Abstract
The distribution of soil invertebrates from different taxa and the adaptive potential of all three cold hardiness strategies in the cold climate of northeastern Asia are analyzed. The correlation between the resistance to low temperatures and the overwintering conditions in habitats of some species is studied. The mechanisms and degree of cold hardiness are shown generally to be unrelated to the taxonomic proximity. The effect of the resistance to low wintering temperatures on the habitat distribution and faunogenesis of soil invertebrates in permafrost regions is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the first part of our article (Berman and Leirikh, 2017), the cold hardiness of 64 species of soil-dwelling invertebrates of northeastern Asia, belonging to six taxonomic groups, was considered. This number of species is clearly not sufficient for constructing a general picture of invertebrate adaptation to extremely low winter temperatures in the region. However, further accumulation of material without comprehending the results would not be advisable. The task of this article is to give a comparative characterization of the cold resistance of soil invertebrates and to assess its role in limiting the landscape distribution and geographical distribution of these animals.
EFFECTIVENESS AND OCCURRENCE OF THE COLD-HARDINESS MECHANISMS
As reported in the first part of our work (Berman and Leirikh, 2017), in northeastern Asia, all three currently known cold-resistance strategies have been identified in soil invertebrates: protective dehydration, supercooling, and extracellular freezing of liquids (Fig. 1). Apparently, protectivedehydration can be considered the most effective mechanism of resistance to low temperatures, in extreme cases ensuring the survival of cocoons of the earthworm Dendrodrillus rubidus tenuis (Eisen 1874) at –196°C (Berman et al., 2010b) (Fig. 2). The proportion of invertebrates among the studied taxa that use this mechanism is small: apart from the cocoons of earthworms, these are the eggs of three species of slugs and the larvae of several click beetles. Their dimensions are small, and the covers are permeable to water, which is probably necessary for this way of cold protection. Another indispensable condition is also the possibility to accumulate polyols (cocoons of earthworms) or polyols in combination with reserve lipids (larvae of click beetles).
Freeze tolerance can ensure the survival of invertebrates at temperatures down to –83°C (Miller, 1982; Lee, 2011), and probably lower. In our studies, only Tetrix fuliginosa (Zetterstedt 1828) and the larva of Denticollis varians (Germar 1846) withstood temperatures below –40°C in the frozen state. The resistance of other freezing organisms ranged from –5°C (the earthworm Aporrectodea caliginosa (Savigny 1826)) to –35°C (the amphipod Traskorchestia ditmari (Derzhavin 1923)). This mechanism is common in a larger number of taxa than the previous one; it is also used by all the earthworms that survive negative temperatures (five species out of 13 studied), the click beetle Hypnoidus hyperboreus (Gyllenhal 1827), four species of millipedes, and two species of slugs.
The ability to withstand freezing in earthworms is provided by the synthesis of polyols and glucose from stored glycogen. However, few species are capable of accumulating significant amounts of it, due to the peculiarities of the structure of the chloragogenic tissue, and initially, the reserve glycogen seems to have provided survival in the summer diapause (Byzova, 1977).
The supercoolingmechanism prevails in soil-dwelling invertebrates. Owing to the low temperatures of supercooling (supercooling point, SCP), the following wintering species do not freeze: ants, eggs of locusts and harvestmans, the majority of larvae of click beetles, scale insect Arctorthezia cataphracta (Shaw 1794), and terrestrial gastropods shell mollusks. The effectiveness of this mechanism is comparable to the previous two: the lowest average SCP of some species reaches –40…–44°C, and the minimum values are below –50°C: the ants Leptothorax acervorum (Fabricius 1793), L. muscorum (Nylander 1846), and Camponotus herculeanus (Linnaeus 1758). In most of the species studied, the mean SCP amounts to approximately –30°C. The smallest resistance is observed in the majority of shell mollusks and four of five species of ants of the genus Formica. All the eggs of insects studied to date overwinter in this state.
SEASONAL DYNAMICS OF COLD HARDINESS
The nature of seasonal changes in cold hardiness is not similar even in the animals that use the same mechanism, let alone different ones.
The seasonal degree of the ability to supercool was traced in detail in ants. Changes in SCP from summer to winter in the species of the genus Formica amounted to 6–12°C and in the other ants, 22–30°C. The decrease in SCP was accompanied by a considerable stabilization of the supercooled state in Formica: in summer, a 24-hour exposure at a temperature that is 10–12°C higher than SCP is fatal for ants; in winter, it is fatal if the temperature is only 3–5°C higher. The cold hardiness increased gradually from August to December, in larvae, it increased at a faster rate than in the working individuals. Over the next four months, SCP remained stably low, rising only after reaching –10°C in the nests.
Measurements of the cryoresistant parameters of other invertebrates are usually carried out in the middle of summer (July) and late winter (March), in the presence of a sufficient number of animals, and also in August–September. For the invertebrates overwintering in the supercooled state in the postembryonic stages of development, the seasonal changes in the supercooling temperature and the ratio of SCP and the thresholds of the temperatures endured fit into the range given above for the ants. The smallest changes are observed in the species with low winter cold hardiness (shell mollusks) or in those that have summer nonspecific resistance (scale insects); more significant changes are observed in the larvae of click beetles. In locusts and harvestmans, the difference between the SCP of freshly laid and overwintering eggs does not exceed 3°C and is, possibly, determined not by the temperature drop in fall, but by the diapause stage.
Earthworms of the 9th genetic line of the nominative subspecies Eisenia nordenskioldi (Eisen 1879), the only one in the northeast (Shekhovtsov et al., 2015), do not tolerate freezing in summer; this ability appears at the end of August. By the middle of winter, worms accumulate up to 0.3% of glycerin and can withstand cooling to –35°C. On the contrary, the centipede Lithobius steingeri Bollman 1893, which overwinters in a frozen state, can withstand cooling by 2°C lower than SCP even in summer, and in winter, by more than 30°C lower.
The seasonal changes in the cold hardiness of eggs of slugs hibernating in the state of protective dehydration differ from those described above for supercooling locust embryos. The change in SCP of the latter as a result of acclimation is small, whereas the cold resistance of the pre-acclimated eggs of Deroceras reticulatum (Müller 1774) (below –30°C) exceeds the threshold of nonacclimated eggs by more than two times (Berman et al., 2011).
CHEMICAL SUPPORT OF THE COLD HARDINESS MECHANISMS
The chemical support of the three above-mentioned cold resistance mechanisms can be either different or even the same within a single family of invertebrates. The lowest average SCP (about –40°C) is characteristic of three species of ants: Leptothorax acervorum, L. muscorum, and C. herculeanus; their capacity for significant supercooling is associated with the accumulation of 16–20% of polyols (Berman et al., 2010c) (Table 1). The C. herculeanus population from the upper Kolyma is more cold-resistant than the population from the more southern and warmer North American region (Pincher Creek, Alberta, Canada, 50° N, 114° W), where the mean SCP was –28.7°C and the glycerin content was 5.8% (Sømme, 1964). Among the previously studied species of the genus Camponotus, the mean SCP values for the imago of the Kolyma population are the lowest (Berman et al., 2017); i.e., these ants probably have a cold resistance that is close to the maximum for the genus. Apparently, the Kolyma population lives here at the limit of its physiological capabilities, as evidenced by the significant winter death in almost every one of the 20 nests surveyed.
The survival of representatives of other taxa that bear temperatures below –30°C is provided by other mechanisms. E. nordenskioldi, T. fuliginosa, amphipods, and centipedes are resistant to freezing; the cocoons of earthworms have protective dehydration. The cold-protective agents can be polyols playing the role of cryoprotectants, as in ants (Berman and Leirikh, 1985), or substances of an unidentified nature, as in the groundhoppers, amphipods, and D. varians larvae (Berman et al., 1989, 1990, 2013).
In the animals with moderate cold resistance, the mechanisms of maintenance are equally diverse. For the most part, they overwinter in a supercooled state with a SCP of about –30°C. The decrease in SCP in winter can also be accompanied by the accumulation of polyols and sugars, but in a smaller amount than in the first group, up to 10% in the ants Myrmica kamtschatica Kupianskaya 1986 (Berman et al., 2012). In the overwintering eggs of locusts and harvestmans, a strong shell, which prevents inoculation of ice from the outside, contributes to the low values of SCP, as well as the absence of internal crystallization centers; hence, they do not need to accumulate antifreeze (Leirikh et al., 2009). The larvae of click beetles overwinter both in the state of protective dehydration and in the supercooled state; both mechanisms can be associated with an increase in the concentration of polyols or can be provided without their involvement (Berman et al., 2013).
The species the least resistant to cold are characterized by SCP values in the range of –17…–22°C and small contents of low-molecular antifreezes; cold hardiness is achieved, in particular, in the ants of the genus Formica due to the higher stability of the supercooled state compared to the summer one (Berman et al., 1984, 1987, 2010c).
Several species resistant to freezing are characterized by glycoproteins, which play the role of cryoprotectants, which can prevent the recrystallization of ice (Low Temperature…, 2010). These compounds previously found in Lithobius forficatus (Linnaeus 1758) (Tursman and Duman, 1995) are likely to contribute to the cold hardiness of the centipedes studied, as well as Tetrix fuliginosa and amphipods. This is indirectly indicated by the absence of an increase in the concentration of low-molecular cryoprotectants from summer to winter.
For the species that can use any of the three survival routes at negative temperatures, but their resistance is ensured by the accumulation of polyhydric alcohols, the decisive factor for producing maximum cold resistance is probably the presence of reserve glycogen by the time negative temperatures occur. However, the reserves accumulated for the fall can be spent on metabolism during the period before the substrate in which they overwinter freezes. It can be assumed that in cases of prolonged fall transition or overwintering at low positive temperatures (which is frequent in the European part of Russia), the maximum resistance possible for a species may not develop. The interannual variability of the characteristics of cold hardiness is apparently associated with different weather conditions during summer and fall, which determines the conditions for the accumulation and consumption of reserves. A convincing argument in favor of the foregoing is the considerable variability of the mean SCP of ants from different (in terms of overwintering conditions) habitats in the same locality (Berman and Zhigul’skaja, 1995; Berman et al., 2010c).
POPULATION DIFFERENCES IN COLD HARDINESS
Proceeding from the above, it is difficult to expect similar cryoresistance in animals from populations inhabiting different climates; it is more likely only in the species that hibernate at the egg stage. In the Northern Hemisphere, more than 450 species of insects that hibernate in a supercooled state have been studied. For only 25 species was the temperature of maximum supercooling (SCP) of two or more populations geographically removed from each other obtained (Turnock and Fields, 2005). The temperature characteristics of the winter shelters of the vast majority of these species are not given. For only three of them, which overwinter in the crowns or under the bark of trees (i.e., above the snow level) was a correlation between SCP and the temperatures of the environment shown. In general, the available data are absolutely insufficient to draw conclusions about the geographical variability of the cold hardiness of invertebrates in the supercooled state.
Unfortunately, we have studied very few of the objects studied in other regions: the ants Camponotus herculeanus (Sømme, 1964), slugs Deroceras laeve (Müller 1774) (Storey et al., 2007) and D. reticulatum (Bale, 1985; Cook, 2004), earthworms Eisenia nordenskioldi (Holmstrup and Petersen, 1997), grasshoppers Chorthippus fallax (Zubovski 1900) (Hao and Kang, 2004). In these species, except for Ch. fallax (eggs), in the populations from the Northeast, a significantly greater resistance to negative temperatures was found.
It should be noted, however, that it is practically impossible to make such a comparison correctly, since different authors use, as a rule, slightly different techniques of collecting and keeping specimens, as well as acclimating, cooling and heating rates, etc.
We also studied several species of invertebrates hibernating in biotopes differing in the minimum soil temperatures within the same region and in regions with different climatic characteristics. In the coldest of them, in the upper Kolyma, a statistically significant difference of SCP was found in the Leptothorax acervorum ants at 2.0°C and their larvae at 6.2°C in two batches of eight nests with a difference of 8–10°С in the minimum temperatures at the depth of the overwintering chambers in the soil of the habitats (Berman et al., 2010c). Larger differences in SCP that correlate with the overwintering conditions were found in the L. acervorum ants (up to 20°C) and in three species of the genus Formica (up to 12°C): Formica exsecta Nylander 1846, F. gagatoides Ruzsky 1904, and F. lemani Bondroit 1917 from the populations of the upper Kolyma, the environs of Magadan, and from Finland (Berman et al., 2010c). However, in the ants of the genus Myrmica, no such connection of SCP with the environmental temperatures was found (Berman et al., 2012).
Estimation of the population differences in the cold hardiness of invertebrates overwintering in the frozen state is even more difficult, as less standardized methods and criteria for evaluation are used and the number of studied species is small. According to our data, in the earthworms Dendrobaena octaedra and their egg cocoons from different climatic zones, the values of cold hardiness are similar (Meshcheryakova and Berman, 2014). The extreme tolerable temperatures of Lumbricus rubellus cocoons hibernating in a state of protective dehydration are also similar. In the species that endure freezing from other groups from geographically distant populations, these characteristics are, probably, similar.
THE TAXONOMIC LOCATION, ONTOGENETIC STAGES, AND MECHANISMS OF COLD RESISTANCE
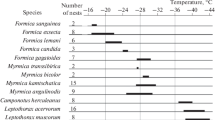
The mechanisms of cold resistance and its possible limits are not related to the taxonomic proximity of the animals (Fig. 3). Here are the most striking examples. The cold hardiness of five species of the genus Formica inhabiting northeastern Asia proved to be different: their average SCP values range from –17 to –30°C. On the contrary, four species of the genus Myrmica have almost identical resistance. Recall that the mechanism ensuring the cold resistance of all ants is the same: supercooling (Berman et al., 2010c).
In another insect family, the click beetles (Elateridae), all three known cold hardiness strategies have been identified with a very close result: the larvae endure temperatures of about –25°C, except for Denticollis varians larvae, which survive in a frozen state below –40°C (Berman et al., 2013).
Two species of slugs of the same genus have different mechanisms of cold hardiness (as well as different life cycles): frozen Deroceras laeve survives cooling to –28°C, while D. reticulatum successfully hibernates in the egg stage in the state of protective dehydration at temperatures down to –35°C (Berman et al., 2011).
The earthworm of the 9th line of the nominative subspecies Eisenia nordenskioldi hibernates in both stages and can withstand up to –35°C (Berman and Leirikh, 1985); another species of the genus, E. fetida, does not tolerate negative temperatures at any stage (Berman et al., 2009). In contrast, the earthworms Dendrobaena octaedra (Savigny 1826) (Opisthopora, Lumbricidae) and Drawida ghilarovi Gates 1969 (Moniligastrida, Moniligastridae), which belong to different orders, overwinter in a frozen state and have the same thresholds of endured temperatures of about –16°C (Berman et al., 2002, 2010a).
The eggs of various invertebrate animals investigated by us do not survive freezing (Leirikh et al., 2009; Berman et al., 2011), as well as all eggs (more than 70 species) of insects, ticks, spiders (Sømme, 1982), enchytraeids (Bauer et al., 2001), and earthworms (Holmstrup et al., 2002; Meshcheryakova and Berman, 2014) studied to date. They can hibernate only in supercooled (eggs of insects and harvestmans) and dehydrated (eggs of earthworms, slugs) states.
The strategy ensuring the cold hardiness of the insect larvae overwintering in the Northeast is different. The larvae of some insects may be supercooled (ants, Arctic scale insect, click beetles: Orithales serraticornis (Paykull 1800), Sericus brunneus (Linnaeus 1758), Limonius koltzei Reitter 1895, Selatosomus melancholicus Fabricius 1798, S. gloriosus (Kishii 1955), S. impressus (Fabricius 1792, Prosternon sericeum (Gebler 1824), Ampedus sp.). The larvae of others (Denticollis varians) can freeze or significantly dehydrate (the click beetles of the genus Oedostethus and the species Hypnoidus hyperboreus).
The imagoes of ants, scale insects, and some click beetles (Orithales serraticornis, Selatosomus melancholicus, and Sericus brunneus) are supercooled; those of one of the species of click beetles (H. hyperboreus) are capable of freezing, like all postembryonic stages of Tetrix fuliginosa.
In representatives of other studied taxa of invertebrates (centipedes, amphipods, slugs and their eggs, earthworms and their egg cocoons), the ability to freeze or protective dehydration predominate (Fig. 1). Only small terrestrial gastropods mollusks and the eggs of harvestmans overwinter in a supercooled state. Centipedes, lithobiids of two species, and one species of geophilids withstand cooling to ‒25…‒31°С in the frozen state. The amphipod Traskorchestia ditmari also freezes (at –6°C) and successfully overwinters in this state at temperatures down to –35°C.
The earthworms of five out of 13 taxa studied survive negative temperatures owing to their resistance to freezing, while their egg cocoons survive in a state of protective dehydration. However, the cocoons of some species cannot effectively dehydrate and dehydration does not always serve as a guarantee of cold resistance (Meshcheryakova and Berman, 2014).
COLD RESISTANCE AND BIOTOPICAL DISTRIBUTION
By the degree of adaptation to the winter conditions of the continental part of northeastern Asia, the animals studied can be divided into several groups.
The first group consists of species with high cold hardiness, which withstand temperatures of –30… ‒35°C and below. They are almost unrestricted by winter temperature conditions in their biotopic distribution and dominate in the background landscapes and biotopes, the common features of which are the high location of the permafrost mirror (50‒60 cm), excessive moistening of the soil, and predominance of the moss–lichen–shrub cover. These include two species of ants (Leptothorax acervorum and Camponotus herculeanus), a groundhopper (Tetrix fuliginosa), and a click beetle (Denticollis varians). These insects winter in the surface horizon of the soil, under the litter and snow, which noticeably softens the temperatures (from –50°C in the air to –20…–25°C at a depth of 5 cm in the soil).
Several species of this group, possessing equally significant cold hardiness, are found locally because of the limitations imposed by summer conditions (distribution of moisture, pH, etc.). Among them are the earthworm Eisenia nordenskioldi, the amphipod Traskorchestia ditmari, and the ant Leptothorax muscorum.
The group can also include two lithobiids with LT50% of –30°C; their maximum tolerated temperatures are below –35°C, but their exact value could not be determined due to the lack of necessary equipment. Thus, we consider nine out of 64 studied species as belonging to the group of invertebrates the most resistant to cold. Among them, the organisms that overwinter in a frozen state are predominant. Separately, mention should be made of species not included in this group with the cold hardiness of egg cocoons below –35°C, but less resistant at the postembryonic stages. These are the earthworms Dendrobaena octaedra and Dendrodrilus rubidus tenuis, and the slug Deroceras reticulatum. In the continental regions of the Northeast, they are absent owing to the insufficient duration of the warm season for the completion of the life cycle “from egg to egg.”
The second group includes 36 species, which can withstand temperatures of –20…–25°C in winter. It consists mainly of insects that are overwintering at different stages in the supercooled state: adults and larvae of ants of the genus Myrmica, imagoes of Formica gagatoides, locust eggs, scale insects Arctorthezia cataphracta, and most larvae of click beetles (9 of 13 species). Among the representatives of other taxa, there are the harvestmans hibernating at the egg stage and the terrestrial mollusk Vertigo modesta (Say 1824). The freezing geophilid Escaryus japonicas Attems 1927 and the slug Deroceras laeve can also be included. The overwintering of the named ants and the larvae of click beetles takes place at a slightly greater depth, i.е., in milder temperature conditions than for the species of the first group. The biotopic distribution of all these invertebrates is hardly limited by winter temperatures in the belt of sparse forests; they are numerous and are among the dominants of the population. Some species are found locally, but their distribution is due not to insufficient cold hardiness, but to unfavorable summer conditions: low heat availability, the depth of the possible arrangement of the overwintering chambers of ants, etc.
The third group includes species with limited cold resistance, which withstand temperatures of ‒10…‒15°С. These are four of five species of ants of the genus Formica and four species of gastropods of land shell mollusks. They all hibernate in a supercooled state and are found locally in warmer areas. Some species of ants settle in places that quickly and deeply thaw in spring, and in winter, owing to the snow cover, preserve a higher temperature of the horizons than the background ones.
Several species of animals inhabiting the coast of the Sea of Okhotsk (vicinity of Magadan) with a milder climate but not penetrating the continental areas of the region have the same cold hardiness. Among them, the earthworm Dendrobaena octaedra, which hibernates in a frozen state and survives temperatures to –14°C, and two invasive species of slugs: Deroceras altaicum (Simroth 1886) and D. agreste (Linnaeus 1758).
Finally, two types of earthworms—Eisenia fetida and Dendrodrillus rubidus tenuis—are found only in the anthropogenic conditions in the Northeast. The first, owing to the inability to survive temperatures below zero at both stages of development, lives exclusively in nonfreezing locales: along poorly insulated heating pipelines, in “streams” of constant discharge of warm technical waters, in greenhouses heated year round, etc. The cocoons of the second species survive extremely low temperatures, but the life cycle in natural habitats cannot be completed in one season (because of its shortness). In the greenhouses heated in spring (which prolongs the vegetation period), the number of the worms D. rubidus tenuis can reach many hundreds of individuals in August, and that of cocoons, tens of thousands per 1 m2.
Cold resistance is certainly not the only adaptation of invertebrate animals in northeastern Asia, contributing to successful existence in extreme climatic conditions. A number of other adaptations are also traced. In ants, this is a reduction in the need for summer heat supply due to changes in the life cycle: the lack of rapid brood and larvae development over two to three years (Zhigul’skaya et al, 1989, 1992). In addition, the least cold-resistant species survive in relatively warm sites (at the depth of the overwintering chambers) that occupy a small area in general (Berman et al., 2010c).
The overwintering of the immature stages, characteristic of the groundhopper Tetrix fuliginosa and the slug Deroceras laeve, allows them to stretch their development over two seasons or more and, thus, not depend on the weather conditions of the short northern summer (Berman et al., 1989, 2011). The groundhopper has an imaginal diapause (Berman et al., 1989), which was not found in slugs. In England, the slugs D. reticulatum of different ages overwinter, and usually two overlapping generations are present in the population (Bale, 1985). In the conditions of Magadan, the life cycle of this species is seasonally synchronized: the slugs die in the first colds, while the eggs have an embryonic diapause. In contrast, Deroceras laeve is able to tolerate negative temperatures only in the postembryonic stages and it would be natural to expect this species to have an imaginal diapause.
The embryonic perennial diapause (“superdiapause”) undoubtedly facilitates the survival of locusts in northeastern Asia (Bei-Bienko and Mishchenko, 1951; Uvarov, 1966; Berman and Leirikh, 2006). In the southern regions, this property contributes to the survival of the population in arid years (Uvarov, 1966); in the north, it helps survive cold years or the years with abundant summer precipitation.
COLD HARDINESS AND THE GEOGRAPHICAL DISTRIBUTION
The role of cold hardiness in the geographical distribution of animals could be traced in separate species of a small number of groups, since for most of the species studied there are no data on the cold resistance in the southern part of the range.
Ants. The high cold hardiness of the ants of the hypoarctic complex allows them to survive extremely severe winters in the surface horizons of the soil throughout northern Eurasia, including the pole of cold of the Northern Hemisphere in Oimyakon and Verkhoyansk. On the contrary, the limited ability to supercool in the ants of the genus Formica is the reason for the depletion of their fauna in the upper reaches of the Kolyma River and even more so in the upper reaches of the Yana and Indigirka rivers with even lower winter temperatures.
In general, the obtained cold resistance characteristics for most species are apparently close to the maximum possible for ants. This is indirectly indicated by the aforementioned impoverishment of the fauna in the upper Indigirka basin relative to the upper Kolyma basin and, even more so, Central Yakutia (Berman et al., 2010c). The toughening of winter conditions in northeastern Yakutia combined with lower summer heat supply in comparison with the mentioned regions proves insurmountable for many species of the genus Formica, and possibly Myrmica.
Thus, the insufficient resistance of ants to low temperatures is a powerful factor of faunogenetic processes, along with a lack of summer heat for ontogenesis, biotic relationships, and historical reasons.
Slugs. Three of the four species studied by us (except for Deroceras laeve) are invaders; they were discovered near Magadan not only locally, but also separately from each other. In the interior of the continent, these species were not found, whereas the cold resistance of eggs, at least in D. reticulatum, is more than sufficient for a successful overwintering in continental areas. The absence of these species is associated with circumstances other than the overwintering conditions, especially with the short, frost-free period. The limiting factors are probably insurmountable, as the invasion has probably lasted at least 75–80 years, since the beginning of the intensive development of the region (Berman et al., 2011).
Harvestmans. The revealed cold hardiness of the hibernating eggs of harvestmans shows that Mitopus morio (Fabricius 1799) and especially Homolophus arcticus Banks 1893 are not limited by low winter temperatures not only in the vicinity of Magadan, but in many biotopes of the continental areas of Magadan oblast as well (Leirikh et al., 2009). The preliminary data obtained on the rate of development indicate that the time (and heat supply) for completing the life cycle of M. morio and H. arcticus is sufficient for one season; and in warm years there may even be some reserve. In cold summers, some animals probably do not have time to lay eggs before winter. In general, both species of harvestmans are common in the vicinity of Magadan every year and their populations are stable.
Earthworms. The species diversity of earthworms on the Russian plain is decreasing to the north and east. From western Siberia to the east, not counting the valleys of large rivers, mountains (Altai-Sayan system, Sikhote-Alin, etc.), and disturbed territories, one or two species are found almost everywhere (Vsevolodova-Perel’, 1997).
The most cold-resistant earthworm is the 9th genetic line Eisenia nordenskioldi nordenskioldi, inhabiting the north of Asia and Yakutia; it is not limited by the temperature conditions of overwintering. The reaction to low negative temperatures of earthworms of the Eisenia nordenskioldi complex of other lines (Berman and Meshcheryakova, 2013; Shekhovtsov et al., 2015, 2017; Berman et al., 2016) has not been studied. Eisenia sibirica Perel et Graphodatsky 1984 tolerates at least –12°C, and its limited abilities to overwintering at low temperatures prevent the expansion of the range of this endemic species (Berman et al., 2016). The severity of the winters, which increases from west to east, prevents the penetration of the cosmopolitan species of Dendrobaena octaedra into the tundra and taiga of Siberia farther than the isotherms of minimum soil temperatures at a depth of 3 cm –12…–14°C (Berman et al., 2002). The overwintering conditions also control the progress of the earthworm Drawida ghilarovi from the south to the north of Khabarovsk krai, which withstands temperatures of up to –16°C in the frozen state (Berman et al., 2010a). The spread of Aporrectodea caliginosa is limited by resistance to cold in the phase of the worm (–5°C) and the possibility of completion of ontogenesis (including the deposition of cocoons) in one season. The habitat of the egg cocoons of species with a high cold resistance (Dendrodrilus rubidus tenuis, Octolasion lacteum, Lumbricus castaneus, L. rubellus) is bounded to the east by the biotopes that either do not freeze in winter (the phase of the worm does not survive at temperatures below zero) or are so warm in summer that they ensure completion of the life cycle. The distribution of species that do not survive cooling to –2…–5°C at any stage of development is also associated with the habitats that do not freeze from the surface (Eisenia fetida, Eiseniella tetraedra, and Aporrectodea rosea) or with deep soil horizons (Lumbricus terrestris) (Mescheryakova and Berman, 2014).
Our research allows us to answer unequivocally the question of the primary role of winter or summer climatic conditions in the formation of regional faunas of the investigated groups. Thus, the considerable similarity of the winter temperature (and also permafrost) conditions in the Upper Kolyma basin and Central Yakutia and the striking difference between their faunas indicate that the impoverishment of the species diversity of ants and orthopterans of the upper reaches of Kolyma consists primarily in the lack of summer heat supply. The poverty of the diversity of slugs and earthworms in the continental regions of northeastern Asia is caused not only by the insufficient resistance of the wintering stages, but also by the short duration of the warm period, which prevents the achievement of the cold-resistant life cycle stages by the onset of cold weather (different species may have different stages). The role of historical causes is also not excluded: the possibility of restoration of a species extinct due to, for example, an anomalously cold snowless winter on the gigantic territory of northeastern Asia is small, as, unlike in Central Yakutia, there is no close source of secondary (reparative) invasion.
The most important conclusion from the work done is that in taxonomically close species there may be unequal mechanisms and values of cold resistance. Different ontogenetic stages of the species can also have different resistance to negative temperatures, determined by different mechanisms. From what has been said, it follows that nowadays, as a rule, it is impossible to predict the cold-resistance of even the next species of the genus, knowing that of others. At best, we can guess (not predict!) the type of mechanism of cold protection.
This pessimistic conclusion, of course, does not mean the inevitability of a total scanning of all invertebrates, but certainly obliges us to search for heuristic approaches. We would like to draw attention to four of them, which seem to be promising for studying the most common issues of cold resistance.
(1) The emergence of cold resistance was not discussed in the text of this article. The available information on the prevalence of strategies in different arthropod taxa is insufficient to formulate hypotheses about their evolution (Choun and Sinclair, 2010).
Significant variations in resistance within the genus can be seen both as the youth of the manifestation of the trait, and as its conservatism. There is an obvious temptation to link the origin of cold resistance to the last glaciation. One of the particular pieces of evidence for this is the high cryoresistance of earthworms of the tropical genus Drawida (Berman et al., 2010a), which penetrated into Primor’e. The formation of cryoresistance of D. ghilarovi can be clarified by studying this trait in close relatives inhabiting Central China and its southern regions.
In favor of the ancient origin, one can consider the cold resistance of different species as a reflection of their former habitat in cold regions. Recall that cryochrons in the history of the Earth are by no means a rarity. During the Pleistocene (1.7 million years), there were at least 16 large (“great”) glaciations. But the age of most invertebrates with which we are dealing is much greater. Moreover, the morphological features of Pleistocene insects that preserved in the permafrost and the same species that exist now are not distinguishable (Kiselev, 1981; Matthews, 1977, 1977a).
Cryochrons are known to have existed much earlier than the Pleistocene (Zimy…, 1982). The localization of the glaciers was by no means identical, which determined the difference in the distribution of landscapes, the distribution of organisms, and, in the end, the formation of cold resistance that interests us. Thus, the roots of the development of adaptive strategies to the cold, probably, go deep into history. In this case, it is necessary to talk about adaptations that have arisen beyond the limits of the time possible for discussion.
Among such cases will be pre-adaptations: adaptations that have emerged as a “by-product” of adapting to a factor other than cold. For example, it seems obvious that protective dehydration could have arisen on the path to adaptation to dryness.
(2) In the corresponding section of this article and in the previously published works (Meshcheryakova and Berman, 2014), it was shown that the cryoresistance of earthworms and their cocoons is in no way connected and that their ratio can be anything. Recall, for example, that the cocoons of Dendrodrilus rubidus tolerate staying in liquid nitrogen, and worms cannot withstand cooling below zero, although worms and cocoons overwinter in the same horizon, in the litter. On the contrary, the two phases of Eisenia nordenskioldi of the 9th genetic line are extremely resistant to low temperatures.
The reasons for the lack of connection lie in the fundamentally different mechanisms ensuring cold resistance. Worms of most of the studied taxa (11 of 15) remain unfrozen only until –5…–3°C (i.e., supercooled); with a further decrease in temperature, they become frozen and die. Representatives of the other four taxa belonging to different genera, on the contrary, survive freezing, i.e., the formation of ice in the body.
The cocoons of all species, except Eisenia fetida (Savigny 1986), have a mechanism of protective dehydration. Resistance to low temperatures in this case is associated with the loss of almost all osmotically active water. Switching mechanisms from the protective dehydration of cocoons to the ability of worms to withstand freezing or supercooling occurs simultaneously upon hatching. Apparently, it is the appearance of the centers of crystallization on the surface of the body that is the trigger of this process.
To what extent are the values of cryoresistance of worms and their cocoons obtained in the experiment variable? Do they depend on the temperature conditions of overwintering? In other words: is the measured cold resistance variable or temperature-dependent and does it reflect the adaptive “behavior” that is within the reaction norm, or is this parameter a stable species trait? The materials on the resistance of worms from geographically distant populations remote from one another that are available at our disposal allow us to regard it as a species-specific, slightly variable feature, i.e., evolutionarily fixed. It follows that the evolution of resistance to negative temperatures of worms and their cocoons has probably proceeded in different ways, although both ontogenetic stages inhabit one environment: the soil.
This case is another example of the independent evolution of two ontogenetic stages of one organism (embryo and imago), previously shown for larvae and adults of insects inhabiting the soil and terrestrial layers, respectively (Gilyarov, 1949).
Owing to the above information, an in-depth parallel study of the relation of imagoes and preimaginal stages of the same species using different mechanisms of ensuring cold resistance to negative temperatures is extremely interesting and important.
(3) On the basis of the above materials and the synthesis of the few existing literature sources, it can be assumed that in the animals that survive negative temperatures in the supercooled state and in freeze tolerant species, the geographic variability of cold hardiness is different.
Invertebrates that hibernate above the snow level generally survive freezing, which occurs, as a rule, at small negative temperatures: ‒5…‒10°С. Apparently, their ultimate resistance is initially “tuned” to very low air temperatures in both moderate and extracontinental climates.
The cold resistance of animals that hibernate in the supercooled state, on the contrary, depends on the actual temperatures of the environment: the lower it is, the lower the maximum supercooling temperature, the greater the cold resistance. It varies in soil-dwelling invertebrates inhabiting even neighboring biotopes with different thicknesses of the snow cover, i.e., depending on the degree of thermal insulation of the overwintering sites (Berman and Zhigulskaja, 1995). The degree to which the ability of animals from regions with mild and severe winter climate conditions to respond quickly to changes in temperature differs (or, which is the same, the significance of the genetic component fixed by selection) remains unclear.
To test this hypothesis, it would be advisable to compare the cold hardiness of the marginal (eastern and western) populations, which survive freezing, and the supercooling widely distributed species, as the January isotherms are mainly oriented by the geographic longitude. In general, the problem is reduced to determining the resistance of both groups of animals from the regions that differ drastically in terms of climate. The studied populations from the continental regions of the northeast (as one of the coldest regions) could be used as a reference.
(4) It should be borne in mind that the identification of geographic variability of cold resistance must be carried out with caution, as it can be hampered by fluctuations associated with the weather conditions not only in winter, but also in preceding seasons. It depends on the dynamics of the formation, time, and character of spending reserve substances used by the organism for the synthesis of cold-protective substances. Their accumulation in the warm season depends on the temperature and humidity conditions. If the overwintering passes for most of the time at positive or near-zero temperatures, reserves can be spent on vital functions. Glycogen serves as a universal reserve substance, from which both sugars (trehalose) and polyhydric alcohols can be synthesized in the body of insects. Glycerin is the most common and metabolically beneficial of them; in detail, the ways of synthesizing polyols and sugars from glycogen were discussed by Storey and Storey (Storey, K.B. and Storey, J.M., 1991, 2012). With the early onset of temperatures that inhibit metabolism, reserves serve as the basis for the synthesis of cold-protective substances (Storey, K.B. and Storey, J.M., 1991, 2012), which provide an additional increase in resistance to cold. Figuratively speaking, the capacity of the battery is finite, and the charge can be spent on different purposes, but each time to the detriment of others. This idea is supported by many facts, unfortunately, scattered, obtained at different times, and from different sites. Therefore, at the moment, it cannot be strictly proven, but it seems very promising. It appears that the described approach is more applicable to animals that are wintering in the supercooled state rather than in the frozen one.
Part of the above conclusions depends to a large extent on the animal groups studied. These are the conclusions about the prevalence of the resistance mechanisms (supercooling, the ability to tolerate freezing, and protective dehydration), the biotopic distribution depending on the cold hardiness, the “tuning” of soil animals in general to the level of soil temperatures, etc. With another group of animals, the conclusions could be slightly different. In particular, the inclusion of a completely untouched mass component of almost any type of soil fauna—the Diptera larvae—in the work could significantly affect the results. The cold hardiness of the overwintering stages of representatives of the vast majority of beetle families has not been studied. Of course, it would be inadmissible to mix together inhabitants of strata different in depth: soil, litter, under snow, and above snow tiers of vegetation.
In conclusion, we emphasize that the predictions of a shift in the range of species under different scenarios of climate change for a territory with negative winter temperatures are impossible without an assessment of the cold hardiness of organisms. Unfortunately, we are still very far from solving such problems.
REFERENCES
Bale, J.S., Supercooling characteristics of adults, juveniles and eggs of the slug Deroceras reticulatum (Mull.), CryoLetters, 1985, vol. 6, no. 6, pp. 400–401.
Bauer, R., Worland, M.R., and Block, W., Experimental studies on cold survival of enchytraeid cocoons, Pedobiologia, 2001, vol. 45, pp. 561–571.
Bei-Bienko, G.Ya. and Mishchenko, L.L., Saranchevye fauny SSSR (Acridoidea of the Fauna of the USSR), Opredeliteli po faune (Identification Guides to Fauna), Moscow: Akad. Nauk SSSR, 1951, vol. 38, part 1; vol. 40, part 2.
Berman, D.I. and Leirikh, A.N., The ability of the earthworm Eisenia nordenskioldi (Eisen) (Lumbricidae, Oligochaeta) to endure subfreezing temperatures, Doklady Biological Sciences, Proceedings of the Academy of Sciences of the USSR. Biol. Sci. Sections, 1985, vol. 285, no. 1–6, pp. 845–848.
Berman, D.I., Alfimov, A.V., and Leirikh, A.N., Wintering conditions and cold resistance of the amphipod Traskorchestia ditmari on the coast of the Sea of Okhotsk, Biol. Morya, 1990, no. 5, pp. 31–36.
Berman, D.I. and Leirikh, A.N., Factors affecting the population dynamics of Acridoidea (Orthoptera: Insecta) of North-East Asia, in Populyatsionnaya ekologiya zhivotnykh. Materialy Mezhdunar. konf. “Problemy populyatsionnoi ekologii zhivotnykh” (Population Ecology of Animals: Proc. Int. Conf. “The Problems of Population Ecology of Animals”), Tomsk: Tomsk. Gos. Univ., 2006, pp. 275–277.
Berman, D.I. and Leirikh, A.N., Cold hardiness of mass soil invertebrate animals of northeastern Asia: 1. Cold hardiness and the mechanisms of its maintenance, Biol. Bull. (Moscow), 2018, vol. 45, no. 7, pp. 53–63.
Berman, D.I. and Meshcheryakova, E.N., Ranges and cold hardiness of two earthworm subspecies (Eisenia nordenskioldi, Lumbricidae, Oligochaeta), Biol. Bull. (Moscow), 2013, vol. 40, no. 9 pp. 719–727.
Berman, D.I. and Zhigulskaja, Z.A., Cold-resistance of the ants of the North-east and the North-west of the Palaearctic region, Acta Zool. Fenn., 1995, vol. 199, pp. 73–80.
Berman, D.I., Zhigul’skaya, Z.A., and Leirikh, A.N., Biotopic distribution and cold hardiness of Formica exsecta (Formicidae) in the northeastern boundary of the range (upper reaches of the Kolyma River), Byull. Mosk. Obshch. Ispytat. Prirody, Otd. Biol., 1984, vol. 89, no. 3, pp. 47–63.
Berman, D.I., Zhigul’skaya, Z.A., and Leirikh, A.N., Winter ecology of polar ant (Formica gagatoides) in the upper reaches of the Kolyma River, Zool. Zh., 1987, vol. 66, no. 3, pp. 373–384.
Berman, D.I., Leirikh, A.N., and Yakimchuk, N.V., Wintering and related biological characteristics of Tetrix fuliginosa (Orthoptera, Tetrigidae) in the Northeast of the USSR, Zool. Zh., 1989, vol. 68, no. 9, pp. 86–95.
Berman, D.I., Meshcheryakova, E.N., Alfimov, A.V., and Leirikh, A.N., The distribution of the earthworm Dendrobaena octaedra (Lumbricidae: Oligochaeta) in the north of the Holarctic is limited by its insufficient frost hardiness, Zool. Zh., 2002, vol. 81, no. 10, pp. 1210–1221.
Berman, D.I., Alfimov, A.V., Zhigul’skaya, Z.A., and Leirikh, A.N Overwintering and Cold-Hardiness of Ants in the Northeast of Asia, Sofia-Moscow: Pensoft, 2010c.
Berman, D.I., Leirikh, A.N., and Meshcheryakova, E.N., Cold hardiness of ontogenetic stages of the redworm Eisenia fetida (Oligochaeta, Lumbricidae), Zool. Zh., 2009, vol. 88, no. 3, pp. 272–279.
Berman, D.I., Meshcheryakova, E.N., Leirikh, A.N., and Kurenshchikov, D.K., Geographic range and cold hardiness of the earthworm Drawida ghilarovi (Oligochaeta, Moniligastridae), Biol. Bull. (Moscow), 2010a, vol. 37, no. 9, pp. 895–904.
Berman, D.I., Meshcheryakova, E.N., and Leirikh, A.N., Egg cocoons of the earthworm Dendrodrilus rubidus tenuis (Lumbricidae, Oligochaeta) withstand the temperature of liquid nitrogen, Dokl. Biol. Sci., 2010b, vol. 434, pp. 347–350.
Berman, D.I., Meshcheryakova, E.N., and Leirikh, A.N., Cold hardiness, adaptive strategies, and invasion of slugs of the genus Deroceras (Gastropoda, Pulmonata) in Northeastern Asia, Biol. Bull. (Moscow), 2011, vol. 38, no. 8, pp. 765–778.
Berman, D.I., Leirikh, A.N., and Zhigul’skaya, Z.A., A common strategy of cold hardiness in ants of the genus Myrmica (Formicidae, Hymenoptera) in Northeast Asia, Entomological Review, 2012, vol. 92, no. 3, pp. 247–261.
Berman, D.I., Leirikh, A.N., and Bessolitzina, E.P., Three strategies of cold tolerance in click beetles (Coleoptera, Elateridae), Dokl. Biol. Sci., 2013, vol. 450, pp. 168–172.
Berman, D.I., Bulakhova, N.A., and Meshcheryakova, E.N., The cold hardiness and range of the earthworm Eisenia sibirica (Oligochaeta, Lumbricidae), Sib. Ekol. Zh., 2016, no. 1, pp. 56–64.
Berman, D.I., Zhigulskaya, Z.A., and Leirikh, A.N., Cold resistance of an ant Camponotus herculeanus (Hymenoptera, Formicidae) in various climates in North-East Asia, CryoLetters, 2017, vol. 38, no. 1, pp. 17–28.
Byzova, Yu.B., The hemoglobin content in the worms Allolobophora caliginosa (Sav.) (Lumbricidae, Oligochaeta) during the summer rest period, Dokl. Akad. Nauk SSSR, 1977, vol. 236, no. 3, pp. 763–765.
Choun, S.L. and Sinclair, B.J., The macrophysiology of insect cold-hardiness, in Low Temperature Biology of Insects, Denlinger, D.L. and Lee, R.E., Eds., New York: Cambridge Univ. Press, 2010, pp. 191–222.
Cook, R.T., The tolerance of the field slug Deroceras reticulatum to freezing temperatures, CryoLetters, 2004, vol. 25, no. 3, pp. 187–194.
Gilyarov, M.S., Osobennosti pochvy kak sredy obitaniya i ee znachenie v evolyutsii nasekomykh (Features of Soil as a Habitat and Its Importance in the Evolution of Insects), Moscow: AN SSSR, 1949.
Hao, S.G. and Kang, L., Supercooling capacity and cold hardiness of the eggs of the grasshopper Chorthippus fallax (Orthoptera: Acrididae), Eur. J. Entomol., 2004, vol. 101, pp. 231–236.
Holmstrup, M., Bayley, M., and Ramløv, H., Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates, Proc. Natl. Acad. Sci. U. S. A., 2002, vol. 99, no. 8, pp. 5716–5720.
Holmstrup, M. and Petersen, B.F., Freeze-tolerance in the subarctic earthworm Eisenia nordenskioeldi (Eisen), CryoLetters, 1997, vol. 18, pp. 153–156.
Kiselev, S.V., Pozdnekainozoiskie zhestkokrylye severo-vostoka Sibiri (Late Cenozoic Beetles of Northeastern Siberia), Moscow: Nauka, 1981.
Leirikh, A.N., Meshcheryakova, E.N., Kuz’minykh, G.V., and Kurenshchikov, D.K., Cold hardiness and development rate as elements of adaptive strategies of phalangiid harvestmen (Opiliones, Phalangiidae) in Northeastern Asia, Entomological Review, 2009, vol. 89, no. 3, pp. 323–331.
Li, N.G., Some physiological features of the strategy of frost hardiness of Aporia crataegi L. (Lepidoptera: Pieridae) and Upis ceramboides L. (Coleoptera, Tenebrionidae) inhabiting Central Yakutia, Entomol. Obozr., 2011, vol. 82, pp. 3–10.
Low Temperature Biology of Insects, Denlinger, D.L. and Lee, R.E., Eds., New York: Cambridge Univ. Press, 2010.
Matthews, J.V., Jr., Tertiary Coleoptera fossils from the North American Arctic, Coleopterists Bull., 1977, vol. 31, pp. 297–308.
Matthews, J.V., Jr., Coleoptera fossils: their possible value for dating and correlation of late Cenozoic sediments, Can. J. Earth Sci., 1977a, vol. 14, pp. 2339–2347.
Meshcheryakova, E.N. and Berman, D.I., The cold hardiness and geographic distribution of earthworms (Oligochaeta, Lumbricidae, Moniligastridae) Entomological Review, 2014, vol 94, no. 4, pp. 486–497.
Miller, K., Cold-hardiness strategies of some adult and immature insects overwintering in interior Alaska, Comp. Biochem. Physiol., 1982, vol. 73A, no. 4, pp. 595–604.
Miller, L.K. and Werner, R., Extreme supercooling as an overwintering strategy in three species of willow gall insects from interior Alaska, Oikos, 1987, vol. 49, no. 3, pp. 253–260.
Shekhovtsov, S.V., Berman, D.I., and Pel’tek, S.E., Phylogeography of the earthworm Eisenia nordenskioldi nordenskioldi (Lumbricidae, Oligochaeta) in northeastern Eurasia, Dokl. Biol. Sci., 2015, vol. 461, pp. 85–88.
Shekhovtsov, S.V., Bazarova, N.E., Berman, D.I., Bulakhova, N.A., Golovanova, E.V., et al., DNA barcoding: how many earthworm species are there in the South of West Siberia?, Russ. J. Genet.: Appl. Res., 2017, vol. 7, no. 1, pp. 57–62.
Sømme, L., Effect of glycerol on cold-hardiness in insects, Can. J. Zool., 1964, vol. 42, pp. 87–101.
Sømme, L., Supercooling and winter survival in terrestrial arthropods, Comp. Biochem. Physiol., 1982, vol. 73A, no. 4, pp. 519–543.
Storey, K.B. and Storey, J.M., Biochemistry of cryoprotectants, in Insects at Low Temperature, New York: Chapman and Hall, 1991, pp. 64–93.
Storey, K.B. and Storey, J.M., Insect cold hardiness: metabolic, gene, and protein adaptation, Can. J. Zool., 2012, vol. 90, pp. 456–475.
Storey, K., Storey, J., and Churchill, T., Freezing and anoxia tolerance of slugs: a metabolic perspective, J. Comp. Physiol. B, 2007, vol. 177, no. 8, pp. 833–840.
Turnock, W.J. and Fields, P.G., Winter climates and cold hardiness in terrestrial insects, Eur. J. Entomol., vol. 102, no. 4, pp. 561–576.
Tursman, D. and Duman, J.G., Cryoprotective effects of thermal hysteresis protein on survivorship of frozen gut cells from the freeze-tolerant centipede Lithobius forficatus, J. Exp. Zool., 1995, vol. 272, no. 4, pp. 249–257.
Uvarov, B.P., Grasshoppers and Locusts. A Handbook of General Acridology, Cambridge: Cambridge Univ. Press, 1966, vol. 1.
Vsevolodova-Perel’, T.S., Dozhdevye chervi fauny Rossii: kadastr i opredelitel’ (Earthworms of the Russian Fauna: Inventory and Identification Guide), Moscow: Nauka, 1997.
Zhigul’skaya, Z.A., Kipyatkov, V.E., and Kipyatkova, T.A., Experimental study of seasonal cycles of the development of ants Leptothorax acervorum and Myrmica sp. 1 under Subarctic conditions, in Problemy okhrany i ratsional’nogo ispol’zovaniya poleznykh nasekomykh. Materialy Vseros. Soveshch. (Problems of Protection and Sustainable Use of Useful Insects: Proc. All-Russia Conf.), Voronezh, 1989, pp. 32–35.
Zhigul’skaya, Z.A., Kipyatkov, V.E., and Kipyatkova, T.A., Seasonal cycle of development of the ant Myrmica aborigenica in the upper reaches of the Kolyma River, Zool. Zh., 1992, vol. 71, no. 5, pp. 72–83.
Zimy nashei planety (Winters on Our Planet), John, B., Ed., Moscow: Mir, 1982.
ACKNOWLEDGMENTS
This study was supported by the Russian Foundation for Basic Research, project nos. 01-04-48921-а, 04-04-48187-а, 07-04-00362-а, 07-04-07028-d, 10-04-00425-а, 13-04-00156-a, and 16-04-00082-a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Smolina
Rights and permissions
About this article
Cite this article
Berman, D.I., Leirikh, A.N. The Cold Hardiness of Mass Soil Invertebrates of Northeastern Asia: 2. The Cold Hardiness of Soil Invertebrates as Adaptation to Climate. Biol Bull Russ Acad Sci 45, 680–690 (2018). https://doi.org/10.1134/S106235901807004X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106235901807004X