Abstract
Nonrandom distribution of meiotic recombination events along the chromosomes shapes the diversity of potential genetic combinations among the offspring. To redistribute the chromosomal regions involved in recombination events, it was proposed to use meiosis-specific genes of Spo11 proteins (generating double-strand DNA breaks) from phylogenetically different organisms. For these purposes, transgenic tomato plants expressing native SPO11 genes from yeast (Saccharomyces cerevisae) or Arabidopsis thaliana under the control of constitutive 35S CaMV promoter were constructed. Genetic analysis showed that expression of both target SPO11 genes partly disturbed the monogenic inheritance of marker Wv:wv alleles in chromosome 2 and suppressed the crossing over in the region between the wv and d genes. A stable negative correlation between the target gene expression levels and the decrease in the frequency of crossing over in the analyzed chromosomal region was found. The possible genetic mechanisms underlying the redistribution of crossovers along chromosome 2 resulting from the target SPO11 gene expression are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Meiotic recombination plays an important role in the evolution of eukaryotic organisms and is the major source of genetic variation in combinative plant breeding. The genetic variation that arose in heterozygotes in the course of meiosis is the combined result of the interaction of several fundamental mechanisms. One of these mechanisms is well known and is realized in the form of independent recombination of homologous chromosomes during the formation of haploid gametes. Two other mechanisms are based on the redistribution of single- and double-stranded DNA regions between homologs and are initiated in prophase of meiosis by programmed double-strand DNA breaks (DSBs), which throughout the genome are created by meiosis-specific DNA topoisomerases VI, consisting of the TopoVIA subunit, known as Spo11 [1], and TopoVIB subunit [2, 3].
The DSB repair pathways separate after the formation of the D-loop and lead to different recombination products [1]. The double-strand break repair path leads to crossing over, which is essential for the formation of chiasmata between homologs and their proper segregation in anaphase I [4]. Not all cases of DSB repair end with crossing over; for example, in Arabidopsis thaliana, about 200–300 DSBs per cell arise, but only about 10 crossovers are observed in meiosis [5]. To maintain the genome integrity, DSBs that are not restructured by crossing over are repaired via the alternative synthesis-dependent strand annealing pathway [6] or using sister chromatids as a matrix [7], leading to noncrossover recombination products. The third mechanism for the formation of variability is realized through the repair of unpaired bases in heteroduplex DNA, arising in crossover and noncrossover recombination products, and subsequent gene conversion events [8].
In A. thaliana, mutations or the increased dosage of recombination modifier protein genes (FANCM, FIGL1, RECQ4, HEI10) led to the increase in recombination [9–12]. In our studies, expression of the bacterial recA gene also locally stimulated the frequency of crossing over in chromosome 2 of tomato [13]. The described approaches increase the frequency of crossing over in natural regions of the genome, but do not allow for its redistribution to the DSB-cold regions. In this case, the methods providing the DSB stimulation in different genomic regions are required.
In the yeast (Saccharomyces cerevisae) spo11∆ mutants, expression of the chimeric GAL4BD-SPO11 gene induced new DSBs at the Gal4 protein binding site [14]. The chimeric Spo11 proteins with various DNA-binding modules (transcription factors, Cas9 nuclease, etc.) are able to stimulate crossing over in the genomic regions with low natural activity [15]. In the latter case, the authors suggest their own strategy for increasing the genetic variation of gametes in plant breeding.
However, it is difficult to use higher organisms with the knockout of their own SPO11 genes in breeding programs. In A. thaliana, similar mutations led to the absence of fully synapsed homologs in prophase I, their random segregation, production of a large proportion of nonfunctional gametes, and an order of magnitude lower genetic recombination [16]. In mice (M. musculus) with Spo11–/– genotype, complete absence of DSBs, chromosome asynapsis, and infertility were observed [17]. The expression level of recombinant isoform of the mouse Spo11β gene was crucial for chromosome synapsis and successful completion of meiosis [18]. These results show that, in higher organisms, within the framework of the proposed strategy, only overexpression of recombinant SPO11 genes seems to provide the redistribution of exchanges between homologous chromosomes.
At present, there are no experimental data on the dependence of the frequency of crossing over and its distribution along chromosomes on overexpression of recombinant SPO11 genes. The present study is focused on assessing the effect of overexpression of phylogenetically different SPO11 genes on the frequency of crossing over between marker wv and d genes of the tomato chromosome 2.
MATERIALS AND METHODS
Cloning of the S. cerevisiae SPO11 gene was described in our earlier study [19]. The A. thaliana SPO11-1 gene was kindly provided by M. Grelon [16]. Translated regions of the ScSPO11 and AtSPO11-1 genes were cloned into the p35S-recA plasmid (based on the pBI121 vector) [20] at the BamHI and XbaI sites to obtain the p35S-ScSPO11 and p35S-AtSPO11-1 plasmids, in which their expression was controlled by the constitutive 35S RNA promoter of CaMV virus. The Agrobacterium-mediated transformation of cultivated tomato plants (Solanum lycopersicum) of the Marglobe line with dominant alleles of the chromosome 2 Wv and D genes was carried out according to previously published results [20]. To evaluate the frequency of crossing over, the Mo938 marker line of cultivated tomato with linked, with the frequency of 29%, recessive alleles of the wv (white virescent) and d (dwarf) genes on chromosome 2, as well as with the recessive “anthocyanin absence” gene located outside chromosome 2, was used [21].
Isolation of DNA and RNA and synthesis of gene cDNAs were performed in accordance with previously published data [20]. Molecular analysis of transformants and hybrids was performed using polymerase chain reaction (PCR) and primers to the ScSPO11 or AtSPO11-1 and tomato actin gene sequences (Table 1). To exclude the contamination of total plant DNA specimens with Agrobacterium tumefaciens, the previously developed primers virE (plus) and virE (minus) were used [20]. The amounts of ScSPO11 and AtSPO11‑1 mRNAs with normalization relative to the tomato actin gene were determined simultaneously by combining the reverse transcription with subsequent real-time PCR (“in one tube”). For these purposes, the reaction mixture was supplemented with linear destructible TaqMan ROX (5(6)-carboxy-X-rhodamine)/BHQ2 (for actin) and FAM (5(6)-carboxyfluorescein)/BHQ1 (for the target gene) probes, primers (Table 1) complementary to the actin gene region (Fwd-act and Rev-act) and to the region of ScSPO11 (Fwd-scs and Rev-scs) or AtSPO11-1 (Fwd-ats and Rev-ats), total RNA as a template, and MMLV-RT reverse transcriptase (Syntol, Russia). The temperature profile of the reaction was the following: 45°С for 900 s; 95°C for 300 s; 50 cycles of 95°C for 15 s, 60°C for 40 s. The fluorescence level was recorded at the end of each cycle using a CFX96 Touch Real Time System Amplifier (Bio-Rad, United States). Real-time PCR data were normalized using the 2−ΔΔCT method [22]. Measurements were repeated five times in triplicate using young leaves 3.5 ± 0.5 cm in size.
To determine the nucleotide sequence of tomato DNA flanking the right border (RB) sequence of T‑DNA, the method of flanking sequence tags (FSTs) isolation with the use of the PstI restriction endonuclease was applied [23]. Amplification was carried out in two stages: at the first stage with “external” pbi1m and pbi4m primers; at the second stage with pbi1m and “internal” pbi3m primer (Table 1). For direct amplification of the tomato genomic sequence flanking the T-DNA RB, pbi3m and pbi5m primer generated on the basis of determined genomic DNA sequence were used. The search for plasmid DNA fragments in the genome of transgenic plants was carried out using the technique and primers (BD1–BD10) to different regions of the pBI121 vector [24].
Statistical treatment of the data with the chi-square test (χ2) and calculation of the recombination frequency (rf) using the method of maximum likelihood were performed in accordance with the recommendations reported in [25]. Analysis of the offspring was conducted using F2 populations with germination capacity of more than 90%.
RESULTS
Expression and Inheritance of the ScSPO11 or AtSPO11-1 Target Genes in Hybrids
To obtain transgenic tomato plants, Agrobacterium-mediated transformation of the Marglobe tomatoes was performed using p35S-ScSPO11 and p35S-AtSPO11-1 plasmids carrying the ScSPO11 and AtSPO11-1 target genes under the control of the 35S CaMV promoter. Independent transformational events resulted in the production of the two groups of kanamycin-resistant regenerates, of which 19 transformants without Agrobacterium contamination and with the target gene sequences were selected using PCR and specific primers. Analysis of their total RNA by means of reverse transcription with subsequent PCR made it possible to select plants with the target gene expression, Т0-ScSPO11 and Т0-AtSPO11-1 (Fig. 1).
Pollination with pollen from the tomato line Mo938 resulted in the production of F1 hybrids from transgenic plants nos. 6, 11, and 16 belonging to the T0-ScSPO11 group and from transgenic plants nos. 6, 19, and 24 of the T0-AtSPO11-1 group. All F1 hybrids had about 90% fertile pollen and were indistinguishable with respect to this parameter. Using PCR analysis of DNA, hybrids with target genes were selected. In the offspring of each transformant, there were non-transgenic hybrids resulting from segregation, which were later used as a control. In all transgenic F1-ScSPO11 and F1-AtSPO11-1 hybrids, target gene mRNA sequences were detected. To compare gene expression levels, the amounts of their mRNAs were measured relative to the mRNA of the tomato actin reference gene using real-time PCR (Fig. 2).
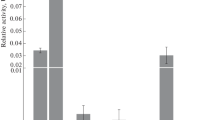
In F1-ScSPO11 hybrids, target gene expression ranged from 0.2 to 3.3 units. (Fig. 2a). The lowest values, ranging from 0.2 to 0.9 units, were observed in the offspring of transformant no. 6; medium values from 0.9 to 1.3 units were in the offspring of transformant no. 16; and the highest values from 2.3 to 3.3 units were in the offspring of transformant no. 11. In F1‑AtSPO11-1 hybrids, target gene expression ranged from 3.6 to 15.9 units and was generally higher than the expression of the ScSPO11 gene (Fig. 2). Eight of the nine hybrids expressed AtSPO11-1 at a level from 3.6 to 7.4 units. Hybrid no. 19-4 showed the expression level 2–4 times higher than that of other F1-AtSPO11-1 plants.
To exclude T-DNA insertion near the markers of chromosome 2, in the offspring of each transgenic hybrid, from 68 to 110 plants of F2 populations were analyzed using PCR and primers to the target gene sequences (Table 1).
In each population, transgenic and non-transgenic plants were represented in a ratio of 3 : 1, respectively (Table 2). The monogenic segregation pattern of the target gene implied the T-DNA insertion at a single locus in the genome of transgenic F1 hybrids. In all F2 populations, with the exception of the offspring of hybrid no. 24-8 from the F1-AtSPO11-1 group, target genes were inherited independently of the chromosome 2 D:d locus alleles (Table 2), as well as the alleles of the Wv:wv marker loci and the “anthocyanin presence:absence” locus (data not shown). These results suggested that transgenic F1 hybrids did not contain T‑DNA insertion near the chromosome 2 markers.
In the offspring of hybrid no. 24-8, monogenic marker segregation was observed only in relation to the “anthocyanin coloration” trait. Namely, among 110 plants, 77 had anthocyanin coloration, while 33 plants were anthocyanin-free. Moreover, the target gene was co-inherited (χ2 corresponds to 8.46) with the anthocyanin coloration locus, but with abnormally high crossover frequency (65%). Since at the time of the experiments on the determination of the T-DNA integration locus, insufficient amounts of hybrid no. 24-8 DNA were available, DNA from its two transgenic F2 offspring was used. Successive stages of amplification with “external” pbi1m and “internal” pbi3m primers resulted in cloning of a 774-bp DNA fragment from both plants (Fig. 3).
The nucleotide sequence amplified from the DNA of the offspring of hybrid no. 24-8 (treated with the PstI restriction endonuclease). The vector sequence is represented in regular font. In it, the pbi1m primer (top) and the sequence complementary to pbi3m primer (bottom) are indicated in dark gray, the remnant of RB sequence is underlined, and the PstI restriction endonuclease recognition site is in bold. The italics indicate the sequence from the tomato genome (between PstI and RB), in which the pbi5m primer sequence is shown in light gray color.
Sequencing data showed the presence of a sequence partly corresponding to that of the pBI121 vector between the sequences of pbi1m and pbi3m primers (Fig. 3). Between the PstI restriction endonuclease recognition site and the RB remnants belonging to T-DNA, there was a 501-bp sequence from the tomato genome. To prove the existence of a chimeric sequence in the genomes of transgenic offspring of hybrid no. 24-8, native DNA (without treatment with the PstI restriction endonuclease) from 26 transgenic F2 plants was amplified using pbi3m and pbi5m primers. As a result, the 538-bp DNA fragments that completely corresponded to the region between pbi3m and pbi5m were obtained. In the case where DNA from other F1-AtSPO11-1 group hybrids was used as a template, no amplification was observed. Using the BLAST program, for the nucleotide sequence between the PstI restriction endonuclease recognition site and RB, three 98–99% identical DNA sequences were found in the GenBank database on chromosome 10 of cultivated tomato S. lycopersicum (CP023766.1 and HG975522.1) and on chromosome 10 of Solanum pennellii (HG975449.1). No additional plasmid DNA sequences were found in the genome of transgenic offspring of hybrid no. 24-8 using BD1–BD10 primers to different regions of the pBI121 vector [24].
Inheritance of Marker Genes in the Offspring of Hybrids Expressing ScSPO11 or AtSPO11-1 Genes
In each F2 population, the correspondence of allele segregation at the Wv:wv, D:d, and “anthocyanin presence:absence” loci to the Mendelian pattern, according to which three quarters of the plants should have dominant characters and one quarter should be recessive, was tested. No deviations in the allele inheritance at the three loci in all nine populations from the control F1 hybrids were found. In the offspring of all transgenic hybrids, no inheritance defects at the “anthocyanin presence:absence” locus were detected. At the same time, in one and two of the four populations obtained from F1-ScSPO11 nos. 6-1 and 6-11, as well as in two of the three populations obtained from F1‑AtSPO11-1 no. 6-6 hybrid, abnormal segregation at the Wv:wv locus was observed (Table 3).
From Table 3, it follows that, in populations nos. 6-1-1 and 6-11-2, the abnormality was determined by the reduced proportion of recessive wvwv genotypes (segregation of about 4–7 : 1), and in populations nos. 6-11-1, 6-6-1, and 6-6-2, by their increased proportion (segregation of about 2 : 1). In addition, in population no. 6-11-2, the proportion of recessive dd genotypes at the D:d locus was also reduced (segregation of 4 : 1). In all offspring populations of hybrid no. 24-8 from F1‑AtSPO11-1, the proportion of dominant genotypes was reduced by about 15 times at the Wv:wv locus (segregation of 0.2 : 1) and by about 4 times at the D:d locus (segregation of 0.7 : 1 ) of chromosome 2. The only exception was one population without abnormalities at the D:d locus.
The Frequency of Crossing Over between Marker Wv and D Genes of Chromosome 2 in Hybrids Expressing ScSPO11 or AtSPO11-1 Genes
The frequency of crossing over was examined using F2 populations with monogenic segregation at all marker loci (Table 4).
From Table 4 it follows that, in individual control hybrids, the frequency of crossing over between the wv and d genes was from 25.4 to 27% and was generally similar to our previously reported data for the crossing combination (Marglobe × Mo938) [21, 26]. In individual F1-ScSPO11 and F1-AtSPO11-1 transgenic hybrids, the frequency of crossing over varied in a wider range, from 17.9 to 28.8%, and averaged 21.8 and 22.0%, respectively, which was considerably lower than in the control (26.7%).
The frequency of crossing over decreases with the increase in the expression level of the target genes (Fig. 4). In both cases, an average negative correlation was observed (r = –0.4–0.5). However, the regression coefficient for the yeast gene (–1.3) was approximately 3 times higher than that upon the use of the plant gene (–0.4).
DISCUSSION
Previously, in cultivated tomato hybrids with the expression of the bacterial recA gene, no effect of T‑DNA insertions on the inheritance patterns of the chromosome 2 marker loci and the “anthocyanin presence:absence” locus in the offspring was observed [13]. However, in the eukaryotic genomes, adjacent DNA insertions can disturb marker segregation [27, 28]. The independent inheritance of the ScSPO11 and AtSPO11‑1 genes relative to the marker loci in the offspring of all tomato transgenic hybrids, with the exception of no. 24-8 from F1-AtSPO11-1 (Table 2), made it possible to exclude the nonspecific effect of T-DNA localization on the marker inheritance and the crossing over between them.
It can be suggested that genetic transformation of the Marglobe line resulted in that transformant no. 24 from the T0-AtSPO11-1 group acquired at least two T‑DNA insertions: one insertion into the region of the Wv and D genes on chromosome 2 and another insertion into chromosome 10. Hybrid no. 24-8, derived from this transformant, also contained these two insertions, but transmitted to the offspring only the last of them. It seems likely that the T-DNA insertion near the Wv and D genes of chromosome 2 in the new genetic environment appeared to be harmful to the offspring and led to the elimination of dominant alleles linked to it. The 3 times more pronounced elimination of Wv than D suggests the T-DNA location closer to Wv and more distant from D. The crossing over between T-DNA and Wv and D partially compensates for the negative selection, which is determined by the genetic linkage, and ensures the appearance of the proportional share of dominant genotypes among the offspring (Table 3). A similar process of elimination of the chromosome 2 linked genes was observed in our study among in the offspring of interspecific hybrids of the (S. lycopersicum × Solanum cheesmaniae) combination and was associated with negative epistatic interactions between the genomes of different tomato species [26]. However, in the latter case, a recessive pair of wv and d markers of chromosome 2 underwent negative selection.
In the tomato Mo938 line, the “anthocyanin absence” gene is located outside chromosome 2, but among the offspring of cross combination (Marglobe × Mo938), it is inherited together with the d gene of chromosome 2 owing to the “quasi-linkage” effect [21]. In the offspring of hybrid no. 24-8, the AtSPO11-1 gene located on chromosome 10 is inherited linked to the gene determining the “anthocyanin absence” trait (Table 2), but also to the frequency of crossing over considerably higher than that upon independent inheritance. According to the data of the Tomato Genetic Resource Center (http://tgrc.ucdavis.edu), tomato chromosome 10 contains only one ag (anthocyanin gainer) gene, which determines the absence of anthocyanin. The phenotypic manifestation of ag allows for the presence of anthocyanin on the cotyledons and the underside of leaves. At the same time, in the Mo938 line, anthocyanin is completely absent from all organs and under any growing conditions. Therefore, linked inheritance with the abnormally high frequency of crossing over also does not exclude the “quasi-linkage” effect between the “anthocyanin absence” gene and chromosome 10 loci in the cross combination (Marglobe × Mo938).
In mei-W681 (spo11) mutants of Drosophila melano-gaster, expression of heterologous SPO11 genes from A. thaliana or rice (Oryza sativa) increased the number of meiotic DSBs, although it was insufficient for normal completion of meiosis [29]. These results suggest that, in the cells of hybrid tomato constitutively expressing the ScSPO11 and AtSPO11-1 genes, recombinant Spo11 proteins could also have been involved in the formation of DSBs. This resulted in the manifestation of two interrelated effects within the same region of tomato chromosome 2. Namely, in most of the hybrids, the frequency of crossing over between the wv and d genes decreased (Table 4) and abnormal inheritance of the Wv:wv alleles in some of their offspring was observed (Table 3).
Among the offspring of interspecific hybrids (S. lycopersicum × S. cheesmaniae), abnormal segregation at the Wv:wv and D:d loci simultaneously was expressed as a proportional decrease of linked wv and d recessive alleles of chromosome 2, i.e., as elimination of whole chromosomes [26]. Against this background, abnormal segregation only at the Wv:wv locus of chromosome 2 among the offspring of linear hybrids F1-ScSPO11 and F1-AtSPO11-1, both in the direction of increase in the proportion of recessive genotypes and in the direction of its decrease (Table 3), is considerably different from previously discovered effects. High pollen fertility of F1-ScSPO11 and F1‑AtSPO11-1, as well as monogenic inheritance of the linked D:d locus of chromosome 2, prevented negative selection of the whole chromosomes.
In heterozygotes, the change in the allele ratio in the products of meiosis and the disruption of Mendelian inheritance among offspring may be the consequence of the preferable formation of DSBs in one of the locus alleles, the repair of which using the homolog is accompanied by gene conversion events [8]. In the latter case, in F1 hybrid (A/J × DBA/2J) mice, at the A3 recombination hot spot, the formation of DSBs mainly in DBA/2J chromosome compared to A/J homolog was observed. The conversion events associated with crossing over resulted in the increased transmission of A/J chromosome alleles to gametes. Therefore, abnormal segregation at the Wv:wv locus among the offspring of tomato transgenic hybrids could have resulted from gene conversion of the dominant allele into the recessive allele and vice versa (Table 3) because of the preferable formation of DSB at one of the alleles of tomato hybrids expressing the ScSPO11 or AtSPO11-1 genes. At the same time, the reasons for selectivity and the direction of gene conversion are unclear and may be associated with the heterozygosity of genetic determinants, relative to which the isogenicity of the Marglobe and Mo938 lines was not tested.
It is known that, in yeast spo11∆ mutants, expression of the chimeric GAL4BD-SPO11 gene (derived from the yeast SPO11 gene) stimulated 3–4 times higher meiotic crossing over [30] and increased gene conversion by an order of magnitude [14]. Crossing over increases diversity among the offspring by creating new allele combinations, but does not affect their population frequencies [31]. The latter opinion should be accepted in the absence of mass crossing over and gene conversion events within the marker allele sequence. In these studies, deviations in the segregation of marker Wv:wv locus could have resulted from the recombination events within the alleles. In the formation of DSBs, heterologous Spo11 proteins could have had an advantage over endogenous proteins owing to the fact that their genes were under the control of strong constitutive promoter, which was active in most of the plant tissues at different stages of development. Since the observed effects in marker inheritance did not have obligatory manifestations in all hybrids (as in the case of crossing over suppression), the competition or interaction of heterologous and endogenous protein factors can be suggested, which was previously described in the expression of chimeric SPO11 genes in yeast [15].
A decrease in the frequency of crossing over at the chromosome 2 region between the Wv:wv and D:d loci suggests the DSB repair at the Wv:wv locus using crossing over. The latter circumstance is explained in terms that at least 70–80% of crossing overs in tomato are realized along the interference-prone Pathway 1 [32], and the crossing overs arising at the Wv:wv locus interfere with neighboring ones located between Wv:wv and D:d. This supposition is proved for all transgenic hybrids in the offspring of which mass disturbances of Wv:wv segregation were observed (Tables 3 and 4). Thus, in contrast to the recA expression, which stimulated the existing crossing overs in tomato [13], the expression of ScSPO11 and AtSPO11-1 led to their redistribution along chromosome 2.
In tomato plants, the expression level of the eukaryotic ScSPO11 and AtSPO11-1 genes was 1–2 orders of magnitude higher than the earlier observed expression of the bacterial recA gene of Escherichia coli [26]. An approximately 5-fold lower yeast gene mRNA level led to a comparable decrease in crossing over, as with the higher level of Arabidopsis gene mRNA (Figs. 2 and 4). In the F1-AtSPO11-1 group, the lower value of the crossing over regression coefficient is a consequence of the high expression level in hybrid no. 19-4, which is 2–4 times higher compared to the values in other hybrids of this group. Even without taking into account specimen no. 19-4, the regression coefficient in this group remains 2 times lower than that in F1‑ScSPO11.
Therefore, the effect of expression of the yeast gene on the suppression of crossing over in tomato is higher than that of the Arabidopsis gene. According to the BLAST program, the protein products of the ScSPO11 and AtSPO11-1 genes are 24 and 61% identical to the amino acid sequence of the putative tomato Spo11 (XM 0103259702), respectively. It is possible that the mechanism underlying the influence of heterologous expression can also be associated with the dominant negative effect of the interaction of heterologous Spo11 with endogenous protein factors of tomato.
REFERENCES
Keeney, S., Spo11 and the formation of DNA double-strand breaks in meiosis, Genome Dyn. Stab., 2008, vol. 2, pp. 81—123. https://doi.org/10.1007/7050_2007_026
Vrielynck, N., Chambon, A., Vezon, D., et al., A DNA topoisomerase VI–like complex initiates meiotic recombination, Science, 2016, vol. 351, pp. 939—943. https://doi.org/10.1126/science.aad5196
Robert, T., Nore, A., Brun, C., et al., The TopoVIB-like protein family is required for meiotic DNA double-strand break formation, Science, 2016, vol. 351, pp. 943—949. https://doi.org/10.1126/science.aad5309
De Boer, E., Jasin, M., and Keeney, S., Analysis of recombinants in female mouse meiosis, Methods Mol. Biol., 2013, vol. 957, pp. 19—45. https://doi.org/10.1007/978-1-62703-191-2_2
Choi, K., Zhao, X., Kelly, K.A., et al., Arabidopsis meiotic crossover hot spots overlap with H2A.Z nucleosomes at gene promoters, Nat. Genet., 2013, vol. 45, pp. 1327—1336. https://doi.org/10.1038/ng.2766
Lam, I. and Keeney, S., Mechanism and regulation of meiotic recombination initiation, Cold Spring Harbor Perspect. Biol., 2014, vol. 7, no. 1. a016634. https://doi.org/10.1101/cshperspect.a016634
Goldfarb, T. and Lichten, M., Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis, PLoS Biol., 2010, vol. 8, no. 10. e1000520. https://doi.org/10.1371/journal.pbio.1000520
Cole, F., Keeney, S., and Jasin, M., Preaching about the converted: how meiotic gene conversion influences genomic diversity, Ann. N.Y. Acad. Sci., 2012, vol. 1267, pp. 95—102. https://doi.org/10.1111/j.1749-6632.2012.06595.x
Crismani, W., Girard, C., Froger, N., et al., FANCM limits meiotic crossovers, Science, 2012, vol. 336, pp. 1588—1590. https://doi.org/10.1126/science.1220381
Girard, C., Chelysheva, L., Choinard, S., et al., AAA-ATPase FIDGETIN-LIKE 1 and helicase FANCM antagonize meiotic crossovers by distinct mechanisms, PLoS Genet., 2015, vol. 11, no. 7. e1005369. https://doi.org/10.1371/journal.pgen.1005369
Seguela-Arnaud, M., Crismani, W., Larcheveque, C., et al., Multiple mechanisms limit meiotic crossovers: TOP3alpha and two BLM homologs antagonize crossovers in parallel to FANCM, Proc. Natl. Acad. Sci. U.S.A., 2015, vol. 112, pp. 4713—4718. https://doi.org/10.1073/pnas.1423107112
Ziolkowski, P.A., Underwood, C.J., Lambing, C., et al., Natural variation and dosage of the HEI10 meiotic E3 ligase control Arabidopsis crossover recombination, Genes Dev., 2017, vol. 31, pp. 306—317. https://doi.org/10.1101/gad.295501.116
Komakhin, R.A., Komakhina, V.V., Milyukova, N.A. et al., Analysis of the meiotic recombination frequency in transgenic tomato hybrids expressing recA and NLS-recA-licBM3 genes, Russ. J. Genet., 2012, vol. 48, no. 1, pp. 23—31. https://doi.org/10.1134/S1022795411110093
Peciña, A., Smith, K., Mézard, C., et al., Targeted stimulation of meiotic recombination, Cell, 2002, vol. 111, no. 2, pp. 173—184. https://doi.org/10.1016/S0092-8674(02)01002-4
Sarno, R., Vicq, Y., Uematsu, N., et al., Programming sites of meiotic crossovers using Spo11 fusion proteins, Nucleic Acids Res., 2017, vol. 45, no. 19. e164. https://doi.org/10.1093/nar/gkx739
Grelon, M., Vezon, D., Gendrot, G., and Pelletier, G., AtSPO11-1 is necessary for efficient meiotic recombination in plants, EMBO J., 2001, vol. 20, pp. 589—600. https://doi.org/10.1093/emboj/20.3.589
Baudat, F., Manova, K., Yuen, J.P., et al., Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11, Mol. Cell, 2000, vol. 6, pp. 989—998. https://doi.org/10.1016/S1097-2765(00)00098-8
Kauppi, L., Barchi, M., Lange, J., et al., Numerical constraints and feedback control of double-strand breaks in mouse meiosis, Genes Dev., 2013, vol. 27, pp. 873—886. https://doi.org/10.1101/gad.213652.113
Komakhin, R.A., Komakhina, V.V. Compartmentalization of Spo11p in vegetative cells of yeast Saccharomyces cerevisiae, Mol. Biol. (Moscow), 2008, vol. 42, no. 3, pp. 436—441. https://doi.org/10.1134/S0026893308030126
Komakhin, R.A., Komakhina, V.V., Milyukova, N.A., et al., Transgenic tomato plants expressing resA and NLS-resA-lisVM3 genes as a model for studying meiotic recombination, Russ. J. Genet., 2010, vol. 46, no. 12, pp. 1440—1448. https://doi.org/10.1134/S1022795410120069
Komakhin, R.A., Strelnikova, S.R., and Zhuchenko, A.A., Genetic characteristics of the marker line for cultivated tomato Mo938, Russ. J. Genet., 2019, vol. 55, no. 1, pp. 60—69. https://doi.org/10.1134/S1022795419010083
Muller, P.Y., Janovjak, H., Miserez, A.R., and Dobbie, Z., Processing of gene expression data generated by quantitative real-time RT-PCR, Biotechniques, 2002, vol. 32, pp. 1372—1374, 1376, 1378—1379.
Pogorelko, G.V. and Fursova, O.V., A highly efficient miPCR mehtod for isolating FSTs from transgenic Arabidopsis thaliana plants, J. Genet., 2008, vol. 87, pp. 133—140. https://doi.org/10.1007/s12041-008-0020-8
Permyakova, N.V. and Deineko, E.V., Vector DNA fragments integrating into the genome of transgenic carrot plants during agrobacterial transformation, Vestn. Tomsk. Gos. Univ., Biol., 2015, vol. 32, pp. 145—161.
Orlova, N.N., Geneticheskii analiz (Genetic Analysis), Moscow: Moscow Gos. Univ., 1991.
Komakhin, R.A., Milyukova, N.A., Strelnikova, S.R., et al., Inheritance of marker genes among progeny of interspecific tomato hybrids expressing the recA Escherichia coli gene, Russ. J. Genet., 2019, vol. 55, no. 4, pp. 433—443. https://doi.org/10.1134/S1022795419040069
Hammarlund, M., Davis, M.W., Nguyen, H., et al., Heterozygous insertions alter crossover distribution but allow crossover interference in Caenorhabditis elegans, Genetics, 2005, vol. 171, pp. 1047—1056. https://doi.org/10.1534/genetics.105.044834
Yunusov, Z.R., Solov’ev, A.A., Mikhailenko, S.N., et al., Effect of transgenes on meiotic recombination in higher eukaryotes exemplified by tomato, S.-kh. Biol., 2009, vol. 44, pp. 52—59.
Yoshinori, S., Tokai, T., Agawa, Y., et al., The double-stranded break-forming activity of plant SPO11s and a novel rice SPO11 revealed by a Drosophila bioassay, BMC Mol. Biol., 2012, vol. 13, no. 1. https://doi.org/10.1186/1471-2199-13-1
Murakami, H. and Nicolas, A., Locally, meiotic double-strand breaks targeted by Gal4BD-Spo11 occurs at discrete sites with a sequence preference, Mol. Cell Biol., 2009, vol. 29, pp. 3500—3516. https://doi.org/10.1128/MCB.00088-09
Jeffreys, A. and Neumann, R., Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot, Nat. Genet., 2002, vol. 31, pp. 267—271. https://doi.org/10.1038/ng910
Anderson, L.K., Lohmiller, L.D., Tang, X., et al., Combined fluorescent and electron microscopic imaging unveils the specific properties of two classes of meiotic crossovers, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, pp. 13415—13420.https://doi.org/10.1073/pnas.1406846111
Funding
Construction of transgenic plants was supported by the Russian Foundation for Basic Research (grant no. 11-04-00873-a); determination of the T-DNA integration locus was carried within the framework of the state contract no. 0574-2019-0001 (state registration no. AAAA-A18-118051890110-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by N. Maleeva
Rights and permissions
About this article
Cite this article
Komakhina, V.V., Krinitsina, A.A., Milyukova, N.A. et al. Expression of Recombinant SPO11 Genes Locally Alters Crossing Over in Tomato. Russ J Genet 56, 1079–1089 (2020). https://doi.org/10.1134/S1022795420090124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420090124