Abstract
The possibility of using the horseradish peroxidase (HRP)–phenol enzyme–substrate system in the development of an amperometric immunoenzyme sensor for the determination of the mycotoxin zearalenone (ZEA) was shown. New immunoenzyme amperometric sensors based on printed graphite electrodes, including those modified with a fullerene/gold nanoparticles and HRP composite, were proposed for the determination of ZEA in a concentration range from 1 × 10–11 to 1 × 10–6 M, and cd was 5 × 10–12 M The binding constant of immune complexes (Ka = (5.3 ± 0.2) × 108 mol–1) and the percentage of cross reactions, which was <2.5% for patulin and <1.3% for deoxynivalenol, were estimated. A procedure for the determination of the mycotoxin ZEA in food products at an MPC level or below with RSD of no higher than 0.063 using the proposed immunoenzyme sensors was developed and tested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
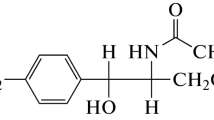
Among mycotoxins, zearalenone (ZEA) (Scheme 1), which is synthesized by fungi from the Fusarium genus (F. graminearum, F. tricinctum), stands out with its carcinogenic, estrogenic, and teratogenic properties and its wide prevalence; it belongs to resorcylic acid lactones and is characterized by anabolic and estrogenic activities.

Scheme 1 . Structural formula of zearalenone (6,10-hydroxy-6-oxo-1-undecyl-β-resorcylic acid lactone).
Zearalenone is a mycotoxin, which mainly infects crops and can accumulate in crops before harvest. This mycotoxin was found in food products and animal feed in a wide range of concentrations depending on environmental state and storage conditions. Acute and chronic poisoning and even cancer can be associated with the consumption of food and feed contaminated with ZEA [1].
Arbitration methods for the quantitative determination of mycotoxins are gas–liquid chromatography (for T-2 toxin), HPLC with UV detection (for deoxynivalenol), and HPLC with fluorescence detection (for aflatoxins and ZEA). These methods are rapid and convenient for routine analysis, and they allow fast and reliable separation of contaminated and uncontaminated samples. Screening methods include thin layer chromatography and a fluorescent method for determining mycotoxins in contaminated grain [2–4].
Currently, biochemical (including immunochemical) methods for the determination of mycotoxins are under development. These methods of analysis are convenient for the primary screening of large batches of products due to their simplicity, rapidity, and relatively low cost. However, studies on the use of bio- and immunosensors for the determination of mycotoxins are still few [5–8]. Interest in the methods of immunochemical analysis is also associated with the possibility of relatively simple variation of the selectivity of analysis for a number of compounds mainly due to the use of antibodies (Abs) with different specificities.
Devices whose operation is based on a combination of the principles of biocatalytic and immunochemical interactions and voltammetric detection of analytical signals associated with the use of enzymes make it possible to develop simple and economically affordable methods for the determination of mycotoxins [8, 9]. In the literature, there are only a few references to the use of horseradish peroxidase (HRP) as a label for the determination of the mycotoxin zearalenone [10]. In the version of an immunosensor described by Panini et al. [10], carbon nanotubes were used as a modifier. This made it possible to reach the limit of detection at a level of n × 10–9 M, which does not always satisfy the requirements for determining trace amounts of mycotoxins.
A current approach to the improvement and development of new amperometric bio- and immunosensors, including those for the determination of mycotoxins, is associated with the use of various nanomaterials to modify the surface of primary transducers. Among carbon nanomaterials, carbon nanotubes are most frequently used as modifiers [8, 11–13]. Other carbon materials are still used very limitedly in the composition of immunosensors. In particular, information on the use of fullerene is still scarce. Gold nanoparticles (Au NPs) are among the most studied and well-established nanomaterials; however, the use of a nanocomposite based on fullerene and Au NPs as an electrode surface modifier for the determination of mycotoxins was not described in the literature.
It was found previously [8, 9] that mycotoxins (aflatoxin B1, patullin, and ochratoxin) have an inhibitory effect on enzymes (cholinesterase, alkaline phosphatase, and tyrosinase). In addition, the cited works explored the possibility of using enzymes from different classes to develop immunosensors for the determination of ZEA. In most cases, the test mycotoxin inhibited the catalytic activity of immobilized enzymes (cholinesterase and tyrosinase), which did not make it possible to achieve the required selectivity of determinations.
Horseradish peroxidase (HRP) is an enzyme frequently used in the development of immunosensors. For example, HRP was successfully used in the determination of aflatoxin B1 and ochratoxin [8, 14]. There is no data on the effect of ZEA on the catalytic activity of HRP; therefore, it seems appropriate to study the action of the mycotoxin in the antigen (Ag) (ZEA)–Ab–enzyme systems. Information on the effect of ZEA on the HRP–phenol enzyme–substrate system will allow one to judge the analytical capabilities of this enzyme and its various options for solving a problem of the selective determination of the individual mycotoxin in complex organic matrices.
The aim of this work was to evaluate the possibility of using HRP as a label in the development of immunosensors based on planar electrodes modified with various nanostructured materials for the determination of the mycotoxin ZEA in food products.
EXPERIMENTAL
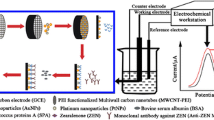
The developed immunosensors were based on printed graphite electrodes of the 3 in 1 design consisting of working, auxiliary, and reference electrodes obtained on a polymer substrate by printing technologies (manufactured at the Department of Analytical Chemistry of Kazan Federal University). The surface material of the working electrode, on which the modifier and the enzyme were immobilized, was graphite ink (Gwent Electronic Materials, the United States). The auxiliary electrode was also made of graphite ink. The reference electrode was AgCl/Ag paste [15]. The working cell volume of the system was 2.0 mL. All measurements with the use of these electrodes were performed with a µAutolab Type III potentiostat/galvanostat (the Netherlands). The electrode surface was electrochemically stabilized and cleaned by cycling the potential in a range from 0 to 1000 mV; for this purpose, five to seven cyclic voltammograms were recorded in a 0.01 M solution of KCl at a sweep rate of 100 mV/s.
To prepare a dispersion of fullerene in an amino derivative of polyether polyol (H20-NH2) and Au NPs in chitosan, an ultrasonic bath model S30H with a frequency of 37 kHz (Elmasonic, Germany) was used.
The presence of nanomaterials on the electrode surfaces was detected by obtaining images using a Solver P47H atomic force microscope (AFM) (ZAO NT-MDT, Russia). To detect the presence of Au NPs, a BioSpectrometrKinetic single-beam spectrophotometer (Eppendorf, Germany) was used.
Chemically pure phenol was used as a substrate, and its solutions were prepared by dissolving accurately weighed portions in a working buffer solution and used within 3 h. Peroxidase from horseradish roots with an activity of 250–330 U/mg (Sigma-Aldrich, the United States), monoclonal Abs against ZEA with an initial concentration of 1.0 mg/mL (Sigma-Aldrich, the United States), 1% solution of glutaraldehyde (GA) (ICN, the United States) and H20-NH2 polyether polyol (1%) (prepared at the Department of Inorganic Chemistry, Kazan Federal University), and fullerene functionalized with hydroxyl groups (Aldrich, the United States) were used. A chromatographically pure ZEA mycotoxin preparation (ZEA solution in benzene) from a state standard reference sample (manufacturer: All-Russian Research Institute of Veterinary Sanitation, Hygiene, and Ecology of the Russian Academy of Agricultural Sciences, Moscow, Russia) was used. To prepare working solutions from the ZEA standard reference sample, the organic solvent (benzene) was vacuum-distilled off at room temperature. The resulting ZEA preparation was used to prepare working solutions by dissolving in twice distilled water. Phosphate (pH 6.86 ± 0.05 and 7.0 ± 0.2), Tris–HCl (pH 7.60 ± 0.05), and acetate (pH 4.00 ± 0.05 and 5.50 ± 0.05) buffer solutions were used. The values of pH in aqueous solutions were determined with a pH-150 pH meter with a glass electrode calibrated using standard buffer solutions.
The following working electrodes were used: an immunosensor with horseradish peroxidase as a label (IS no. 1), an immunosensor based on electrodes modified with C60 fullerene (IS no. 2), and an immunosensor based on electrodes modified with a С60 fullerene/Au NP composite (IS no. 3)
Preparation of the biosensitive part of the immunoenzyme sensor. HRP was applied to the surface of the working electrode together with Abs against ZEA. For this purpose, a mixture containing the enzyme, Abs, H20-NH2 polyether polyol, a phosphate buffer solution (50 mM, pH 7.0 ± 0.2), and a 1% solution of GA. Glutaraldehyde was added last, and 1 µL of this mixture was applied to the electrode surface after vigorous stirring. The biosensors thus obtained were left overnight in a closed Petri dish at +4°C. The next day, the sensors were washed with water, dried in air, and then stored in a refrigerator for no more than 15 days. The proposed immobilization method facilitated the retention of the catalytic activity of HRP and Ab for at least 15 days from the date of manufacture of the immunoenzyme sensor. The measurement error of analytical signals from electrode to electrode did not exceed 5–8%, which indicates good reproducibility of the surfaces of the biosensitive parts of the sensors.
Determination of the concentration of zearalenone unbound in the immune complex in order to calculate the binding constants. The concentration of ZEA that was not bound into the immune complex was determined in the solution after the formation of the Ab–Ag (ZEA) immune complex on the surface of the immunosensor after its incubation for 10 min in a solution of the mycotoxin. The mycotoxin concentration remaining after the formation of the Ab–ZEA immune complex was determined by an amperometric biosensor (enzymatic electrode) based on alkaline phosphatase and 1-naphthyl phosphate at the potential E = +0.35 V, at which the inhibitory effect of this mycotoxin was established previously [16]. The data obtained were used to construct a graph in Scatchard coordinates.
Preparation of gold nanoparticles. Nanoparticles of various metals are increasingly used to modify the surface of electrodes in order to impart certain properties to them. Gold nanoparticles have proven themselves as modifiers. It was of interest to evaluate the effect of the surface modification of electrodes serving as the basis of biosensors with fullerene and fullerene/metal nanoparticle (Au) nanocomposites on the analytical characteristics.
Many methods used for the production of gold nanoparticles are currently known. We decided on a method reported by Tarozaitq et al. [17] because it is simple, allows the synthesis of gold nanoparticles from available reagents under mild conditions, and does not take much time. Optimal conditions for the preparation of gold nanoparticles are presented below (PEG is polyethylene glycol):
Component | HAuCl4⋅4H2O | SnCl2 | Citrate buffer solution, pH | PEG | Chitosan | Color of the resulting solution |
Concentration | 1 × 104 mg/L | 9500 mg/L | 4.3 ± 0.1 | 10 mg/L | 0.75% | Burgundy |
RESULTS AND DISCUSSION
An analysis of published data [5, 6] and preliminary studies of the effect of ZEA on the catalytic activity of enzyme preparations (see above) allowed us to propose an amperometric enzyme immunosensor based on a graphite printed electrode and a biosensitive part including Ab against ZEA, HRP, H20-NH2 polyether polyol, a phosphate buffer solution, and a solution of GA.
Various compounds can act as peroxidase substrates, but phenols are most often used [18]. This is due to the fact that most of the developed peroxidase bio- and immunosensors with HRP as a label were intended specifically for determining the concentration of phenols in environmental materials as pollutants and toxic compounds. At the same time, under the conditions we are considering, the use of phenol as a substrate will make it possible to obtain a signal that is stable in time, well pronounced, and reproducible. Thus, under certain conditions, the peroxidase–phenol enzyme–substrate pair can be used as a recording system in immunoenzyme sensors.
It is well known from the literature [19] that phenol undergoes electrochemical oxidation and enzymatic catalysis under the action of HRP and in the presence of hydrogen peroxide (hydroxylase activity) with the formation of quinone as a product of the enzymatic reaction (oxidase activity) (Scheme 2).
Electrochemical reaction:

Enzymatic reaction:

Scheme 2 . Enzymatic catalysis of phenol under the action of horseradish peroxidase and in the presence of hydrogen peroxide.
The peak at a potential of 0.35 V corresponds to the quasi-reversible oxidation of hydrogen peroxide, and the peak at a potential of 0.75 V most likely refers to the electrochemical oxidation of phenol to the corresponding quinone (Fig. 1), as confirmed by experimental and published data [20].
Voltammograms of the electrooxidation of phenol (1 × 10–3 M), a substrate of horseradish peroxidase (supporting electrolyte, a phosphate buffer solution with pH 7.0): (1) hydrogen peroxide concentration of 5 × 10–5 M (2) in the presence of the mycotoxin zearalenone (1 × 10–8 M) and (3) in the absence of zearalenone. Immunosensor no. 1.
The rate of an enzymatic reaction is largely affected by the conditions under which this reaction proceeds: the nature and pH of the supporting electrolyte and the concentrations of the substrate and hydrogen peroxide. It is well known [21] that HRP exhibits sufficient catalytic activity at pH from 4.0 to 8.0; therefore, we studied the effect of pH on the immunosensor response in acetate (50 mM) (pH 4.0–5.5), phosphate (pH 6.86–7.5), and Tris–HCl (pH 7.6–8.0) buffer solutions. As can be seen in Fig. 2, the highest activity of immobilized HRP (a maximum in the graph) was observed in a phosphate buffer solution with pH 7.0. This was due to the fact that, under these conditions, the maximum possible amount of phenol underwent enzymatic conversion and electrochemical oxidation.
To demonstrate the maximum activity of HRP, it is necessary to create optimal concentrations of the substrate and hydrogen peroxide. With an excess of the substrate, the catalytic activity of this enzyme was inhibited. The substrate and hydrogen peroxide concentrations were varied from 5 × 10–3 to 1 × 10–5 M (Fig. 3). The optimal concentrations of phenol and hydrogen peroxide were 1 × 10–3 and 5 × 10–5 M, and the supporting electrolyte was a phosphate buffer solution with pH 7.0.
Effect of zearalenone on the catalytic activity of immobilized peroxidase. The study of the effect of ZEA on HRP showed that this mycotoxin is not an effector of this enzyme preparation; therefore, it was possible to use HRP as a label in the composition of the corresponding immunoenzyme sensor for detecting immunochemical interactions: Ab against ZEA–ZEA.
The developed immunoenzyme sensor (Fig. 4) was a graphite printed electrode on the surface of which HRP and an immunoreagent (Ab against ZEA) were coimmobilized.
Schematic diagram of the action of an enzyme immunoassay sensor based on immobilized antibodies (Abs) against zearalenone (ZEA) and horseradish peroxidase (HRP): (1) coimmobilized enzyme (HRP) and Ab, (2) antigen (Ag) in a solution of ZEA, and (3) resulting immune complex and variants of the approach of the substrate to the active surface of the enzyme. T is a primary converter (in our case, a printed graphite electrode), E is enzyme (HRP), Abs are antibodies to ZEA, Ag is ZEA, S is the substrate, and P is the product.
It was established that, upon the coimmobilization of Ab with HRP on the electrode surface in the presence of ZEA in solution, the analytical signal decreased in the concentration range (1 × 10–10–1 × 10–6 M); this was apparently associated with the formation of the Ab–Ag immune complex, which was a steric hindrance when the substrate approached the active site of the enzyme. This led to the fact that a smaller number of substrate molecules were involved in the enzymatic process in relation to the control experiment, and the value of the analytical signal decreased.
The greatest inhibitory effect and, consequently, the possibility of detecting analytical signals with a smaller error were achieved with the use of Ab in a dilution of 1 : 50 (a maximum degree of inhibition was (80.2 ± 0.5%). The Ab dilutions of 1 : 1, 1 : 10, 1 : 20, and 1 : 100 provided a narrower range of determined concentrations (1 × 10–9–1 × 10–6 M) and, accordingly, lower degrees of inhibition (68.0–72.0) ± 0.4%. Because the immunoenzyme sensor based on an Ab dilution of 1 : 50 had the best analytical characteristics, this Ab concentration was chosen for the subsequent immunoassays. Table 1 summarizes the analytical characteristics of the immunoenzyme sensor for the determination of ZEA. The correctness of the results of the determination of ZEA obtained using the developed immunoenzyme sensor was confirmed by the standard addition method (Table 2).
Immunosensor modified with C60 fullerene for the determination of zearalenone. The use of various nanostructured materials, in particular, carbon nanomaterials—structures that are a new allotropic carbon species in the form of closed, frame, and macromolecular systems—is a modern trend in the development of biosensor technologies. The addition of carbon nanomaterials with a high specific surface area significantly increases the efficiency of detection of various substances, in particular, with the use of biosensors [22].
Among carbon nanomaterials, carbon nanotubes are currently used most often [23, 24]. Fullerenes and their derivatives are used much less frequently for these purposes [25, 26]. At the same time, the problems of both preserving and purposefully changing the main properties of nanocarbon materials and variants of their combination in the composition of biosensors, for example, by maintaining the corresponding nanosizes by using various substances to obtain dispersions that are stable in time remain unresolved [27].
We used C60 fullerene as an electrode surface modifier. Fullerene is carbon nanospheres, spherical polycyclic structures with a diameter of 40 nm, consisting of carbon atoms linked in six- and five-membered rings. Fullerenes have high chemical stability, and they can be considered as promising candidates for signal amplification in biosensors.
At present, various stable derivatives of C60 have been synthesized to expand the area of their application, in particular, by surface modification with various polar groups (for example, amido, hydroxyl ,and carboxyl groups), which leads to the production of water-soluble and biocompatible compounds.
Various substances including hyperbranched polymers (HBPs), hyperbranched molecules with biosimilar structural fragments and a set of practically useful properties, can be used to obtain dispersions of graphite nanomaterials [28]. A water-soluble amino derivative on the H20-NH2 HBP platform was used to obtain a dispersion of C60 fullerene.
To obtain a modified surface of printed electrodes, the resulting dispersion of fullerene in H20-NH2 and HRP together with Ab against ZEA was deposited onto their surface by drop evaporation (see above on the preparation of the biosensitive part of the immunoenzyme sensors).
The study of the effect of ZEA on the immunoenzyme sensors modified with fullerene showed that the nature of the effect of this compound on immobilized HRP did not change significantly. Modification with fullerene made it possible to expand the range of determined concentrations of the test mycotoxin and improve the correlation coefficient (Table 3). A maximum decrease in the catalytic activity of the enzyme under the action of ZEA on the modified immunosensor under these conditions became somewhat greater and amounted to (83.0 ± 0.9)% in the studied concentration range.
The accuracy of the determination of ZEA in the above concentration ranges with the use of the developed immunosensors was assessed by the standard addition method (Table 2).
Gold nanoparticles as surface modifiers for screen-printed electrodes. The presence of Au NPs of a certain size in solutions (see the Experimental section for the method of preparation) was confirmed by corresponding optical spectra. It was shown that the optical spectra of Au NP solutions contained plasmon absorption bands with maximums characteristic of the absorption of spherical Au NPs in the presence of chitosan at λ = 580 nm (particle size, about 50 nm) [29].
Preliminary studies have shown that the modification of electrode surfaces with C60 fullerene suspensions and the C60/Au NPs composite changed the analytical capabilities of biosensors. Atomic force microscopy (AFM) is a convenient method for visual analysis of changes in the electrode surface upon modification with fullerene and Au NPs. The use of AFM makes it possible to obtain images of the modified electrode surface at different stages of obtaining the biosensitive part of the sensors under different conditions (Fig. 5). Judging from the AFM images of the electrode surfaces, the surface became more developed upon the deposition of Au NPs, and the NPs were fairly homogeneous and evenly distributed over the electrode surface.
Varying the amount of a solution of Au NPs deposited onto the surface of a printed electrode made it possible to establish that 1 μL of the solution gave a more reproducible uniform surface, which provided a sufficient analytical signal; therefore, this amount of the solution of Au NPs was subsequently used (Fig. 6). Then, Ab and HRP were immobilized on this modified surface.
Effect of the modification of electrode surfaces with C60 fullerene and gold nanoparticles on the analytical capabilities of the immunosensor. The study of the effect of ZEA on IS no. 3 modified with the C60/Au NPs composite showed that the corresponding analytical signal decreased in wider concentration ranges compared to that of the unmodified analogue (Table 2). The percentage decrease in catalytic activity under the action on IS no. 3 was (90.0 ± 0.6)% in the studied concentration range. The use of modification with the C60/Au NPs composite made it possible to improve the analytical characteristics of the developed sensor.
The accuracy of the determination of ZEA in the indicated concentration ranges using the developed immunosensors was assessed by the standard addition method (Table 1).
Evaluation of the binding constants of immune complexes and the specificity of immunochemical interactions on IS no. 3. In the development of immunochemical analysis methods based on the Ag–Ab reaction, the knowledge of physicochemical parameters of specific interactions is very important because it makes it possible to evaluate the sensitivity and specificity of the method and to select reagents for analysis. Graphical processing of experimental data in Scatchard coordinates allows one not only to determine the constant of formation of immune complexes but also to calculate the concentration of active Abs (Ag) in the system.
We performed a series of experiments to determine the concentrations of free and bound mycotoxin at various initial concentrations of ZEA and a constant concentration of Ab in the system. The mycotoxin–Ab interaction presented in Scatchard coordinates indicated the predominant existence of an Ab population with a sufficiently high specificity for the mycotoxin to be determined. This shape of the curve is typical for monoclonal Abs. The binding constant of Ab to ZEA (Ka1 = (5.3 ± 0.2) × 108 mol–1) lies in an optimal range of binding constants [16]. Data on the binding strength of the immunological pairs under consideration are currently not available in the literature. At the same time, the found values of the constants of formation of immune complexes indicate a fairly strong binding of Ag to the corresponding Abs, which allows one to use the developed immunoenzyme sensors for the highly sensitive and selective determination of the mycotoxin ZEA.
Nonspecific cross interaction of Abs is one of the main problems in the development of new variants of immunoassays. The quality of Abs is often the main obstacle to increasing the sensitivity of immunodetection and to shorten the duration of analysis due to a sample preparation procedure.
In this work, we investigated the cross reactivity of Ab against ZEA to other mycotoxins, patulin and deoxynivalenol, which were detected in the same foods as ZEA. We found that the Abs used have very little cross reactivity to the test antigens (patulin, <2.5%; deoxynivalenol, <1.3%). This confirms the specificity of the studied Abs only to the corresponding Ag, which makes it possible to selectively determine ZEA independently of other mycotoxins.
Determination of zearalenone in food products. The proposed immunoenzyme sensors can be used to determine the ZEA content of food products. Fungi have the highest producing capacity on corn, oats, rice, and sorghum.
The fungi F. graminearu producing ZEA are common in the southern part of the Russian Federation and in many countries that grow corn for grain. The spores of the fungus live in the soil, from where they fall on vegetative plants and, under favorable conditions (high humidity), germinate affecting the ear or cob and forming their metabolic products—mycotoxins.
The developed immunosensor was used to determine ZEA in corn and barley and corn flour processing products. Samples were prepared using standard recommendations [30] for the separation of ZEA by chromatographic analysis.
Procedure for the extraction (isolation) of zearalenone from cereals. A weighed sample of 1 g (cereals or grains) was ground into powder, which was suspended in a mixture of acetonitrile and water (5 : 1) to determine ZEA. According to Zhanga et al. [31], in this case, a sufficiently complete extraction of the components to be determined should be achieved. The mixture was stirred with a magnetic stirrer for at least 30 min. Then, it was centrifuged for 20 min at a speed of 7000 rpm, and the supernatant was used to prepare aqueous working solutions by sequential dilution for the subsequent determination of ZEA using an immunoenzyme sensor modified with the fullerene/Au NPs composite (IS no. 3).
It was preliminarily established that, upon the repeated extraction of a mixture remaining after a single extraction, a solution that did not cause a change in the analytical signal was obtained; that is, it did not contain components that reduce the catalytic activity of immobilized peroxidase. Thus, the mycotoxin was completely recovered from the sample already as a result of a single extraction. The analytical signal acquired a constant value after 30 min when the extracting mixture was mixed with the sample.
It was found that the concentration of an organic solvent used for the most complete extraction of ZEA from cereal samples did not exceed (8–10)% of the volume of the analyzed solution. This concentration of acetonitrile had no effect on the catalytic activity of HRP; that is, it had no inhibitory or activating effect. This allowed us to use the resulting extract for the determination of ZEA in the samples.
Procedure for the determination of the zearalenone content of cereal samples. The solutions of the sample obtained as described above, phenol (c = 1 × 10–3 M), and hydrogen peroxide (c = 5 × 10–5 M), a phosphate buffer solution (pH 7.0), and IS no. 3 were added to a 2000-μL cell. The solutions were incubated for 10 min. Then, the current was measured at a potential of +0.75 V.
The concentrations of ZEA in the samples were determined according to a calibration curve (see Table 3, IS no. 3). The samples of food products containing corn were also tested using polarization fluorescence analysis (Table 4), which is currently often used to determine mycotoxins. A comparison of the results obtained by the two methods using F- and t-values showed that the methods are equally accurate (Fcalc < Ftabl), and the discrepancies between the average values are insignificant.
In almost all cases, the found amounts of the mycotoxin ZEA in the analyzed samples of food products were lower than the MPC. However, it should be noted that the presence of ZEA in baby food is not allowed.
A comparison of the analytical characteristics of the developed amperometric immunoenzyme sensor and similar methods described in the literature (Table 5) showed that the developed immunosensor is superior to other methods for the determination of ZEA. We can conclude that the developed immunoenzyme sensor can be used for the quality control of a number of food products and, especially, baby food.
* * *
Thus, we demonstrated the applicability of the HRP–phenol enzyme–substrate system to the development of an amperometric immunoenzyme sensor for the determination of ZEA, the action of which is based on a combination of immunological, enzymatic, and electrochemical reactions leading to a decrease in the analytical signal, which most closely corresponds to the term quasi-inhibition. We proposed new amperometric immunoenzyme sensors based on printed graphite electrodes modified with the fullerene/Au NPs composite and HRP for the determination of the mycotoxin ZEA, which make it possible to expand the range of determined concentrations, decrease cd, and improve the coefficient of correlation. The operating conditions for the developed biosensors are as follows: buffer solution pH 7.0 ± 0.2; phenol substrate concentration, 1 mM; and hydrogen peroxide concentration, 50 μM. The developed immunosensors make it possible to determine ZEA in the following concentration ranges: IS no. 1, from 1 × 10–10 to 1 × 10–6 M; IS no. 2, from 1 × 10–11 to 5 × 10–6 M; and IS no. 3, from 1 × 10–11 to 1 × 10–6 M. The lower limit of the determined concentrations is at a level of (1–7) × 10–11(–12) M). The estimated binding constant of the immune complex is Ka = (5.3 ± 0.2) × 108 mol–1, and the percentage of cross reactions is <2.5% for patulin or <1.3% for deoxynylvalenol (IS no. 3). Procedures for the determination of ZEA in food products (cornmeal and cornbread) at a level below the MPC with sr no higher than 0.048 were developed with the use of the proposed immunosensors.
REFERENCES
Haque, M.A., Wang, Y., Shen, Z., Li, X., and He, C., Microb. Pathog, 2020, vol. 142, 104095.
Yang, Y., Li, G., Wu, D., Liu, J., Li, X., Luo, P., Hu, N., Wang, H., and Wu, Y., Trends Food Sci. Technol., 2020, vol. 96, p. 233.
Silva, A.S., Brites, C., Pouca, A.V., Barbosa, J., and Freitas, A., Curr. Res. Food Sci., 2019, vol. 1, p. 1.
González-Jartín, J.M., Alfonso, A., Rodríguez, I., Sainz, M.J., Vieytes, M.R., and Botana, L.M., Food Chem., 2019, vol. 275, p. 703.
Goud, K.Y., Sunil, K.V., Hayat, A., Gobi, K.V., Song, H., Kim, K.-H., and Marty, J.L., J. Electroanal. Chem., 2019, vol. 832, p. 336.
Fernández, O., Vicario, A., Villarroel-Rocha, J., Sapag, K., Messina, G.A., Raba, J., and Bertolino, F.A., Microchem. J., 2018, vol. 141, p. 388.
Oliveira, I.S., Junior, A.G.S., Andrade, C.A.S., and Oliveira, M.D.L., Curr. Opin. Food Sci., 2019, vol. 29, p. 64.
Varlamova, R.M., Medyantseva, E.P., Khamidullina, R.R., and Budnikov, H.C., J. Anal. Chem., 2019, vol. 74, p. 59.
Medyantseva, E.P., Tkhi Tkhan Mai, N., Varlamova, R.M., Tarasova, E.Yu., Sakhapova, G.R., Nikolaeva, O.V., and Budnikov, H.C., Zavod. Lab., Diagn. Mater., 2014, vol. 80, no. 2, p. 5.
Panini, N.V., Bertolino, F.A., Salinas, E., Messina, G.A., and Raba Panini, J., Biochem. Eng. J., 2010, vol. 51, p. 7.
Feng, W. and Ji, P., Biotechnol. Adv., 2011, vol. 29, p. 889.
Le, V.T., Vasseghian, Y., Dragoi, E.N., and Moradi, M., Food Chem. Toxicol., 2021, vol. 148.
Yadav, N., Yadav, S.S., Chhillar, A.K., and Rana, J.S., Food Chem. Toxicol., 2021, vol. 152.
Alonso-Lomillo, M.A., Renedo, O., Torno-de Romõn, L., and Arcos-Martnez, M.J., Anal. Chim. Acta, 2011, vol. 688, p. 49.
Mai Tkhi Tkhan, Kh., Medyantseva, E.P., Varlamova, R.M., Sakhapova, G.R., and Nikolaeva, O.V., Vestn. Kazansk. Tekhnol. Univ., 2012, no. 15, p. 149.
Egorov, A.M., Osipov, A.P., Dzantiev, B.B., and Gavrilov, E.M., Teoriya i praktika immunofermentnogo analiza (Theory and Practice of Enzyme Immunoassay), Moscow: Vysshaya Shkola, 1991.
Tarozaitq, R., Juskunas, R., Kurtinaitienu, M., Jagminienu, A., and Vaskelis, A., Chemija, 2006, vol. 17, nos. 2–3, p. 1.
Andreeva, V.A., Ferment peroksidaza: Uchastie v zashchitnom mekhanizme rastenii (Peroxidase Enzyme in Plant Defense Mechanism), Moscow: Nauka, 1988.
Gibson, D.M. and Lin, E.H., Arch. Biochem. Biophys., 1978, vol. 186, no. 3, p. 317.
Mustafanova, G.K., Konurbaev, A.E., and Baeshov, A., Tekh. Nauki Teor. Prakt., 2016, vol. 12, no. 60, p. 167.
Rogozhin, V.V., Peroksidaza kak komponent antioksidantnoi sistemy zhivykh organizmov (Peroxidase as a Component of the Antioxidant System of Living Organisms), St. Petersburg: GIORD, 2004, p. 22.
Shtykov, S.N., Nanoob"ekty i nanotekhnologii v khimicheskom analize (Nanoobjects and Nanotechnologies in Chemical Analysis), vol. 20 of Problemy analiticheskoi khimii (Problems of Analytical Chemistry), Moscow: Nauka, 2015.
Nano- and Biocomposites, Lau, A.K.-T., Hussain, F., and Lafdi, Kh., Eds., Boca Raton: CRC, 2010.
Mahmoudpour, M., Dolatabadi, J.E.N., Torbati, M., Tazehkan, A.P., Homayouni-Rad, A., and Guardia, M., Biosens. Bioelectron., 2019, vol. 143, 111603.
Camargo, J.R., Baccarin, M., Raymundo-Pereira, P.A., Campos, A.M., Oliveira, G.G., Fatibello-Filho, O., Oliveira, J.O.N., and Janegitz, B.C., Anal. Chim. Acta, 2018, vol. 1034, p. 137.
Shetti, N.P., Mishra, A., Basu, S., and Aminabhavi, T.M., Mater. Today Chem., 2021, vol. 20, 100454.
You, H., Mu, Z., Zhao, M., Zhou, J., Yuan, Y., and Bai, L., Sens. Actuators, B, 2020, vol. 305, 127483.
Yates, C.R. and Hayes, W., Eur. Polym. J., 2004, vol. 40, p. 1257.
Shashkanova, O.Yu. and Ermolaeva, T.N., Sorbtsionnye Khromatogr. Protsessy, 2009, vol. 9, no. 5, p. 677.
GOST (State Standard) 28001-88: Fodder Grain, Products of Its Processing, Mixed Feeds. Methods for Determination of Micotoxins: T-2 Toxin, Zearalenon (F-2) and Ochratoxin A, Moscow: Izd. Standartov, 1988.
Zhanga, F., Liua, B., Shenga, W., Zhanga, Y., Liua, Q., Lia, S., and Wanga, S., Food Chem., 2018, vol. 255, p. 421.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Medyantseva, E.P., Beilinson, R.M., Nikolaenko, A.I. et al. Horseradish Peroxidase: Analytical Capabilities in the Determination of Zearalenone. J Anal Chem 77, 671–680 (2022). https://doi.org/10.1134/S1061934822060090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934822060090