Abstract
An electrochemical immunosensor based on screen-printed graphite electrodes is developed for the determination of the antibiotic chloramphenicol in water and milk samples. It is shown that the immobilization of chloramphenicol-specific antibodies in the liquid-crystal layer of the membrane-like didodecyldimethylammonium bromide preserves the mobility and accessibility of active centers of antibodies, and the addition of gold nanoparticles improves the electron transfer from the electrode surface to the redox centers of horseradish peroxidase, which is used as a label. The limit of detection of chloramphenicol is 0.02 μg/L in water and 0.04 μg/L in milk. This method can be used to determine the residual amounts of chloramphenicol in animal products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chloramphenicol (CAP, ([R–(R*,R*)]-2,2-di-chloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]acetamide, levomycetin, chloromycetin, synthomycin) belongs to the class of aromatic compounds of amphenicols (Fig. 1); it is a broad-spectrum antibiotic. The CAP bacteriostatic activity is manifested in the binding of the antibiotic to the 23S rRNA subunit of the 50S bacterial ribosome, inhibiting peptidyl transferase and disrupting the RNA translation processes. Chloramphenicol is widely used to treat infections caused by pathogenic Gram-positive and Gram-negative bacteria and to suppress rickettsiae, spirochetes, and chlamydia. Microorganisms acquire resistance to this antibiotic relatively slowly. However, administering chloramphenicol orally or intravenously for treating human infectious diseases can result in toxicity, leading to conditions like aplastic anemia, irreversible suppression of the spinal cord’s hematopoietic function, leukemia, etc. [1–3]. Considering its potential hazards to human health, chloramphenicol should be classified as an antibiotic with restricted usage. Consequently, its application in clinical medicine has been limited to specific fields such as ophthalmology and the treatment of superficial skin inflammations. Due to its effectiveness and relatively low cost, chloramphenicol is still actively used in veterinary medicine, animal husbandry, and poultry farming, including for the prevention of infectious diseases. Therefore, the ingress of the antibiotic itself and its metabolites in animal products remains highly probable. The Food and Drug administrations of the European Union, the United States, China, and the Republic of Belarus have set the minimum required detection limit for chloramphenicol or its metabolites in foodstuffs at 0.3 μg/kg. On the territory of the Customs Union, the residual concentration of chloramphenicol in food products of animal origin should not exceed 10 μg/kg, and it is limited to only 0.3 μg/kg in products intended for baby food [4, 5]. Thus, the development of methods for the highly sensitive and selective determination of trace amounts of chloramphenicol in food products is an urgent task.
Currently, high-performance liquid chromatography, enzyme immunoassay (ELISA), thin-layer chromatography, and microbiological methods are applied for the certification and sanitary and hygienic control of agricultural products for the concentration of chloramphenicol. The highest sensitivity is typical for HPLC with tandem mass spectrometry (the detection limit of chloramphenicol is 0.2 μg/kg), but there is a high relative standard deviation of the determined concentration values in the range from 0.2 to 1.0 μg/kg [4]. The cost of equipment associated with this method poses a significant limitation to its widespread use. Microbiological methods, in contrast, are quite inexpensive and easy to apply, but they are characterized by low sensitivity and specificity, and their detection limits for chloramphenicol are higher than those specified in the standards. The ELISA approach is widely used for the screen inspection of samples of food raw materials for a residual amount of chloramphenicol. These methods are characterized by high levels of specificity and accuracy, enabling the analysis of numerous samples quickly. The limit of determination of chloramphenicol here is 0.3–0.7 μg/kg, depending on the type of product (eggs, milk, meat) [6]. However, small laboratories require portable analytical devices to efficiently monitor the chloramphenicol concentration.
The aim of this study is to develop an immunosensor with electrochemical detection for the determination of chloramphenicol in aqueous solutions and milk. Immunosenors were created using screen-printed graphite electrodes (SPEs) that have a high level of standardization, surface chemical modification capability, a broad range of potentials, minimal background current, and low cost [7]. This study involved several tasks: selecting a principle for determining chloramphenicol, optimizing antibody immobilization on the SPE surface, determining conditions for detecting chloramphenicol, and using the newly developed immunosensor for detecting chloramphenicol in milk and water samples.
EXPERIMENTAL
Materials
We used didodecyldimethylammonium bromide (DDAB), HAuCl4⋅3H2O, sodium borohydride, catechol, horseradish peroxidase (Sigma-Aldrich, United States), hydrogen peroxide, ferrocyanide, casein, acids, alkalis, and inorganic salts—components of buffer systems of high-purity grade (Khimmed, Russia). Buffer solutions were prepared using deionized water (Milli-Q System, United Kingdom). Polyclonal antibodies to chloramphenicol were provided by NVO Immunotek (Russia); and the chloramphenicol conjugated with horseradish peroxidase (HRP) was obtained according to the procedure [8]. Milk samples with 3.2% fat content were purchased from a retail chain in Moscow. We used three-contact electrodes with a graphite working electrode (diameter 2 mm), an auxiliary electrode, and the silver–silver chloride reference electrode (SPE) obtained by the ColorElectronics screen printing procedure (Russia, http://www.colorel.ru). The spectral studies were carried out using a UV 1602 spectrophotometer (Shimadzu, Japan); electrochemical measurements were conducted using an Autolab 12 potentiostat (Metrohm Autolab, Netherlands) with the GPES software (version 4.9.7). Electrochemical studies were performed in a 0.1 M potassium phosphate buffer solution containing 50 mM NaCl, pH 7.4 (PPB) at room temperature. Cyclic voltammograms (CVAs) were recorded at a scan rate of 10 to 100 mV/s. All electrochemical potentials are given relative to the silver–silver chloride (Ag/AgCl) reference electrode.
Synthesis of a DDAB-Stabilized Colloidal Solution of Gold Nanoparticles
A 0.5-mL portion of a 10 mM aqueous solution of HAuCl4⋅3H2O was added to 1 mL of 0.1 M DDAB in chloroform upon stirring. Then, 0.2 mL of a freshly prepared 0.4 M aqueous solution of NaBH4 was slowly added upon vigorous stirring. After 2 h, the colored organic layer was separated and washed with the same volume of water. The colloidal solution of gold nanoparticles (AuNPs), stabilized by DDAB in chloroform, was characterized by absorption spectroscopy. The concentration of gold nanoparticles in 0.1 M DDAB in chloroform was calculated according to the reaction stoichiometry.
Preparation of Immunosensors and Determination of Chloramphenicol
A 2-μL portion of a 5 mM colloidal gold solution in 0.1 M DDAB in chloroform was applied to the SPE working surface. After chloroform evaporation for 10 min, the immunoglobulin fraction of antibodies at a concentration of 5 μg/mL was applied. The fraction was obtained from antiserum by double reprecipitation with ammonium sulfate, followed by dialysis against PPB. The electrodes were left for 12 h at +4°C in a humid chamber to prevent the electrodes from drying completely. The resulting electrode was washed in a flow system for 5 min in a PPB solution containing a 0.5% Tween-20 solution (PPBT) and then in a PPB solution (flow rate 1 mL/min). Then, a solution consisting of a mixture of standard solutions of chloramphenicol and a chloramphenicol–HRP conjugate in a buffer containing a 0.5% casein solution was passed through the electrode for 20 min. The reference chloramphenicol solutions with concentrations of 0, 0.05, 0.1, 0.3, 1.0, 3.0, and 10.0 μg/L were prepared in a buffer solution from an antibiotic stock solution in methanol (1 mg/mL). After washing the electrode in PPBT and PPB solutions in a flow system for 5 min (flow rate 1 mL/min), the electrochemical activity of HRP was determined by passing the substrate of hydrogen peroxide and a catechol mediator. The calibration curves of the dependence of the recorded current on the chloramphenicol concentration were plotted. The detection limit of chloramphenicol was determined as the average of the maximum current of the chloramphenicol-free solution minus three standard deviations.
RESULTS AND DISCUSSION
The Principle of Determining Chloramphenicol with Electrochemical Biosensors
Electrochemical analysis methods are the foundation for portable analytical devices that have gained increasing attention in recent years. These devices require high selectivity and sensitivity and must be compatible with programmable compact recording devices. Furthermore, they should be seamlessly integrated with mobile devices and smartphones [9, 10]. The low dependence on environmental factors is an undeniable advantage of electrochemical biosensors. The combination of specificity and sensitivity of ELISA with a shorter analysis time and a decreased number of reagents used, which are characteristic of electrochemical detection methods, opens up broad prospects for the development of immunosensors for the determination of various biologically active compounds. Using electrodes produced by screen-printing with a working graphite electrode improves the reproducibility of the system, cuts costs, and simplifies the analysis. Such electrodes have a low background current and a wide range of potentials used [7, 11, 12].
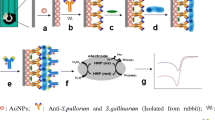
To determine chloramphenicol, we proposed an immunosensor that uses a direct circuit of a competitive enzyme immunoassay, which is fastest (the determination is performed in one stage) and most sensitive for the determination of small haptens. This assay format is based on a competition between native chloramphenicol and the chloramphenicol–HRP conjugate for a limited number of antibody active sites immobilized on the SPE working surface. Schematic representations of the SPE, modification of its surface, and analysis with the use of the electrode are shown in Fig. 2.
Immobilization of Antibodies on the Immunosensor Working Surface
Antibodies immobilized on the electrode surface are the main biorecognizing element of immunosensors. Immobilization of proteins on various surfaces may be accompanied by partial denaturation or inaccessibility for interaction with ligands [13]; therefore, the preservation of the activity and conformation of their active centers, as well as their orientation relative to the electrode surface, are key factors affecting the sensitivity of immunosensors. To improve the stability and preserve the properties of the immobilized proteins, the electrodes are modified with membrane-like synthetic surfactants, for example, didodecyldimethylammonium bromide (DDAB), dihexadecyl phosphate (DHP), lecithin, and dimyristoylphosphatidylcholine, which form stable lyotropic liquid crystal films on the electrode surface [14–17]. In this study, we used DDAB, an amphiphilic surfactant compound that forms a multilayer permeable film on the surface of graphite that simulates biological membranes [18]. Thus, antibodies are placed in the layer formed by the hydrophobic “tails” of a synthetic surfactant, preserving their biological activity. The DDAB film was supplemented with AuNPs, which function as electrical signal transducers and have been demonstrated to facilitate electron transfer between a protein’s redox center and electrode surfaces [19, 20]. Three types of electrodes were used to study the retention of the recognition activity of the active sites of antibodies on the SPE with respect to chloramphenicol:
(1) unmodified graphite electrodes (SPE);
(2) electrodes modified with synthetic membrane-like DDAB (SPE/DDAB);
(3) electrodes modified with DDAB and gold nanoparticles (SPE/DDAB/AuNP).
The antibody immobilization efficiency was calculated based on the electrochemical signal strength after the formation of an immobilized antibody complex with a chloramphenicol–HRP conjugate at a constant concentration. The number of complexes formed on the electrode surface was proportional to the electrochemical activity of the label enzyme (HRP) in the electroreduction of hydrogen peroxide. Catechol was used as a mediator, which does not oxidize by hydrogen peroxide in the absence of peroxidase. The CVAs of the differently modified SPE with immobilized polyclonal antibodies to chloramphenicol, which were obtained after incubating them with the chloramphenicol–HRP conjugate, are presented in Fig. 3.
Cyclic voltammograms of screen-printed graphite electrodes with immobilized antibodies after interaction with chloramphenicol–horseradish peroxidase conjugate. Antibody (Ab) immobilization methods: (1) SPE/Ab, (2) SPE/DDAB/Ab, (3) SPE/DDAB/AuNP/Ab. The measurements were carried out in a 0.1 M potassium phosphate buffer solution (pH 7.4) containing 50 mM NaCl, 0.5 mM hydrogen peroxide, and 0.1 mM catechol. Potential range –0.5 to +0.5 V; scan rate 50 mV/s.
The highest electrochemical activity of HRP in the composition of immune complexes was observed for the electrodes modified with DDAB/AuNP (Fig. 3, curve 3). The maximum amplitude of the peaks of electrooxidation and electroreduction of catechol as a mediator was significantly higher for DDAB/AuNP electrodes compared to electrodes modified only with DDAB: by a factor of for the oxidation peak and by a factor of three for the reduction peak. Thus, the comparison of different methods of immobilization suggests that the use of electrodes modified with DDAB and colloidal gold (DDAB/AuNP) yields an immunosensor with immobilized antibodies that better retain the availability and activity of their antigen-binding sites compared to that modified with only DDAB or unmodified graphite. We assume antibodies immobilized in a DDAB liquid-crystal layer on the SPE surface retain the mobility of their active centers better, which ensures their availability for binding with the antigen. The high permeability of the DDAB layer facilitates electrolyte and mediator particle penetration from the bulk of the solution, while AuNPs boost the electron transfer from the electrode surface to the enzyme redox centers.
Direct Determination of Chloramphenicol Using Label-Free Immunosensors
We studied the possibility of the direct determination of chloramphenicol using electrodes with immobilized antibodies in a layer of DDAB with AuNPs. We recorded the CVA of the electrodes by passing chloramphenicol solutions in PPB with different concentrations (Fig. 4a). A 5 mM K3Fe(CN)6 solution was used as a supporting electrolyte. Changes in the maximum amplitude of the peaks of reduction and oxidation of the electrolyte depended on the chloramphenicol concentration. Using a DDAB liquid crystal layer with AuNPs improves the electron transport properties of the electrolyte. The maximum amplitude of the reduction current depends on the chloramphenicol concentration (Fig. 4a) and can be used as an analytical signal in its determination. Figure 4b shows the calibration curve obtained from the dependence of the cathode current at a voltage of 0.1 V on the chloramphenicol concentration. With an increase in the chloramphenicol concentration, a proportional increase in the cathode amplitude current is observed. The detection limit of chloramphenicol was determined as the maximum amplitude of the electroreduction peak of a K3Fe(CN)6 solution without chloramphenicol, plus three times the standard deviation. The detection limit was 0.2 μg/L.
(a) Cyclic voltammograms of the SPE/DDAB/AuNP/Ab electrodes obtained at different concentrations of chloramphenicol: (1) 1, (2) 100, (3) 10, (4) 1, (5) 0.1, (6) 0.01, and (7) 0. Measurements were carried out in a 5 mM K3Fe(CN)6 solution; scan rate 50 mV/s. (b) Calibration curve for the determination of chloramphenicol in a 0.1 M potassium phosphate buffer solution containing 50 mM NaCl (pH 7.4).
Determination of Chloramphenicol by Competitive Immunosensor Assay
To determine chloramphenicol using immunosensors, we developed a competitive immunoassay procedure: a mixture of standard solutions of chloramphenicol and a chloramphenicol–HRP conjugate at a constant concentration were simultaneously passed through the electrodes with immobilized antibodies. In this case, the number of HRP-labeled immune complexes on the electrode surface is inversely proportional to the chloramphenicol concentration. The number of immune complexes with HRP was determined from the electrochemical activity of the label enzyme in the electroreduction of hydrogen peroxide using catechol as a mediator.
The titration curves of immobilized specific antibodies with chloramphenicol–HRP conjugate on the surface of electrodes modified with a synthetic membrane-like compound DDAB and gold nanoparticles AuNPs (SPE/DDAB/AuNP) revealed the optimal concentrations of immunoreagents for the analysis of chloramphenicol in buffer and milk The concentrations of catechol and hydrogen peroxide, as well as the parameters and conditions of the analysis, were optimized to ensure its optimal sensitivity.
Figure 5 shows the typical calibration curve for the determination of chloramphenicol in buffer using 0.5 mM hydrogen peroxide and 0.1 mM catechol. The detection limit for chloramphenicol was 0.02 μg/L. The analytical range of chloramphenicol concentrations, defined as the region of the 20–80% binding of the conjugate, was 0.02–40 μg/L. The procedure demonstrates high levels of accuracy and reproducibility: the relative standard deviations of the determination of chloramphenicol in buffer solutions containing 0.1, 0.5, and 3.0 μg/L of chloramphenicol amounted to 8.9, 7.6, and 7.1% for three different days (n = 3, P = 0.95), respectively. The SPE modified with DDAB and AuNPs showed operational stability: the residual activity of the electrode was maintained at 95% after 3 h of continuous operation. The reagents required for analysis are stable for at least 6 months if stored at +4°C. The analytical characteristics of the developed method are superior in sensitivity and analytical range to the standard ELISA method using 96-well plates (Table 1).
Since chloramphenicol is widely used in veterinary medicine, animal husbandry, and poultry farming, including for the prevention of infectious diseases, the probability of the antibiotic itself and its metabolites getting into animal products is quite high. The developed immunosensor (SPE/DDAB/AuNP/CAP antibodies) for the quantitative analysis of the antibiotic was used to determine it in milk samples. To eliminate the matrix effect of milk components on the results of the quantitative determination of chloramphenicol, we diluted the milk samples with a buffer solution without additional sample preparation. The dilution of milk samples with 3.2% fat content by a factor of five made it possible to obtain results close to those found from the standard calibration curve in a buffer solution (Fig. 5). The limit of detection of chloramphenicol in milk was 0.04 μg/L; the linear range of determined concentrations was 0.04–80 μg/L; and the recovery rate of chloramphenicol by the standard addition method was 89–116% (Table 2).
Thus, the study demonstrated the advantages of immobilizing antibodies in the liquid crystal layer of DDAB formed on the surface of graphite electrodes and adding AuNPs to them to preserve the immunological activity of antibodies and efficient electron transfer, which is crucial for the analytical sensitivity of the method for determining chloramphenicol. An electrochemical immunosensor based on screen-printed graphite electrodes is developed, which can determine the residual amount of chloramphenicol in food products.
REFERENCES
Liu, Y., Yan, K., Okoth, O.K., and Zhang, J., Biosens. Bioelectron., 2015, vol. 74, p. 1016.
Yan, C., Zhang, J., Yao, L., Xue, F., Lu, J., Li, B., and Chen, W., Food Chem., 2018, vol. 260, p. 208.
Hashkavayi, A.B., Raoof, J.B., Ojani, R., and Asl, E.H., Electroanalysis, 2015, vol. 27, no. 6, p. 1449.
Polyanskikh, E.I., Polonevich, A.G., Belysheva, L.L., and Leshchev, S.M., J. Anal. Chem., 2019, vol. 74, no. 6, p. 601.
Qin, X., Wang, Q., Geng, L., Shu, X., and Wang, Y., Talanta, 2019, vol. 197 P, p. 28.
Fedorova, M.D., Andreeva, I.P., Vylegzhanina, E.S., Komarov, A.A., Rubtsova, M.Yu., Samsonova, Zh.V., and Egorov, A.M., Biotekhnologiya, 2009, no. 6, p. 79.
Kulys, J. and D’Costa, E.J., Biosens. Bioelectron., 1991, vol. 6, p. 109.
Hermanson, G.T., Bioconjugate Techniques, San Diego, CA: Academic, 1996.
Seo, S.E., Tabei, F., Park, S.J., Askarian, B., Kim, K.H., Moallem, G., Chong, J.W., and Kwon, O.S., J. Ind. Eng. Chem., 2019, vol. 77, p. 1.
Patel, M., Agrawal, M., and Srivastava, A., Mater. Adv., 2022, vol. 3, p. 8864. https://doi.org/10.1039/d2ma00427e
Suresh, R.R., Lakshmanakumar, M., Arockia Jayalatha, J.B.B., Rajan, K.S., Sethuraman, S., Krishnan, U.M., and Rayappan, J.B.B., J. Mater. Sci., 2021, vol. 56, no. 15, p. 8951.
Li, M., Li, Y.T., Li, D.W., and Long, Y.T., Anal. Chim. Acta, 2012, vol. 734, p. 31.
Gray, J.J., Curr. Opin. Structur. Biol., 2004, vol. 14, no. 1, p. 110.
Srejber, M., Navratilova, V., Paloncyova, M., Bazgier, V., Berka, K., Anzenbacher, P., and Otyepka, M., J. Biochem., 2018, vol. 183, p. 117.
Panicco, P., Castrignan’o, S., Sadeghi, S.J., Nardo, G.Di., and Gilardi, G., Bioelectrochemistry, 2021, vol. 138, p. 107729.
Karyakin, A.A., Puganova, E.A., Budashov, I.A., Kurochkin, I.N., Karyakina, E.E., Levchenko, V.A., Matveyenko, V.N., and Varfolomeev, S.D., Anal. Chem., 2004, vol. 76, p. 474.
Rusling, J.F., Acc. Chem. Res., 1998, vol. 31, p. 363.
Zhang, Z., Nassar, A.-E.F., Lu, Z., Schenkman, J.B., and Rusling, J.F., J. Chem. Soc., Faraday Trans., 1997, vol. 93, p. 1769.
Silva, N.F.D., Magalhães, J.M.C.S., Barroso, M.F., Oliva-Teles, T., Freire, C., and Delerue-Matos, C., Talanta, 2019, vol. 194, p. 134.
Liu, S., Leech, D., and Ju, H., Anal. Lett., 2003, vol. 36, p. 1.
Funding
This study was supported as part of a state task of the Moscow State University, project no. 121041500039-8 “Molecular design, structural and functional analysis, and regulation of enzyme systems, cell structures, and bionanomaterials: fundamental principles and applications in technology, medicine, and environmental protection” and as part of the long-term (2021–2030) Program of Fundamental Scientific Research in the Russian Federation (project no. 122030100168-2).
Author information
Authors and Affiliations
Contributions
All authors have made an equal contribution to the preparation of this publication.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Zhukova
Abbreviations: AuNP, gold nanoparticles; CAP, chloramphenicol; CVA cyclic voltammogram; DDAB, didodecyldimethylammonium bromide; ELISA, enzyme-linked immunosorbent assay; HRP, horseradish peroxidase; SPE, screen-printed graphite electrode.
About this article
Cite this article
Presnova, G.V., Bulko, T.V., Shumyantseva, V.V. et al. Immunosensor Based on Screen-Printed Graphite Electrodes Modified with Gold Nanoparticles and a Synthetic Membrane-Like Substance for the Determination of Chloramphenicol. Moscow Univ. Chem. Bull. 78, 275–282 (2023). https://doi.org/10.3103/S002713142305005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S002713142305005X