Abstract
The Clark–Nikolsky oxidation potential is used to study the formation of iron(II) and iron(III) coordination compounds in aqueous solutions of α-alanine at a temperature of 298.15 K and an ionic strength of the (Na(H)ClO4) solution of 1.0 mol/L. Experimental curves of dependences of the EMF of the system on the concentration parameters of hydrogen, iron(III), iron(II), and α-alanine ions (pH, рСох, рСred, and pCL, respectively) are recorded. The curves show that complexation in the studied system proceeds stepwise in the wide range of pH 0.5–9.0. Mononuclear coordination compounds [FeHL(H2O)5]3+, [Fe(HL)2(H2O)4]3+, [Fe2(HL)2(OH)4(H2O)6]2+, [FeHL(H2O)5]2+, [Fe(HL)(OH)(H2O)4]+, and [Fe(HL)(OH)2(H2O)3]0 and a heterovalent [FeIIFeIII(HL)2(OH)4(H2O)6]+ complex form. The type and number of coordinated ligands and cations and the total composition of the resulting complex compounds are determined. Chemical models complexation are compiled, and the regions of their dominance are determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Being donors of essential trace elements and biomolecules, coordination compounds of transition metals with organic ligands exhibit pronounced physiological activity [1, 2]. They have strong pharmacological effects and are commonly used in the production of various drugs, microfertilizers, and dietary supplements.
All theoretical and practical achievements of coordination chemistry are closely interconnected with advances in physics and mathematics, the use of the latest physicochemical means of research and calculations, the involvement of software programs and modern computational approaches, and the modeling of processes.
It is therefore extremely important to model chemical equilibria in systems where several processes (particularly reactions of complexation and redox) occur in parallel. It is therefore of considerable advantage to use the oxidation potential [3, 4], which is a highly sensitive, simple, inexpensive, and convenient way of studying stepwise complexation in redox systems [5].
The oxidation potential has been used to study the formation of coordination compounds of acetate, glycinate, and citrate complexes of iron(III) and iron(II), copper, and zinc with salicylic and acetylsalicylic acids using platinum, iron, copper, and zinc electrodes at different ionic strengths of the solution, temperatures, and concentration parameters of components of the analyzed systems [6–10].
EXPERIMENTAL
Processes of complexation in the Fe(II)–Fe(III)–L-Аla–Н2О system were studied under other experimental conditions at an ionic strength of the solution of 1.0 mol/L and a temperature of 298.15 K. Experimental dependences of the system’s EMF on different parameters of concentration were recorded for hydrogen ions, oxidized and reduced species of the metal, and the ligand (рН, рСох, pCred, and pCL, respectively).
An equimolecular mixture of solutions of iron(II) and iron(III) perchlorate salts in a 1 M perchloric acid solution was used in our experiments. The initial reagents were ferrous and ferric perchlorates [11].
Iron(III) and iron(II) concentrations were determined in accordance with respective procedures [12, 13].
To prevent the oxidation of Fe(II), the experiments were performed under a stream of gaseous nitrogen (argon). Sodium perchlorate (NaClO4) was purified via filtration, and the concentration was determined gravimetrically [14].
The system’s electromotive force (EMF) was determined via potentiometry. Platinum, glass, auxiliary, and silver chloride electrodes were placed into the electrolytic cell along with tubes for the inert gas. The EMF of the system was measured using two voltaic cells:
The first voltaic cell is needed to measure the system’s EMF; the second is used to determine the pH of the solution in the electrolytic cell [15]. The working solution was constantly saturated with an inert gas. The EMF values were determined using a 150 MI pH meter.
The first working solution was prepared in a 50-mL volumetric flask. It contained an equimolecular mixture of Fe(II) and Fe(III) perchlorates and α-alanine. The concentrations of the acid and the ferrous and ferric iron were varied in the ranges of 1 × 10−3–1 × 10−4 and 1 × 10−3–3 × 10−4 mol/L, respectively. The constant ionic strength of the working solutions was maintained using perchloric acid. The second working solution contained the same components as those of the first solution, and in the same amounts. The ionic strength of the solution was maintained using perchlorates and sodium hydroxide, so a higher pH value of the second working solution was obtained. The first working solution was then placed into an electrolytic cell, an inert gas was passed through it for 15–20 min, and the EMF of voltaic cells (I) and (II) was measured. When the first solution was titrated with the second, the pH of the system gradually shifted to the alkaline region.
Allowing for the formation of a heterovalent coordination compound, we obtain the expression for the EMF (E):
where Е is the EMF of the system; Е0 is the standard EMF of the system; ν = RT/F; Cred is the concentration of the reduced species of the metal; Coх is the concentration of the oxidized species of the metal; q and p are the numbers of atoms of the oxidized and reduced species, respectively; y is the number of hydroxyl groups in the inner coordination sphere of the Fe(III) complex; and \({v}\) is the number of hydroxyl groups in the inner coordination spheres of the Fe(II) complexes.
Equation (1) is the main equation for the EMF of redox systems, in which hydroxo compounds and mono- and polynuclear complexes form. Analysis of the partial dependences of the experimental EMF on one variable, assuming that all others remain constant, allows us to determine the composition and regions of existence of coordination compounds in solutions, and to calculate the numerical values of the constants of formation [16].
It is evident from Eq. (1) that the EMF should rise as the free concentration of Fe(II) ions falls. Conversely, the EMF should fall along with the free concentration of Fe(III) ions. We can therefore identify complexes that form in the redox system of a given solution composition.
RESULTS AND DISCUSSION
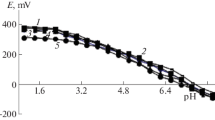
In accordance with the experimental procedure of [6–10], the dependences of the system’s EMF on the pH were recorded first (Fig. 1).
In terms of the theory of redoxmetry, the successive formation of linear portions with slopes of 0, −ν, −2ν, −3ν, −ν, and 0 indicates stepwise complexation of Fe(III) and Fe(II).
The dependence of the system’s EMF on the pH showed that complexation proceeds stepwise in the extremely wide range of pH 0.5–9.0. The start of the hydrolysis of Fe(III) and the formation of a brown precipitate are observed in the working solutions at pH values of more than 9.0.
Experimental curves of the dependence of E on pCох (Fig. 2) and pCred (Fig. 3) were recorded to determine the exact number of atoms of the oxidized and reduced species of the metal in the composition of the resulting complex compounds.
Curve 1 (Fig. 2) was recorded at a pH of 3.0. Slopes of −ν and −ν/2 (−58, −29 mV) were obtained at other pH values of 4.5, 6.0, and 8.0, suggesting that one and two ferric iron atoms participate in complexation (i.e., mono- and binuclear complexes form).
A partial derivative of the general equation for the system’s EMF as a function of the concentration of the oxidized species of the metal has the form
Similar experiments in which the concentration of ferrous iron was varied and all other parameters were constant were also performed (Fig. 3).
It is known that ferrous iron forms coordination compounds at pH values higher than 4, so EMF–рСred dependence curves were recorded at pH values of 4.5 and above.
A single linear portion with a slope of −ν can be drawn through the experimental points of the Е–рСred dependences at different pH values (Fig. 3). This feature corresponds to the formation of mononuclear iron(II) coordination compounds throughout the considered range.
A partial derivative of the general equation for the system’s EMF (Е) as a function of the concentration of the reduced species of iron has the form
and it can be equal to ν only when p = 1.
Experimental curves of the dependence of the system’s EMF on the concentration of aminopropionic acid (рСL) were recorded next (Fig. 4).
The ligand did not coordinate with the central atom of the complexing agent at a pH of 1.5. The slope becomes equal to ν (58 mV) when the pH is raised to 3.0 because one ligand is attached to the complexing atom. One more ligand is attached to the metal when the pH is raised to 4.5 (for a total of two), and the slope of the curves becomes equal to 2ν. This slope of the curves is maintained up to a pH of 6.0. The slope falls to ν when the pH is raised again to 8.0–8.5, and the number of ligands in the inner coordination sphere returns to one.
The number of alaninate ions included in the inner coordination sphere was determined from a joint analysis of the experimental Е–рСL dependences (Fig. 4) and the partial derivative of the general equation for the system’s EMF as a function of the concentration of the ligand:
where х is the number of alaninate ions in Fe(III) complexes and u is the number of alaninate ions in Fe(II) complexes.
Possible versions are
It is obvious that the experimental Е–рСL curves have precisely these slopes.
A partial derivative of the general equation as a function of pH, with all other variables remaining constant, has the form
These slopes were obtained in our experimental E–pH dependences (see Fig. 1).
The numerical values of the slopes of the experimental curves of the dependences of the system’s EMF on concentration were used as a basis for a stoichiometric matrix (mathematical model) of the equilibria that exist in solution (Tables 1, 2).
A complete analysis of the data of the stoichiometric matrix and the determined compositions of the compounds revealed the formation of coordination compounds of Fe(III) and Fe(II) complexes in the studied system. The complexes formed stepwise in solution in the wide range of pH 0.5–9.0.
Data on the stoichiometric matrix were used to construct a chemical model of reactions for the formation of complex compounds (Table 3).
Chemical models can be used to compile software programs and calculate ionic equilibria.
A region of Fe(II) complexation exists at an ionic strength of the solution of 1.0 mol/L and pH values higher than 4.5. [FeHL(H2O)5]2+ and [Fe(HL)(OH)(H2O)4]+ complexes and a heterovalent [FeIIFeIII(HL)2(OH)4(H2O)6]+ complex formed, so complexation occurred up to a pH of 9.0.
CONCLUSIONS
The Clark–Nikolsky oxidation potential was used to study the formation of Fe(II) and Fe(III) coordination compounds in the Fe(II)–Fe(III)–L-Аla–Н2О system at a temperature of 298.15 K and an ionic strength of the solution (Na(H)ClO4) of 1.0 mol/L. Experimental curves of the dependences of the EMF on the concentrations of hydrogen, iron(III), iron(II), and aminopropionic acid ions were recorded. It was shown that the formation of complex compounds proceeds stepwise in the wide range of pH 0.5–9.0. The hydrolysis of Fe(III) and the formation of a brown precipitate were detected at pH values of 9.0 and above. It was established that [FeHL(H2O)5]3+, [Fe(HL)2(H2O)4]3+, [Fe2(HL)2(OH)4(H2O)6]2+, [FeHL(H2O)5]2+, [Fe(HL)(OH)(H2O)4]+, and [Fe(HL)(OH)2(H2O)3]0 complexes and a heterovalent [FeIIFeIII(HL)2(OH)4(H2O)6]+ complex formed in the system. Chemical models of the ionic equilibria of the studied system and possible equations for the formation of these complex compounds were constructed.
REFERENCES
L. R. Nozdryukhina, The Biological Role of Trace Elements in the Body of Animals and Humans (Nauka, Moscow, 1977) [in Russian].
T. N. Litvinova, Biogenic Elements. Complex Compounds (Feniks, Rostov-on-Don, 2009) [in Russian].
W. M. Clark, Oxidation-Reduction Potentials of Organic Systems (Williams and Wilkins, Baltimore, 1960).
B. P. Nikol’skii, Oxredmetry (Khimiya, Leningrad, 1975) [in Russian].
M. S. Zakhar’evskii, Oxredmetry (Khimiya, Leningrad, 1968) [in Russian].
M. M. Rakhimova, T. M. Nurmatov, N. Z. Yusupov, M. A. Ismailova, and E. Faizullaev, Russ. J. Inorg. Chem. 58, 719 (2013).
J. A. Davlatshoeva, G. B. Eshova, M. Rahimova, et al., Am. J. Chem. 7, 58 (2017).
G. B. Eshova, J. A. Davlatshoeva, M. Rahimova, L. V. Kvyatkovskaya, and M. O. Guriev, Russ. J. Inorg. Chem. 63, 561 (2018).
G. B. Eshova, J. A. Davlatshoeva, M. Rahimova, M. O. Guriev, and L. V. Kvyatkovskaya, Russ. J. Inorg. Chem. 63, 772 (2018).
M. Rakhimova, G. B. Eshova, D. A. Davlatshoeva, L. V. Kvyatkovskaya, and F. Miraminzoda, Russ. J. Phys. Chem. A 94, 1560 (2020).
J. C. Schumacher, Perchlorates Their Properties, Manufacture and Uses (Nabu Press, USA, 2011).
R. Přibil, Komplexony v chemicke analyze (NCSAV, Praha 1957).
V. L. Zavorotnyi and N. A. Kalacheva, Methodical Guide to Laboratory Work in Analytical Chemistry. Titrimetric Analysis (RGU Nefti Gaza Gubkina, Moscow, 2007) [in Russian].
V. M. Suslennikova and E. K. Kiseleva, Guidelines for the Preparation of Titrated Solutions (Khimiya, Leningrad, 1968) [in Russian].
R. J. Bates, Determination of pH—Theory and Practice (Wiley, New York, 1964).
Z. N. Yusupov, Resp. Tadzhik. Patent TJ 295, No. 97000501, Byull. Izobret., No. 21 (2000).
Funding
This work was performed as part of a project of the Research Institute of the Tajik National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Eshova, G.B., Davlatshoeva, D.A., Rakhimova, M. et al. Thermodynamic Characteristics of the Formation of Iron(II) and Iron(III) Complexes with L-Alanine in Aqueous Solutions. Russ. J. Phys. Chem. 97, 2443–2448 (2023). https://doi.org/10.1134/S0036024423110079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423110079