshch. Ispytat. Prirody, Otd. Biol., 1984, vol. 89, no. 3, pp. 47–63.
Berman, D.I., Leirikh, A.N., and Yakimchuk, N.V., Wintering and related biological characteristics of Tetrix fuliginosa (Orthoptera, Tetrigidae) in the Northeast of the USSR, Zool. Zh., 1989, vol. 68, no. 9, pp. 86–95.
Berman, D.I., Alfimov, A.V., and Leirikh, A.N., Wintering conditions and cold-resistance of the amphipod Traskorchestia ditmari on the coast of the Sea of Okhotsk // The Soviet Journal of Marine Biology, 1991, pp. 267–271.
Berman, D.I., Meshcheryakova, E.N., Alfimov, A.V., and Leirikh, A.N., The distribution of the earthworm Dendrobaena octaedra (Lumbricidae: Oligochaeta) in the north of the Holarctic is limited by its insufficient frost hardiness, Zool. Zh., 2002, vol. 81, no. 10, pp. 1210–1221.
Berman, D.I., Alfimov, A.V., Zhigul’skaya, Z.A., and Leirikh, A.N., Overwintering and Cold-Hardiness of Ants in the Northeast of Asia, Sofia-Moscow: Pensoft, 2010.
Berman, D.I., Meshcheryakova, E.N., Leirikh, A.N., and Kurenshchikov, D.K., Geographic range and cold hardiness of the earthworm Drawida ghilarovi (Oligochaeta, Moniligastridae), Biol. Bull. (Moscow), 2010a, vol. 37, no. 9, pp. 895–904.
Berman, D.I., Meshcheryakova, E.N., and Leirikh, A.N., Cold hardiness, adaptive strategies, and invasion of slugs of the genus Deroceras (Gastropoda, Pulmonata) in Northeastern Asia, Biol. Bull. (Moscow), 2011, vol. 38, no. 8, pp. 765–778.
Berman, D.I., Leirikh, A.N., and Zhigul’skaya, Z.A., A common strategy of cold hardiness in ants of the genus Myrmica (Formicidae, Hymenoptera) in Northeast Asia // Entomological Review, 2012, vol. 92, no. 3, pp. 247–261.
Berman, D.I., Leirikh, A.N., and Bessolitzina, E.P., Three strategies of cold tolerance in click beetles (Coleoptera, Elateridae), Dokl. Biol. Sci., 2013, vol. 450, pp. 168–172.
Berman, D.I., Meshcheryakova, E.N., and Mikhaljova, E.V., Cold hardiness and range of the myriapod Angarozonium amurense (Polyzoniidae, Diplopoda, Arthropoda) in permafrost environments, CryoLetters, 2015, vol. 36, no. 4, pp. 237–242.
Canal, J., Delatre, J., and Gilard, M.L., Acquistisus nouvelles dans le dosage des lipids totaux de serum: description d’une methode nephelometruqui, Ann. Biol. Clin., 1972, vol. 30, no. 4, pp. 325–332.
Danks, H.V., Key themes in the study of seasonal adaptations in insects. I. Patterns of cold hardiness, Appl. Entomol. Zool., 2005, vol. 40, no. 2, pp. 199–211.
Gornye tundry khrebta Bol’shoi Annachag (verkhov’e Kolymy) (Mountain Tundras of the Bol’shoi Annachag Ridge (Upper Reaches of the Kolyma River)), Vladivostok: Dal’nevost. Nauch. Tsentr Akad. Nauk SSSR, 1980.
Hao, S.G. and Kang, L., Supercooling capacity and cold hardiness of the eggs of the grasshopper Chorthippus fallax (Orthoptera: Acrididae), Eur. J. Entomol., 2004, vol. 101, pp. 231–236.
Hara, A. and Radin, N.S., Lipid extraction of tissues with a low toxicity solvent, Anal. Biochem., 1978, vol. 90, pp. 420–426.
Holmstrup, M., Polyol accumulation in earthworm cocoons induced by dehydration, Comp. Biochem. Physiol., 1995, vol. 111A, no. 2, pp. 251–255.
Holmstrup, M. and Westh, P., Dehydration of earthworm cocoons exposed to cold: a novel cold hardiness mechanism, J. Comp. Physiol. B, 1994, vol. 164, pp. 312–315.
Holmstrup, M. and Zachariassen, K.E., Physiology of cold hardiness in earthworms, Comp. Biochem. Physiol., 1996, vol. 115A, no. 2, pp. 91–101.
Leirikh, A.N., Cold hardiness of the arctic ensign scale Arctorthezia cataphracta (Homoptera, Coccinea: Ortheziidae) in the Northeast of Asia, Entomological Review, 2015, vol. 95, no. 8, pp. 1061–1065.
Leirikh, A.N. and Meshcheryakova, E.N., Methods for the study of cold hardiness of invertebrates, Zool. Zh., 2015, vol. 94, no. 8, pp. 972–984.
Leirikh, A.N., Meshcheryakova, E.N., and Berman, D.I., Mechanisms and ecological implications of cold hardiness in cocoons of the Earthworm Dendrobaena octaedra (Lumbricidae, Oligochaeta), Entomological Review, 2005, vol. 85, suppl. 2, pp. S220–S227.
Leirikh, A.N., Meshcheryakova, E.N., Kuz’minykh, G.V., and Kurenshchikov, D.K., Cold hardiness and development rate as elements of adaptive strategies of phalangiid harvestmen (Opiliones, Phalangiidae) in Northeastern Asia, Entomological Review, 2009, vol. 89, no. 3, pp. 323–331.
Low Temperature Biology of Insects, Denlinger, D.L. and Lee, R.E., Eds., New York: Cambridge Univ. Press, 2010.
Meshcheryakova, E.N. and Berman, D.I., The cold hardiness and geographic distribution of earthworms (Oligochaeta, Lumbricidae, Moniligastridae), Entomological Review, 2014, vol 94, no. 4, pp. 486–497.
Mikhaljova, E.V. and Marusik, Yu.M., New data on taxonomy and fauna of the millipedes (Diplopoda) from the Russian Far East, Siberia and Mongolia, Far East. Entomol., 2004, no. 133, pp. 1–12.
Miller, K., Cold-hardiness strategies of some adult and immature insects overwintering in interior Alaska, Comp. Biochem. Physiol., 1982, vol. 73A, no. 4, pp. 595–604.
Mosin, A.F. and Petrova, K.M., Some biochemical and hematological parameters in voles and lemmings in normal state and in starvation, in Ekologiya mlekopitayushchikh Severo-Vostochnoi Sibiri (Ecology of Mammals of Northeastern Siberia), Moscow: Nauka, 1980, pp. 90–97.
Poyas redkolesii verkhovii Kolymy (raion stroitel’stva Kolymskoi GES) (The Sparse Forest Belt of the Upper Reaches of the Kolyma River (the Kolyma HPP Construction Area)), Vladivostok: Dal’nevost. Nauch. Tsentr Akad. Nauk SSSR, 1985.
Prozorova, L.A. and Kavun, K.V., New species of terrestrial malacofauna of Southeastern Siberia and the Far East, in Bioraznoobrazie ekosistem Vnutrennei Azii: Tezisy Vserossiiskoi konferentsii s mezhdunarodnym uchastiem Ulan-Ude (Rossiya), 5–10 sentyabrya 2006 g. (Biodiversity of Ecosystems of Inner Asia: Proc. All-Russia Conf. with Int. Particip. Ulan-Ude (Russia), September 5–10, 2006), Ulan-Ude: Buryat. Nauch. Tsentr Sib. Otd. Ross. Akad. Nauk, 2006, vol. 1, pp. 167–168.
Pryce, Y.D., A simple, rapid method for determining glucose in blood of plasma, Analyst, 1967, vol. 92, no. 1092, p. 198.
Shekhovtsov, S.V., Berman, D.I., and Pel’tek, S.E., Phylogeography of the earthworm Eisenia nordenskioldi nordenskioldi (Lumbricidae, Oligochaeta) in northeastern Eurasia, Dokl. Biol. Sci., 2015, vol. 461, no. 1, pp. 85–88.
Sinclair, B.J., Insect cold tolerance: how many kinds of frozen?, Eur. J. Entomol., 1999, vol. 96, no. 2, pp. 157–164.
Vaskovsky, V.E. and Isay, S.V., Quantitative determination of formaldehyde liberated with periodate oxidation, Anal. Biochem., 1969, vol. 30, pp. 25–31.
Zambin, I., The cold hardiness of the Asiatic locust eggs, Zashch. Rast., 1939, vol. 19, pp. 48–56.
ACKNOWLEDGMENTS
The authors are grateful to their colleagues in the laboratory A.V. Alfimov, A.P. Bel’ger, Z.A. Zhigul’skaya, G.V. Kuz’minykh, E.N. Meshcheryakova, and A.A. Poploukhin for many years of fruitful cooperation. We are grateful to all specialists who assisted in determining the species and unselfishly shared knowledge on the biology of the objects studied: E.P. Bessolitzina, L.L. Budnikova, Т.S. Vsevolodova-Perel’, E.M. Dantzig, N.T. Zalesskaya, I.G. Kritskaya, L.A. Prozorova, and Ya.I. Starobogatov.
This study was supported by the Russian Foundation for Basic Research (project nos. 01-04-48921-а, 04-04-48187-а, 07-04-00362-а, 07-04-07028-d, 10-04-00425-а, 13-04-00156-а, and 16-04-00082-а).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Smolina
Rights and permissions
About this article
Cite this article
Berman, D.I., Leirikh, A.N. Cold Hardiness of Mass Soil Invertebrate Animals of Northeastern Asia: 1. Cold Hardiness and the Mechanisms of Its Maintenance. Biol Bull Russ Acad Sci 45, 669–679 (2018). https://doi.org/10.1134/S1062359018070038
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359018070038