Abstract
Green microalgae Lobosphaera sp. IPPAS С-2047 and Micractinium simplicissimum IPPAS С-2056 were examined for the first time for cell tolerance to elevated concentrations of manganese applied in the form of MnCl2. Analyses of cell photosynthetic activity by chlorophyll fluorometry and the dynamic patterns of absorbance changes of cell suspensions at the peak of chlorophyll absorption revealed differential tolerance of two microalgae species to manganese. The acute toxicity assayed in 4-day treatments became apparent at manganese concentrations equal to or higher than 1 g/L for M. simplicissimum cells and at concentrations above 10 g/L for Lobosphaera sp. Transmission electron microscopy and energy-dispersive X-ray spectroscopy were used to study the subcellular distribution of manganese in microalgal cells under elevated nontoxic concentrations of manganese in the medium. The results with Lobosphaera sp. established the lack of individual manganese-containing inclusions on cell surfaces and in the cell interior; the intracellular distribution of manganese was dispersed with elevated accumulation of this element in the region of thylakoids and plastoglobules. The occurrence of manganese and phosphorus in plastoglobules was found for the first time. Apparently, these compartments become accessible for accumulation of Mn and P upon the translocation of thylakoid components during stress-induced disassembling of their structure. The cells of M. simplicissimum were able to oxidize the exoplasmic Mn2+ with the formation of manganese nanoparticles in the intercellular matrix as well as on the cell surface and within the cell walls. In addition, the manganese permeating into the cells was shown to compartmentalize in vacuoles and bind to the polyphosphate granules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Biotechnologies based on the use of oxygenic phototrophic microorganisms—cyanobacteria and microalgae—are becoming widely spread in the production of biomass enriched with target products, such as proteins, carbohydrates, polyunsaturated fatty acids, minerals, vitamins, antioxidants, and mycosporin-like amino acids [1]. These microorganisms can also be used for purification of water areas and wastewater from heavy metals, biogenic elements, and organic compounds as well as for the creation of biosensors to assess the pollution of aquatic environments [2, 3].
Oxygenic phototrophic microorganisms, primarily microalgae, are frequently used in the field of environmental protection because they are suitable to trace the impact of adverse factors on individual cells and cell populations over many generations and to unveil the long-term consequences of such an impact [4, 5]. Studies on the sensitivity of microalgae to various treatments require the search for algal species serving as pollution indicators as well as the species capable of rapid absorption, accumulation, and bioinactivation of pollutants in water bodies (bioremediation).
The problem of environmental pollution with heavy metals is becoming increasingly urgent [6]. Current technologies for wastewater treatment actively apply oxygenic phototrophic microorganisms whose cells can accumulate high concentrations of many elements, including heavy metals [7]. Manganese is one of the most abundant heavy metals in natural environments; it is a dangerous pollutant of aquatic ecosystems and wastewater [6]. The most common causes of metal ingress into wastewater include the enrichment of oxidized manganese ores, the manufacture of galvanic cells and ceramics, and organic syntheses. It should be noted that manganese is widely used as a component of fuel additives to increase the octane number [8].
When heavy metals permeate into the cells, they react with functional groups of proteins and other compounds. These interactions may provide a detoxification mechanism (formation of metallothioneins and phytochelatins) and, at the same time, they can lead to numerous metabolic disorders underlying the high toxicity of heavy metals [9]. There is evidence for toxic effects of high manganese concentrations on plant cells [10]. However, there are hardly any studies in which the toxicity tests were combined with the analysis of intracellular manganese distribution in microalgae.
The search for microalgal cultures tolerating high manganese concentrations, the determination of concentration ranges at which microalgae retain high metabolic activity, and the studies of manganese intracellular distribution have both practical and fundamental importance since they expand our knowledge of the mechanisms for adaptation of microalgal cells to this heavy metal.
In this work, the influence of high manganese concentrations on photosynthetic activity of green microalgae Micractinium and Lobosphaera was studied to assess the ranges of their tolerance. In addition, the subcellular distribution of the toxicant was characterized by means of energy dispersive X-ray spectroscopy (EDX).
MATERIALS AND METHODS
Algal Strains and Culture Conditions
Suspension cultures of green microalgae Lobosphaera sp. IPPAS С-2047 (hereafter Lobosphaera sp.) and Micractinium simplicissimum IPPAS С-2056 (hereafter M. simplicissimum) were used. Microalgae were subcultured at regular intervals and grown in 750-mL glass flasks filled with BG-11 medium [11]. The culture vessels were mounted on an Innova 44 incubator shaker (New Brunswick, United States) and stirred at a speed of 150 rpm under constant temperature of 25°C and continuous illumination (40 µmol photons/(m2 s) of photosynthetically active radiation (PAR)).
For cultivating microalgae under excessive amounts of Mn2+ (hereafter manganese), the cells in the preculture were precipitated by centrifugation (3000g, 5 min) on a shaker (Eppendorf, Germany) and resuspended in BG-11 medium to a density of 30 mg chlorophyll/L. The chlorophyll (Chl) content in cell samples was assayed as previously described [12]. Solutions of manganese chloride (MnCl2) in BG-11 medium were prepared separately for each given concentration. The incubation of microalgae at elevated manganese concentrations was performed using 12-well plates (Eppendorf, Germany). Cultivation in plates was carried out in accordance with the recommendations for determining the culture tolerance to various pollutants [13, 14]. One milliliter of a concentrated cell suspension and 2 mL of BG-11 medium with a given manganese concentration were added to the wells of the plates. Thus, each well of the plate contained 3 mL of cell suspension of the same density (10 mg Chl/L) and different concentrations of manganese.
Cell suspensions of Lobosphaera sp. were incubated in media containing the following concentrations of manganese: 0.25, 0.5, 1.0, 5.0, 10.0, 15.0, and 25.0 g/L. The suspensions of M. simplicissimum cells were incubated in media with 0.05, 0.1, 0.5, 1.0, 3.0, and 5.0 g/L manganese. The above ranges of manganese concentrations were based on preliminary experiments that showed substantially different levels of lethal Mn2+ concentrations for these microalgae in acute toxicity tests. The cells of Lobosphaera sp. exhibited higher tolerance of manganese concentrations compared to M. simplicissimum. The control cultures were grown in a standard BG-11 medium containing 0.005 g/L manganese. The Mn-treated cells were cultured in a luminostat under continuous irradiance of 40 μmol photons/(m2 s) PAR and 23°C for 4 days.
To analyze the extracellular and subcellular distribution of manganese at elevated Mn concentrations in outer media using energy dispersive X-ray spectroscopy (EDX), microalgal cells were sedimented by centrifugation (3000g, 5 min) and resuspended in BG-11 medium to a density of 10 mg Chl/L. The suspensions of Lobosphaera sp. and M. simplicissimum were then supplemented with 5.0 and 0.5 g/L of manganese, respectively. The cells were cultured for 5 days under the conditions described above.
Determining Optical Density of Microalgal Suspensions
The physiological condition of microalgae was monitored directly in the wells of cell culture plates using an Infinite M200PRO spectrophotometer (Tecan, Austria). For this purpose, the difference in optical density (OD) of cell suspensions at 678 nm (the peak of chlorophyll absorption in cell cultures) and 750 nm (the spectral region of non-specific absorption) was determined. The OD was first recorded in 3 h and then after 2 and 4 days of cultivation in media with elevated manganese concentrations.
Determining Photosynthetic Activity
The photosynthetic activity (PSA) of algal cells was assessed by pulse-modulated chlorophyll fluorometry [15] using a FluorCam FC 800-C fluorometer (Photon Systems Instrument, Czech Republic). The PSA was assessed from the maximum photochemical quantum yield of photosystem II: φ(PSII) = Fv/Fm [16]. Microalgae cells were dark-adapted for 10 min prior to PSA measurements.
Transmission Electron Microscopy (TEM)
The ultrastructural organization of microalgae was inspected using conventional TEM of ultrathin (<70 nm) sections. The elemental analysis was performed by analytical TEM, namely, the EDX of semithin (200–250 nm) sections. The cells were sedimented by centrifugation (3000g, 5 min) and fixed according to a standard protocol [17]. The pellets were successively treated with 2% (v/v) glutaraldehyde solution in 0.1 M cacodylate buffer (pH 6.8–7.2 depending on pH of the culture) for 30 min and then stained with 1% solution (w/v) of osmium tetroxide in the same buffer for 4 h at room temperature.
The fixed specimens were dehydrated in a series of ethanol–water solutions with increasing ethanol concentrations from 30 to 96% (v/v) followed by the triple dehydration with 100% ethanol (Sigma, United States). The last dehydration procedure was combined with contrasting by 2% uranyl acetate solution (w/v) in absolute ethanol (Sigma, United States) for 1 h at room temperature. The samples were embedded in Araldite epoxy resin (Sigma–Aldrich, United States) and polymerized at 56°C. The sections of a given thickness were obtained by means of an LKB-8800 ultramicrotome (LKB, Sweden). The sections were mounted on copper grids for electron microscopy that were covered with an ultrathin formvar resin support (Ted Pella, United States). To study the cell ultrastructure by TEM, the sections were additionally contrasted with lead citrate solution [18]. Images were obtained using a JEM-1011 electron microscope (JEOL, Japan). The dimensions of structures on TEM micrographs were determined using the Fiji (ImageJ) software v. 20200708-1553 (NIH, United States).
Energy-dispersive X-ray spectroscopy (EDX) of semithin sections was performed as previously described [19] with a JEOL-2100 analytical electron microscope (JEOL, Japan) equipped with a bright-field detector for operation in the scanning TEM (STEM) mode (JEOL, Japan). The microscope was also equipped with an X-Max silicon drift detector having an active crystal area of 80 mm2 (Oxford Instruments, United Kingdom). Energy-dispersive X-ray spectra from the selected points or zonal regions of the specimen were recorded in the X-ray energy range from 0 to 10 keV using the bright-field STEM mode. The signal acquisition time for one spectrum was 120 s. Energy-dispersive spectra were recorded and processed in the “Point & ID mode” using the INCA program (Oxford Instruments, United Kingdom). The resulting spectra were displayed for the energy range of 0.15–7.5 keV that comprises the most intense peaks of all biologically significant elements, including Mn.
Statistical Processing of Results
All experiments were performed in three biological replicates. Significant differences between the treated and control samples were revealed with the Student’s t-test. Differences were considered significant at P ≤ 0.05. The results were processed using the Microsoft Excel program. Figures show the mean values and standard deviations of the means.
RESULTS
Assessment of Culture Tolerance
Tolerance of microalgae cultures was assessed according to the standard bioassay procedure as recommended by state regulations, GOST P 54496-2011 [20]. In our experiments, we determined the manganese concentrations in the range from minimally active to toxic for algal cells. According to the GOST rules, the decrease of analyzed parameters in microalgae cells by 20% compared to normal physiological values indicates a nontoxic concentration of the pollutant. The decrease of the analyzed parameter by more than 50% with respect to the control value is considered as a manifestation of acute toxicity.
After 3-h incubation of Lobosphaera sp. in the BG-11 medium with the highest manganese concentration of 25.0 g/L and after incubation of M. simplicissimum in the presence of 3.0–5.0 g/L manganese, the optical density of cell suspensions decreased by 31–32%, which indicated the approaching manganese toxicity for both cultures (Fig. 1a). It should be noted that, at external manganese concentrations ≤15.0 g/L, the OD of Lobosphaera sp. suspensions decreased by no more than 20%, which indicates an extremely high tolerance of the studied culture to this heavy metal.
Optical densities of cell suspensions of (a, b, c) Lobosphaera sp. IPPAS С-2047 and (d, e, f) Micractinium simplicissimum IPPAS С-2056 after cultivation of microalgae for (a, d) 3 h, (b, e) 2 days, and (c, f) 4 days in the BG-11 medium containing 0.005 g/L manganese (Control, C) or elevated Mn concentrations. Data are presented as mean values and standard deviations for three biological replicates. Significant differences were revealed by the Student’s t-test (*Р ≤ 0.05, **Р > 0.05).
During the next 2 days of cultivation of Lobosphaera sp. in the BG-11 medium supplemented with 1.0–25.0 g/L manganese, the OD of the suspension decreased significantly (by 37–50%) with respect to the control value (Fig. 1b). For M. simplicissimum cells, the decrease in OD by 38–42% was observed at manganese concentrations above 3.0 g/L (Fig. 1e). On the fourth day, the OD of the Lobosphaera sp. cultures continued to decrease at all tested manganese concentrations as compared with that on the 2nd day (Figs. 1b, 1c). The OD of M. simplicissimum cell suspension on the fourth day of incubation at manganese concentrations up to 0.5 g/L was almost the same as on the second day, while it continued to decrease at manganese concentrations of 1.0–5.0 g/L (Figs. 1e, 1f).
Fluorometric studies of photosynthetic apparatus in microalgae cells at increasing manganese concentrations showed that the photosynthetic activity of Lobosphaera sp. diminished by more than 30% at manganese concentrations above 10 g/L, whereas a similar reduction in M. simplicissimum cells was attained at concentrations above 1.0 g/L (Fig. 2).
Photosynthetic activity of (a) Lobosphaera sp. IPPAS С-2047 and (b) Micractinium simplicissimum IPPAS С-2056 cells after 4-day culturing in BG-11 media containing 0.005 g/L manganese (Control, C) or elevated Mn concentrations. Data are presented as mean values and standard deviations for three biological replicates. Significant differences were revealed by the Student’s t-test (*Р ≤ 0.05, **Р > 0.05).
Data in Table 1 illustrate the comparative toxicity of manganese to Lobosphaera sp. and M. simplicissimum cells, based on the decrease in photosynthetic activity and OD (in percents) at various Mn concentrations with regard to the same parameters of cell suspensions in the standard medium. The results show that manganese concentrations up to 5.0 g/L had no acute toxic effect on Lobosphaera sp., indicating a high tolerance of this culture to the tested heavy metal. On the other hand, at manganese concentrations ranging from 10.0 to 25.0 g/L, the OD decreased by ≥55% and photosynthetic activity reduced by 32–54% depending on Mn level in the medium (Figs. 1, 2, Table 1). Similar reductions of analyzed parameters in M. simplicissimum cells were observed at manganese concentrations of ≥1.0 g/L, which indicates a higher sensitivity of this species to elevated amounts of manganese.
Subcellular Distribution of Manganese in Microalgae Cells
The ultrastructural organization of the studied microalgae corresponds to that of representatives of related taxa. The main features of these algae include unicellularity, the presence of a single nucleus and a single parietal chloroplast. Each chloroplast contains a pyrenoid surrounded by a starch sheath having the form of an egg shell in M. simplicissimum or many elongated grains in Lobosphaera sp. (Figs. 3a, 3b). The cell wall in both cultures consists of 2–3 layers differing in electron density, but there is no three-lamellar structure. The cytoplasm contains regular organelles, such as ribosomes, mitochondria, elements of the Golgi apparatus, and vacuoles. The storage structures comprise starch grains, the cytoplasmic oleosomes, vacuolar inclusions, and plastoglobules in the chloroplast. Cultivating the algae in media with an elevated nontoxic manganese concentration (5.0 g/L for Lobosphaera sp. and 0.5 g/L for M. simplicissimum) produced no principal changes in the cell ultrastructure. However, analytical microscopy revealed genus-specific subcellular distribution of manganese in the studied microalgae.
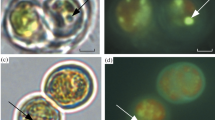
Ultrastructural organization in microalgae (a) Lobosphaera sp. IPPAS С-2047 and (b) Micractinium simplicissimum IPPAS С-2056 (TEM images of ultrathin sections); (c–g) representative images of cell regions analyzed by EDX (STEM images of semi-thin sections): (c) chloroplast thylakoids of Lobosphaera sp. IPPAS С-2047, (d) plastoglobules in Lobosphaera sp., (e, g) nanoparticles on the cell surface and in the intercellular matrix of M. simplicissimum IPPAS С-2056, (f) nanoparticles in the cell wall. V—vacuole; SG—starch grain; CW—cell wall; M—mitochondrion; P—pyrenoid, Os—oleosome; Chl—chloroplast; T—thylakoids. Nanoparticles are indicated by arrowheads. Scale bars: (a, b) 1 µm; (c–g) 0.2 µm.
Analysis of semi-thin sections of Lobosphaera sp. cells grown on a medium with 5.0 g/L Mn did not reveal the formation of individual manganese-containing inclusions in any cellular (sub)compartments (cell wall, chloroplasts, oleosomes, vacuoles, cytosol). All EDX spectra plotted in the range of 0.15–7.50 keV (Fig. 4) featured the lines characteristic of X-ray emission of carbon (Kα = 0.28 keV) and oxygen (Kα = 0.53 keV) that are the main elements of organic compounds occurring in biological samples and the epoxy resin used for the preparation of specimens. The spectra also revealed a peak (Lα = 0.93 keV) characteristic of copper, the main component of electron microscopy grids on which specimen sections were mounted before TEM analysis, as well as the peak of osmium (Kα = 1.91 keV) that was used for cell fixation. Only the EDX spectra recorded in the regions of thylakoids (Figs. 3c, 4a) and plastoglobules (Figs. 3d, 4b) contained small peaks at 5.90 and 6.49 keV corresponding to the characteristic emission of manganese. It should be noted that the spectra obtained in the area of plastoglobules of Lobosphaera sp. also contained the characteristic peak of phosphorus with Kα = 2.01 keV (Fig. 4b).
Representative spectra of energy-dispersive X-ray spectroscopy for microalgae cells of (a–d) Lobosphaera sp. IPPAS С-2047 and (e–h) Micractinium simplicissimum IPPAS С-2056 in the areas of (a) thylakoids, (b) plastoglobules, (c) cell wall, (d) starch grain, (e) nanoparticle agglomerates on the cell surface, (f) cell wall, (g) vacuolar inclusions, and (h) pyrenoid.
In the EDX spectra obtained from other cell subcompartments, e.g., the cell wall (Fig. 4c) and starch grains (Fig. 4d), no discernible peak of manganese X-ray emission was recorded. It is possible that the level of characteristic Mn emission in the cytosol and vacuolar inclusions was very weak and did not exceed 10–12 relative units.
Analysis of sections of M. simplicissimum cells revealed the accumulation of electron-dense nanoparticles 10–20 nm in size on the cell surface (Figs. 3e, 3g), in the outer layer of cell wall (Fig. 3f), and in the intercellular matrix (Figs. 3e, 3g). In the latter case, the size of nanoparticles sometimes reached 40–60 nm. The EDX spectra recorded from nanoparticle agglomerates (Figs. 4e, 4f) contained three different intensity peaks characteristic of manganese emission (Lα = 0.64 keV, Kα = 5.89 keV, and Kα = 6.49 keV). The sufficiently high intensity and occurrence of all peaks of the manganese emission provide reliable evidence for the enrichment of analyzed nanoparticles with this element. Judging from EDX spectra of M. simplicissimum (Fig. 4g), the vacuolar inclusions were characterized by the predominance of phosphorus, P (Kα = 2.01 keV) and nitrogen, N (Kα = 0.39 keV); they also featured small peaks corresponding to the emission of Ca (Lα = 3.69 keV) and Мn (Kα = 5.90 keV). Analysis of other compartments of M. simplicissimum cells (cytosol, starch grains, thylakoids, pyrenoid, lipid globules, nucleus, etc.) did not reveal manganese lines in the EDX spectra.
DISCUSSION
One of the most important tasks of microalgae ecophysiology is to study the cell responses to heavy metal ions whose elevated concentrations have a toxic impact on diverse physiological processes. This problem has obvious practical importance in view of ever-increasing environmental pollution with heavy metals and due to the use of microalgae for bioindication and biotesting of aquatic environments [21]. It is also of fundamental significance for clarifying the mechanisms of adaptation and tolerance of microalgae and for studying the bioremoval of heavy metals from water by microalgae that accumulate large quantities of these metals in their biomass.
The photosynthetic apparatus of microalgae is extremely sensitive to the presence in the environment of pollutants, including heavy metals [16]. The study of the photosynthetic apparatus’s condition in microalgae by fluorometry is one of the most popular methods for analyzing PSA [15].
The results of our study revealed the decline of PSA at increasing concentrations of manganese in the medium, which indicates the toxic action of elevated concentrations of this metal on the photosynthetic apparatus in both cultures. The decrease in OD of cell suspensions in the region of maximum Chl absorption might be caused, on the one hand, by stress-induced decline in intracellular Chl content reflecting the reduction of the photosynthetic apparatus and, on the other hand, it might be due to the decreased number of cells in suspensions.
The results of this study showed that cultures of Lobosphaera sp. and M. simplicissimum are characterized by differential tolerance to elevated manganese concentrations in the outer medium.
The upper tolerance limits of manganese concentrations are known to vary for different cultures of oxygenic phototrophic microorganisms [8] that are characterized by different adaptability to high concentrations of metals. The adaptive capacity of cells is determined by their potential to restrict the entry of metal ions from the medium and by their ability to bind or sequester metal ions in the extracellular polymeric matrix or cell surface structures [22].
The results of our experiments showed that M. simplicissimum cells are resistant to external manganese concentrations in the range from 0.05 to 0.5 g/L. This conclusion stems from an insignificant decrease in OD of the suspension compared to the control values on the second day of Mn treatment, the lack of accelerated OD decrease on the fourth day, and from the persistent PSA levels. By contrast, the OD of suspensions decreased continuously throughout the experiment at concentrations above 1.0 g/L. The observed declines in OD and PSA indicate the relatively low tolerance of M. simplicissimum culture to these manganese concentrations. Cultivation of Lobosphaera sp. in a medium containing 0.25–5.0 g/L manganese showed that the OD of suspensions and PSA of cells on the fourth day of treatment were actually independent of the manganese concentration in the medium, while culturing at higher Mn concentrations considerably overwhelmed the adaptive capacity of the culture.
The toxicity of high manganese concentrations for plant cells is predominantly ascribed to oxidative stress, the disturbance of chloroplast oxidative balance in particular. The excess of manganese is known to enhance the activity of Mn-superoxide dismutase [23]; it may also inhibit cell growth and induce chlorosis and tissue necrosis [10, 24]. The different tolerance of Lobosphaera sp. and M. simplimissium to elevated manganese concentrations might be associated with dissimilar mechanisms of acclimation, including the differential subcellular distribution of manganese in their cells.
In microalgae Lobosphaera sp., no formation of individual manganese-containing inclusions was found. The highest intensity of characteristic manganese emission was observed in thylakoids and plastoglobules, while the manganese signal in the cytosol and vacuoles was indiscernible. Other intracellular compartments and the cell wall contained manganese in undetectable amounts. The observed pattern seems to indicate a dispersed intracellular distribution of manganese and its selective accumulation in chloroplasts.
Manganese is a cofactor for a several enzymes and is present in chloroplasts as part of the Mn cluster of PSII oxygen-evolving complex [25]. Examination of sections of Lobosphaera sp. cells cultured at normal Mn concentrations demonstrated the absence of the manganese signal in all compartments, including chloroplasts, which is most likely due to limitations of EDX spectroscopy for detecting low concentrations of this element.
Judging from the available publications, the characteristic X-ray emissions of manganese and phosphorus in plastoglobules have been revealed for the first time. We suppose that the detection of manganese and phosphorus in plastoglobules is determined by the ingress and accumulation of these elements in plastoglobules upon the rearrangement of thylakoid components during their disassembly under stress. Plastoglobuli are lipoprotein microcompartments integrated into plastidial metabolic functions, including the adaptation to variable environmental conditions [26]. Our results clearly demonstrate the previously unknown properties of plastoglobuli and expand the understanding of their phosphorylation networks and their potential under stress conditions.
In M. simplicissimum culture, the manganese nanoparticles were found to accumulate on the cell surface, in cell walls, and interstitial spaces (Figs. 3, 4). It is known that some types of bacteria and fungi are able to form manganese nanoparticles upon the oxidation of Mn2+ to Mn4+ and can produce insoluble manganese oxide [27]. The exoplasmic manganese oxidation and the formation of nanoparticles were also described for some microalgae species, including a representative of the genus Micractinium [8]. Manganese oxidation occurs not only on cell surface structures but also in the extracellular matrix and cultivation medium. These extracellular reactions are facilitated, firstly, by the increase in external pH during photosynthetic carbon fixation and, secondly, by the involvement of superoxide radicals produced by extracellular proteins that are excreted by some microorganisms, including microalgae [8, 28]. The formation of manganese nanoparticles in the medium, on the cell surface, and in cell walls can be regarded as an adaptive strategy of microalgae cells in response to an increased amount of manganese in the medium, which restricts the Mn penetration into the cell.
Current nanobiotechnologies aimed at the synthesis of manganese nanoparticles by microbial cells are being actively advanced [29]. Manganese nanoparticles exhibit electrocatalytic, magnetic, and fluorescent properties and possess antibacterial and antifungal activity. Therefore, they can be used in the production of supercapacitors and lithium rechargeable batteries as well as in medicine, analytical chemistry, detoxification of dangerous micropollutants, and other fields [29].
Analysis of M. simplicissimum sections showed the lack of characteristic peaks of manganese X-ray emission in intracellular subcompartments, except for vacuoles. In vacuoles, manganese was found in the composition of phosphorus-containing inclusions, which are similar in elemental composition to polyphosphate granules [19]. The accumulation of phosphorus reserves in such a form is known for many microalgae [19, 30, 31], including a representative of the genus Lobosphaera [32, 33]. Sanz-Luque et al. [31] explained the tolerance of microalgae to heavy metals Cd and Pb as a result of binding of these elements to vacuolar polyphosphate granules, which prevents the interference of toxicants in cell metabolism. With an example of Chlamydomonas reinhardtii, manganese was shown to be largely located in vacuoles, together with phosphorus and calcium [34]. Although manganese accumulation depended on the content of cellular polyphosphates, only a small amount of Mn2+ formed stable complexes with polyphosphates.
The tolerance of M. simplimissium cells to elevated concentrations of manganese might be determined by several factors. First, the cells can perform exoplasmic oxidation and accumulation of insoluble forms of manganese. Second, manganese penetrating into the cells is compartmentalized in vacuoles or binds to polyphosphate granules or other ligands. The culture tolerance is compromised at manganese concentrations above 1.0 g/L; this might be due to the increased amount of manganese nanoparticles in cell walls, which impairs the functional transporters delivering nutrients into the cell and excreting cellular metabolites [35]. The high tolerance of microorganisms to heavy metals is known to rely on their ability to induce synthesis of specific metal-binding proteins and cysteine-rich peptides, such as metallothioneins and phytochelatins [36]. The formation of metallothioneins and phytochelatins has also been described for members of the Trebouxiophyceae family, in particular, for some species of Chlorella and Micractinium [9]. The mechanisms of cell tolerance to manganese also include its interaction with negatively charged groups of surface structures in microalgae cells [37]. The metal-binding sites in the cell wall of microalgae can be represented by protein thiol groups; amino acids; and the carboxyl groups of pectins, proteins, and uronic acids.
Thus, our study revealed the distinctions in tolerance of Lobosphaera sp. and M. simplicissimum to the increased amounts of manganese. This can be explained by different mechanisms underlying the cell resistance to this heavy metal and by specific features of the subcellular manganese distribution in cells of the studied cultures.
REFERENCES
Borowitzka, M.A., Commercial production of microalgae: ponds, tanks, tubes and fermenters, J. Biotechnol., 1999, vol. 70, p. 313. https://doi.org/10.1016/S0168-1656(99)00083-8
Junior, W.G.M., Gorgich, M., Corrêa, P.S., Martins, A.A., Mata, T.M., and Caetano, N.S., Microalgae for biotechnological applications: cultivation, harvesting and biomass processing, Aquac., 2020, vol. 528, p. 735562. https://doi.org/10.1016/j.aquaculture.2020.735562
Vasilieva, S., Lobakova, E., Grigoriev, T., Selyakh, I., Semenova, L., Chivkunova, O., Gotovtsev, P., Antipova, C., Zagoskin, Y., Scherbakov, P., Lukyanov, A., Lukanina, K., and Solovchenko, A., Bio-inspired materials for nutrient biocapture from wastewater: microalgal cells immobilized on chitosan-based carriers, J. Water Process Eng., 2021, vol. 40, p. 101774. https://doi.org/10.1016/j.jwpe.2020.101774
Boyle, T.P., The effect of environmental contaminants on aquatic algae, in Algae as ecological indicators, Shubert, L.E., Ed., Moscow: Academic press, 1984, p. 237.
Dmitrieva, A.G., Kozhanova, O.N., and Dronina, N.L., Physiology of plant organisms and the role of metals, Moscow: Moscow State University, 2002, 160 p.
Masindi, V. and Muedi, K. L., Environmental contamination by heavy metals, in Heavy metals, Saleh, H. and Aglan, R., Eds., 2018, vol. 10, p. 115. https://doi.org/10.5772/intechopen.76082
De-Bashan, L.E. and Bashan, Y., Immobilized microalgae for removing pollutants: review of practical aspects, Bioresour. technol., 2010, vol. 101, p. 1611. https://doi.org/10.1016/j.biortech.2009.09.043
Chaput, D.L., Fowler, A.J., Seo, O., Duhn, K., Hansel, C.M., and Santelli, C.M., Mn oxide formation by phototrophs: spatial and temporal patterns, with evidence of an enzymatic superoxide-mediated pathway, Sci. Rep., 2019, vol 9, p. 18244. https://doi.org/10.1038/s41598-019-54403-8
Balzano, S., Sardo, A., Blasio, M., Chahine, T.B., Dell’Anno, F., Sansone, C., and Brunet, C., Microalgal metallothioneins and phytochelatins and their potential use in bioremediation, Front. Microbiol., 2020, vol. 11, p. 517. https://doi.org/10.3389/fmicb.2020.00517
Baldisserotto, C., Ferroni, L., Anfuso, E., Pagnoni, A., Fasulo, M.P., and Pancaldi, S., Responses of Trapa natans L. floating laminae to high concentrations of manganese, Protoplasma, 2007, vol. 231, p. 65. https://doi.org/10.1007/s00709-007-0242-2
Stanier, R., Kunisawa, R., Mandel, M., and Cohen-Bazire, G., Purification and properties of unicellular blue-green algae (order Chroococcales), Bacteriol. Rev., 1971, vol. 35, p. 171. https://doi.org/10.1128/br.35.2.171-205.1971
Solovchenko, A., Merzlyak, M.N., Khozin-Goldberg, I., Cohen, Z., and Boussiba, S., Coordinated carotenoid and lipid syntheses induced in Parietochloris incise (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light, J Phycol., 2010, vol. 46, p. 763. https://doi.org/10.1111/j.1529-8817.2010.00849
Blaise, C., Algal microplate toxicity test, in Small-scale freshwater toxicity investigations, Blaise, C. and Ferard, J.F., Eds., Netherlands: Springer, 2005, p. 137.
Pacheco, A., Hernández-Mireles, I., García-Martínez, C., and Álvarez, M.M. Microplates as a microreactor platform for microalgae research, Biotechnol. Progr., 2013, vol. 29, p. 638. https://doi.org/10.1002/btpr.1721
Strasser, R.J., Tsimilli–Michael, M., and Srivastava, A., Analysis of the chlorophyll a fluorescence transient, in Chlorophyll a fluorescence, Papageorgiou, G.C. and Govindjee, Eds., Netherlands: Springer, 2004, p. 321. https://doi.org/10.1007/978-1-4020-3218-9_12
Matorin, D.N. and Rubin, A.B., Fluorescence of chlorophyll in higher plants and algae, Moscow: Izhevsk IKI-RHD, 2012, 256 p.
Gorelova, O.A., Baulina, O.I., Solovchenko, A.E., Chekanov, K.A., Chivkunova, O.B., Fedorenko, T.A., and Lobakova, E.S., Similarity and diversity of the Desmodesmus spp. microalgae isolated from associations with White Sea invertebrates, Protoplasma, 2015, vol. 252, p. 489. https://doi.org/10.1007/s00709-014-0694-0
Reynolds, E., The use of lead citrate at high pH as an electron-opaque stain in electron microscopy, J. Cell Biol., 1963, vol. 17, p. 208. https://doi.org/10.1083/jcb.17.1.208
Shebanova, A., Ismagulova, T., Solovchenko, A., Baulina, O., Lobakova, E., Ivanova, A., Moiseenko, A., Shaitan, K., Polshakov, V., Nedbal, L., and Gorelova, O., Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy, Protoplasma, 2017, vol. 254, p. 1323. https://doi.org/10.1007/s00709-016-1024-5
GOST (State Standard) R 54496-2011: Water. Determination of toxicity using green freshwater unicellular algae, 2012.
Filenko, O.F., Biological methods in environmental quality control, Ecol. sys. devices, 2007, vol. 6, p. 18.
Silver, S. and Phung, L.T., Bacterial heavy metal resistance: new surprises, Annu. Rev. Microbiol., 1996, vol. 50, p. 753. https://doi.org/10.1146/annurev.micro.50.1.753
González, A., Steffen, K.L., and Lynch, J.P., Light and excess manganese: implications for oxidative stress in common bean, Plant Physiol., 1998, vol. 118, p. 493. https://doi.org/10.1104/pp.118.2.493
Mills, R.F., Doherty, M.L., Loґpez-Marque, R.L., Weimar, T., Dupree, P., Palmgren, M.G., Pittman, J.K., and Williams, L.E., ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis, Plant Physiol., 2008, vol. 146, p. 116.
Svensson, B., Tiede, D.M., Nelson, D.R., and Barry, B.A., Structural studies of the manganese stabilizing subunit in photosystem II, Biophys. J., 2004, vol. 86, p. 1807.
van Wijk, K.J. and Kessler, F., Plastoglobuli: plastid microcompartments with integrated functions in metabolism, plastid developmental transitions, and environmental adaptation, Annu. Rev. Plant Biol., 2017, vol. 68, p. 253. https://doi.org/10.1146/annurev-arplant-043015-111737
Tebo, B., Bargar, J., Clement, B., Dick, G., Murray, K., Parker, D., Verity, R., Samuel, M., and Webb, S., Biogenic manganese oxides: properties and mechanisms of formation, Annu. Rev. Earth Planet. Sci., 2004, vol. 32, p. 287.
Learman, D.R., Voelker, B.M., Vazquez-Rodriguez, A.I., and Hansel, C.M., Formation of manganese oxides by bacterially generated superoxide, Nat. Geosci., 2011, vol. 4, p. 95.
Hoseinpour, V. and Ghaemi, N., Green synthesis of manganese nanoparticles: applications and future perspective—a review, J. Photochem. Photobiol. B: Biol., 2018, vol. 189, p. 234.
Solovchenko, A., Khozin-Goldberg, I., Selyakh, I., Semenova, L., Ismagulova, T., Lukyanov, A., Mamedov, I., Vinogradova, E., Karpova, O., Konyukhov, I., Vasilieva, S., Mojzeš, P., Dijkema, C., Vecherskaya, M., Zvyagin, I. et al., Phosphorus starvation and luxury uptake in green microalgae revisited, Algal Res., 2019, vol. 43, p. 101651. https://doi.org/10.1016/j.algal.2019.101651
Sanz-Luque, E., Bhaya, D., and Grossman, A.R., Polyphosphate: a multifunctional metabolite in cyanobacteria and algae, Front. Plant Sci., 2020, vol. 11, p. 938.
Kokabi, K., Gorelova, O., Ismagulova, T., Itkin, M., Malitsky, S., Boussiba, S., Solovchenko, A., and Khozin-Goldberg, I., Metabolomic foundation for differential responses of lipid metabolism to nitrogen and phosphorus deprivation in an arachidonic acid-producing green microalga, Plant Sci., 2019, vol. 283, p. 95. https://doi.org/10.1016/j.plantsci.2019.02.008
Kokabi, K., Gorelova, O., Zorin, B., Didi-Cohen, S., Itkin, M., Malitsky, S., Solovchenko, A., Boussiba, S., and Khozin-Goldberg, I., Lipidome remodeling and autophagic response in the arachidonic-acid-rich microalga Lobosphaera incisa under nitrogen and phosphorous deprivation, Front. Plant Sci., 2020, vol. 11. https://doi.org/10.3389/fpls.2020.614846
Tsednee, M., Castruita, M., Salomé, P.A., Sharma, A., Lewis, B.E., Schmollinger, S.R., Strenkert, D., Holbrook, K.,Otegui, M., Khatua, K., Das, S., Datta, A., Chen, X., Ramon, C., Ralle, M., et al., Manganese co-localizes with calcium and phosphorus in Chlamydomonas acidocalcisomes and is mobilized in manganese-deficient conditions, J. Biol. Chem., 2019, vol. 294, p. 17626. https://doi.org/10.1074/jbc.RA119.009130
Knauer, K., Jabusch, T., and Sigg, L., Manganese uptake and Mn(II) oxidation by the alga Scenedesmus subspicatus, Aquat. Sci., 1999, vol. 61, p. 44.
Arunakumara, K. and Xuecheng, Z., Heavy metal bioaccumulation and toxicity with special reference to microalgae, J. Ocean Univ. Chin., 2008, vol. 7, p. 60.
Csonto, J., Kadukova, J., and Polak, M., Artificial life simulation of living alga cells and its sorption mechanisms, J. Med. Syst., 2001, vol. 25, p. 221.
ACKNOWLEDGMENTS
Electron microscopic studies were carried out using the equipment of the Center for Collective Use of Moscow State University.
Funding
The study of subcellular manganese distribution was supported by the Russian Science Foundation (project no. 21-74-20004). The assessment of the tolerance of microalgae cultures was supported by the Russian Foundation for Basic Research (project no. 18-29-25050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies with human participants or animals performed by any of the authors.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Bulychev
Abbreviations: Chl—chlorophyll; EDX—energy dispersive X-ray spectroscopy; OD—optical density (absorbance); PAR—photosynthetically active radiation; PSII—photosystem II; PSA—photosynthetic activity; STEM—scanning transmission electron microscopy; TEM—transmission electron microscopy.
Rights and permissions
About this article
Cite this article
Vasilieva, S.G., Gorelova, O.A., Baulina, O.I. et al. Subcellular Localization of Manganese in Two Green Microalgae Species with Different Tolerance to Elevated Mn Concentrations. Russ J Plant Physiol 69, 94 (2022). https://doi.org/10.1134/S1021443722050223
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722050223