Abstract
The complexation of the Fe3+ ion with 2-hydroxybenzamide (salicylamide, HL) has been studied, at 25.0 °C, by potentiometric measurements with a glass electrode in 1 mol·dm−3 NaClO4 medium. The concentration of salicylamide (C L) has been varied between (1 × 10−3 and 10 × 10−3) mol·dm−3, while the concentration of metal cation (C M) ranged within (0.5 × 10−3 and 10 × 10−3) mol·dm−3. The ligand to metal concentration ratio has been varied between 1 and 10 and the hydrogen ion concentration was decreased stepwise until the incipient precipitation of a basic salt of the metal, which occurred at different values depending on the specific ligand to metal ratio. The experimental data were consistent with the formation of the complexes FeL2+, Fe(HL)L2+ and \( {\text{FeL}}_{2}^{ + } \). Equilibrium formation constants have been given for the investigated ionic medium as well as for the infinite dilution reference state, evaluated according to the Specific Interaction Theory. The predominant species in neutral solution are \( {\text{FeL}}_{2}^{ + } \) and \( {\text{Fe}}\left( {\text{OH}} \right)_{2}^{ + } \).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the past two decades, our interest has been addressed to a systematic study of the complexation of some bioavailable metal cations with 2-hydroxybenzoic acid (salicylic acid) and its derivatives [1,2,3,4,5], which are the group of drugs extensively used for anti-inflammatory, analgesic and antipyretic activities [6,7,8,9]. Research in the literature show that the complexation behavior of salicylic acid towards several metal cations has been evaluated in a very wide range of experimental conditions (i.e., ionic strengths, media and temperature) [10]. On the contrary its derivatives have been primarily studied in non-aqueous solvents due to lower solubility in aqueous media. Among these, 2-hydroxybenzamide (salicylamide, HL), whose structure is shown in Scheme 1, is an aromatic compound used in different pharmaceutical fields, due to its antimicrobial, analgesic and anti-inflammatory properties [11].
The biochemical characteristics of this ligand have led us to consider it as a possible chelating agent towards bioavailable and toxic metal cations. The chelating agent for a given metal is a drug able to bind it; however, that used is not necessarily the best but only the most common, while specificity and stability are really important in the choice of a chelator. An ideal chelating agent should exhibit low toxicity and weak affinity towards essential metal cations to avoid metal depletion, have low molecular mass to facilitate oral administration, and form hydrophilic complexes with toxic metal ions to improve its urinary elimination. The metal’s toxicity can be classified in three different categories: acute intoxication which derives from direct ingestion of the toxic metal; chronic intoxication which depends on environmental contamination [12]; metal overload due to genetic diseases [13]. The last category includes iron and copper; in particular, iron overload is a common adverse consequence of the chronic transfusion therapies for thalassemic patients. In a previous work [1] the complexation of the Cu2+ ion with salicylamide was studied, at 25.0 °C in NaClO4 media for ionic strength ranging from 0.5 to 3 mol·dm−3; a comparison with results obtained by using salicylic acid as ligand [14] showed that no significant differences exist between the two ligands, which show similar behavior. The purpose of this work was to study the complexing power of salicylamide toward iron(III) at 25.0 °C in 1 mol·dm−3 NaClO4 medium and then to evaluate differences and similarities in behavior with the system Fe(III)–salicylic acid (H2Sal), already investigated under the same experimental conditions [3]. The constant ionic medium method has been demonstrated to be indispensable in equilibrium studies of complicated ionic reactions. The method, which consists of using, as a solvent, concentrated solutions of inert salts, has remarkable effectiveness for the minimization of variations in activity coefficients [15]. The equilibrium constants, calculated from measurements in a given medium, are strictly valid only in that medium; thus, for practical applications, it is necessary determine constants in each of the conditions prevailing in the natural conditions. For this reason, equilibrium formation constants have been given for the investigated ionic constant medium and for the infinite dilution reference state, evaluated according to the specific interaction theory (SIT) [16, 17]. The theory allows a linear extrapolation to zero ionic strength of equilibrium constants determined in the ionic strength range from 0.5 to 3.5 mol·kg−1.
2 Experimental Section
2.1 Chemical Used
Salicylamide has been obtained from Sigma-Aldrich as analytical reagent grade (99%). The purity has been controlled as reported in literature [18], and has been used without further purification. Iron(III) perchlorate has been prepared and standardized as reported by Ciavatta et al. [19]. Perchloric acid, sodium perchlorate and sodium hydroxide stock solutions were prepared and standardized as previously described [14]. All solutions were prepared with doubly distilled water.
2.2 Procedure and Measurements
The potentiometric apparatus and Ag| AgCl electrodes were prepared as described in a previous work [20]. The glass electrodes, manufactured by Metrohm, acquired a constant potential within 15 min, after the addition of the reagents, which remained unchanged within ±0.1 mV for several hours. The titrations were carried out with the same instrument described in a previous work [21]. To avoid the carbonate interference a slow stream of nitrogen gas was passed through three bottles (a–c) containing: (a) 1 mol·dm−3 NaOH, (b) 1 mol·dm−3 H2SO4 and (c) 1.0 mol·dm−3 NaClO4, and then into the stirred test solutions through the gas inlet tube. During the EMF measurements, the cell assembly has been placed in a thermostat kept at (25.0 ± 0.1) °C.
The complexation equilibria have been studied, at 25 °C and in 1 mol·dm−3 NaClO4, by measuring, with a glass electrode (GE), the competition of the salicylamide, HL, for Fe3+ and H+ ions. Measurements have been performed as potentiometric titrations with cell (G)
in which RE, is the reference electrode (Ag|AgCl| 0.01 mol·dm−3 NaCl|0.99 mol·dm−3 NaClO4| 1 mol·dm−3 NaClO4) and the Test Solution contained C M mol·dm−3 Fe(ClO4)3, C L mol·dm−3 HL, C A mol·dm−3 HClO4, C B mol·dm−3 NaOH, and (1 – 3C M – C A – C B) mol·dm−3 NaClO4. The EMF of cell (G) can be written, in mV, at the temperature of 25 °C, as Eq. 1:
where E 0 was constant for each series of measurements and E j is the liquid junction potential, which is a function of [H+] only [15]. E j in the range of hydrogen concentration investigated was at most ±0.2 mV, which represents the reproducibility of the EMF measurements. The concentration of salicylamide (C L) has been varied between (1 × 10−3 and 10 × 10−3) mol·dm−3, while the concentration of metal cation (C M) ranged within (0.5 × 10−3 and 10 × 10−3) mol·dm−3. The ligand to metal concentration ratio was varied between 1 and 10 and the hydrogen ion concentration was decreased stepwise until the incipient precipitation of a basic salt of metal, which occurred at different values depending on the specific ligand to metal ratio. Each titration was divided in two parts. In the first part, E 0 was determined in the absence of Fe3+ and HL, as reported in literature [22]. In the [H+] range 10−4–10−2 mol·dm−3 values constant to within 0.1 mV have been calculated according to the Gran’s method [23, 24]. In the second part, after the addition of the reagents, the acidity was stepwise decreased by adding known volumes of NaOH standard solution. The primary C M, C L, C A, C B and [H+] data form the basis of the treatment to obtain the stability constants.
3 Results and Discussion
The complexation between Fe(III) and HL was evaluated assuming that the reagents act according to the following general equilibrium, Eq. 2:
that takes into account the formation of simple (q = r), mixed (q ≠ r), mononuclear (p = 1) and polynuclear (p > 1) species. The most probable p, q, r values and the corresponding constants β pqr have been determined by processing the primary data (C M, C L, C A, C B, [H+]) through graphical [25] as well as numerical procedures [26]. In the attempt to explain the data in the simplest way, the presence of two mononuclear and simple complexes (p = 1 and r = q) FeL2+ and \( {\text{FeL}}_{2}^{ + } \), formed according to Eq. 3, was assumed:
The validity of this assumption was verified by constructing the graphs Z against log10 [HL]/[H+], Eq. 4:
where [HL] = C L + C A − C B − [H+] + K W/[H+] and [L−] = β 011 [HL]/[H+].
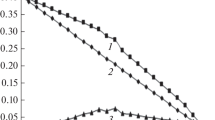
The acidic constant, β 011, of HL was determined in 1 mol·dm−3 NaClO4 and at 25.0 °C in a previous work [1] while K W in our experimental condition has been taken from literature [27]. Thus, if complexes of general formula FeL (3−r) r predominate, the points Z against log10 ([HL]/[H+]), at different C L and C M, should fall on a unique curve. As Fig. 1 shows, all of the experimental points fall on a unique curve which tends to 2, therefore the main complexes are FeL2+ and \( {\text{FeL}}_{2}^{ + } \). The confirmation of the existence of these equilibria in solution was the reversibility of these equilibria during the back titration (symbol □ in Fig. 1).
Z as a function of log10 ([HL]/[H+]). (C M/mmol·dm−3, C L/mmol·dm−3): open circle (1/10), open square (1/10 back titration) and open triangle (1/1). The curve was calculated with the constants obtained by numerical treatment and reported in Table 1
The most probable values β 111 and β 122 were calculated by comparing the experimental graphs with the family of model functions [25]. The best fit was found with the values given in Table 1. The uncertainty, σ, was evaluated taking into account the shift along x axes that still gave an acceptable fit.
A careful inspection of the graph shows that small deviations from the model including FeL2+ and \( {\text{FeL}}_{2}^{ + } \) are observed. These deviations are an indication that some additional species are present. The probable composition of the species responsible of the deviations was obtained by numerical treatment [26]. In the numerical evaluation, the acidic constant of salicylamide [1] and the equilibrium constants for the hydrolytical species of metal ion, Fe(OH)2+, \( {\text{Fe}}\left( {\text{OH}} \right)_{2}^{ + } \) and \( {\text{FeL}}_{2}^{ + } \) \( {\text{Fe}}_{2} \left( {\text{OH}} \right)_{2}^{4 + } \), have been kept fixed [28]. The results are reported in Table 2.
The distribution diagrams reported in Fig. 2 give some confidence to the real presence of such species. As can be seen in Fig. 2a, when the analytical concentration of the ligand is greater than that of the metal, \( {\text{FeL}}_{2}^{ + } \) and Fe(HL)L2+ are the predominant complexes over the whole pH range investigated, while none of the hydrolytic species reaches significant percentages. When the analytical concentrations of ligand and metal are comparable (Fig. 2b) the predominant complex, above pH = 3.5, is the hydrolytic species \( {\text{Fe}}\left( {\text{OH}} \right)_{2}^{ + } \) while the others reach percentages lower than 30%, indicating that ligand is competitive with the hydroxide ion only when its concentration is greater than that of the metal cation.
Distribution diagrams calculated with the values of the constants reported in Table 2. a C L = 10 × 10−3 mol·dm−3, C M = 1 × 10−3 mol·dm−3; b C L = 1 × 10−3 mol·dm−3, C M = 1 × 10−3 mol·dm−3
Modelling in natural systems requires knowledge of the formation constants valid in the dilute reference state. Extrapolation to zero ionic strength was carried out by assuming the validity of the SIT [16, 17]. Since the theory is formulated in terms of molal units, the equilibrium constants and the other quantities in the following treatment have been converted on the molal scale. The conversion factors have been assumed from Baes and Mesmer [28]. According to the theory the activity coefficient, γ i , of the species i with charge z i can be expressed at 25 °C in aqueous solutions as Eq. 5:
where D = 0.2063, and b(i,k) is the specific interaction coefficient of i with species k of molality m k . Interaction coefficients depend on the ionic strength but the variation in the range 0.5 ≤ I ≤ 3.5 mol·kg−1 is sufficiently low that they may be assumed to be constants. As a further simplification, interaction coefficients of ions with the same charge type are nearly zero. According to Eq. 2 and indicating with \( \beta_{pqr}^{\text{o}} \) the constant at zero ionic strength, the variation of the various equilibrium constants determined in this work can be expressed as Eqs. 6–8:
Some b(i,k) values, needed for the calculations, have been deduced from various sources. From Ref. [16] b(H+, \( {\text{ClO}}_{4}^{ - } \)) = 0.14 and b(Fe3+, \( {\text{ClO}}_{4}^{ - } \)) = 0.56, and from Ref. [1] b(Na+, L–) = 0.108. The b(i,k) values for the complexes of stoichiometry (1,−1,1), (1,−2,2) and (1,−1,2) have been evaluated on the basis of empirical rules, suggested elsewhere by Ciavatta [17]. The activity coefficient of the ligand, γ HL, was taken from Ref. [1]. Results of the extrapolation have been collected in Table 3 and the uncertainties assigned to the constants arise mainly from the interaction coefficients of complexes, which are estimated as probable within ±0.05.
The constants given in Table 3 have been used to construct the distribution diagram reported in Fig. 3 to evaluate the predominant species present in the natural conditions.
Distribution diagram calculated with the values of the constants valid at the infinite dilution reference state and reported in Table 3. C L = 5 × 10−4 mol·dm−3, C M = 1 × 10−5 mol·dm−3
Figure 3 shows that complexes with salicylamide can be present in significant amounts in conditions similar to those in natural systems. The prevalent species in neutral solution are \( {\text{FeL}}_{2}^{ + } \) and \( {\text{Fe}}\left( {\text{OH}} \right)_{2}^{ + } \). It is well-known from the literature [28] that the ferric ion hydrolyzes in highly acidic media (pH = 1); however, complexation with salicylamide reduces the percentage of hydrolytic complexes which is approximately 35%.
A comparison was made with results obtained at 25 °C by Ågren [29]. Due to the difference of the ionic strength, the previously reported data have been extrapolated to the infinite dilution reference state (see Table 4).
As can be seen, the value of the stability constant for the complex \( {\text{FeL}}_{2}^{ + } \) obtained in this work is in good agreement with those obtained by Ågren in a previous study. As concerns log10 \( \beta_{1 - 11}^{\text{o}} \) the difference is remarkable. A plausible reason resides in the different speciation profile obtained in this work compared to those of the previous studies. In Figs. 2 and 3, it is evident that the two complexes FeL2+ and Fe(HL)L2+ have the same existence domain and the metal cation is distributed between these two species.
In a previous work [3] it was found that iron(III) ion forms complexes with hydrogen salicylate ion with similar stoichiometry (Table 5).
According to results obtained by Hernández–Gutiérrez and Pulido–Cuchi [30], the complexes between iron(III) ion and salicylamide are more stable than that with salicylic acid, but the low solubility of salicylamide with respect to that of salicylate ion restricts the complex formation to FeL, FeL2 and Fe(HL)L.
4 Conclusions
In this work the complexing power of salicylamide toward iron(III) at 25.0 °C in 1 mol·dm−3 NaClO4 medium was evaluated. The equilibrium constants of the complexes have been calculated at the infinite dilution reference state also. By comparing these results with that previously obtained in the same experimental conditions by studying the system Fe(III)–salicylate ion it was verified that salicylamide forms more stable complexes with this metal cation.
References
Porto, R., Furia, E.: On the complexation of copper(II) ion with 2-hydroxybenzamide. Ann. Chim. 97, 187–198 (2007)
Furia, E., Porto, R.: 2-Hydroxybenzamide as a ligand. Complex formation with dioxouranium(VI), aluminum(III), neodymium(III), and nickel(II) ions. J. Chem. Eng. Data 53, 2739–2745 (2008)
Furia, E., Sindona, G.: Interaction of iron(III) with 2-hydroxybenzoic acid in aqueous solutions. J. Chem. Eng. Data 57, 195–199 (2012)
Furia, E., Napoli, A., Tagarelli, A., Sindona, G.: Speciation of 2-hydroxybenzoic acid with calcium(II), magnesium(II), and nickel(II) cations in self-medium. J. Chem. Eng. Data 58, 1349–1353 (2013)
Porwal, S.K., Furia, E., Harris, M.E., Viswanathan, R., Devireddy, L.: Synthetic, potentiometric and spectroscopic studies of chelation between Fe(III) and 2,5-DHBA supports salicylate-mode of siderophore binding interactions. J. Inorg. Biochem. 145, 1–10 (2015)
Girl, A.K., Adhikari, N., Khan, K.A.: Comparative genotoxicity of six salicylic acid derivatives in bone marrow cells of mice. Mutat. Res. 370, 1–9 (1996)
Velcheva, E.A., Stamboliyska, B.A.: Structural changes caused by the conversion of 2-hydroxybenzamide (salicylamide) into the oxyanion. J. Mol. Struct. 875, 264–271 (2008)
El Kheir, A.A., Belal, S., El Sadek, M., El Shanwani, A.: Spectrophotometric determination of acetaminophen, oxyphenbutazone and salicylamide by nitration and subsequent complexation reactions. Analyst 111, 319–321 (1986)
Fuster Mestrea, Y., Lahuerta Zamoraa, L., Martõ Ânez Calatayudb, J.: Direct flow injection chemiluminescence determination of salicylamide. Anal. Chim. Acta 394, 159–163 (1999)
Pettit, G.: IUPAC: Stability Constant Data Base. Academic Software, Otley (1995)
Ienaúcu, I.M.C., Lupea, A.X., Hãdãruga, D., Hãdãruga, N., Popescu, I.M.: The antimicrobial activity and quantitative structure–biological activity relationships evaluation of some novel 2-hydroxybenzamide derivatives. Rev. Chim. 59, 247–250 (2008)
D’Adamo, P., Ulivi, S., Beneduci, A., Pontoni, G., Capasso, G., Lanzara, C., Andrighetto, G., Hladnik, U., Nunes, V., Palacin, M., Gasparini, P.: Metabonomics and population studies: age-related amino acids excretion and inferring networks through the study of urine samples in two Italian isolated populations. Amino Acids 38, 65–73 (2010)
Nurchi, V.M., Crespo-Alonso, M., Toso, L., Lachowicz, J.I., Crisponi, G.: Chelation therapy for metal intoxication: comments from a thermodynamic viewpoint. Mini-Rev. Med. Chem. 13, 1541–1549 (2013)
Furia, E., Porto, R.: The effect of ionic strength on the complexation of copper(II) with salicylate ion. Ann. Chim. 92, 521–530 (2002)
Biedermann, G., Sillén, L.G.: Studies on the hydrolysis of metal ions. IV. Liquid junction potentials and constancy of activity factors in NaClO4–HClO4 ionic medium. Arkiv Kemi. 5, 425–440 (1953)
Ciavatta, L.: The specific interaction theory in evaluating ionic equilibria. Ann. Chim. 70, 551–567 (1980)
Ciavatta, L.: The specific interaction theory in equilibrium analysis. Some empirical rules for estimating interaction coefficients of metal ion complexes. Ann. Chim. 80, 255–263 (1990)
Crea, F., Falcone, G., Foti, C., Giuffrè, O., Materazzi, S.: Thermodynamic data for Pb2+ and Zn2+ sequestration by biologically important S-donor ligands, at different temperatures and ionic strengths. New J. Chem. 38, 3973–3983 (2014)
Ciavatta, L., Nunziata, G., Sillén, L.G.: Iron(III) acetate complexes in aqueous 3 M(Na+)ClO4 − medium. Acta Chem. Scand. 23, 1637–1652 (1969)
Furia, E., Sindona, G.: Complexation of l-cystine with metal cations. J. Chem. Eng. Data 55, 2985–2989 (2010)
Furia, E., Falvo, M., Porto, R.: Solubility and acidic constants of l-cystine in NaClO4 aqueous solutions at 25 °C. J. Chem. Eng. Data 54, 3037–3042 (2009)
De Stefano, C., Gianguzza, A., Giuffrè, O., Piazzese, D., Orecchio, S., Sammartano, S.: Speciation of organotin compounds in NaCl aqueous solution: interaction of mono-, di- and tri-organotin(IV) cations with nucleotide 5′ monophosphates. Appl. Organomet. Chem. 18, 653–661 (2004)
Gran, G.: Determination of the equivalent point in potentiometric titrations. Acta Chem. Scand. 4, 559–577 (1950)
Gran, G.: Determination of the equivalence point in potentiometric titrations. Part II. Analyst 77, 661–670 (1952)
Sillén, L.G.: Some graphical methods for determining equilibrium constants. II. On “curve-fitting” methods for two-variable data. Acta Chem. Scand. 10, 186–202 (1956)
Gans, P., Sabatini, A., Vacca, A.: SUPERQUAD: an improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalton Trans. 6, 1195–1200 (1985)
Ciavatta, L., De Tommaso, G., Iuliano, M.: The acidic constants of 2-hydroxybenzohydroxamic acid in NaClO4 solutions at 25 °C. Ann. Chim. 94, 295–302 (2004)
Baes, C.F., Mesmer, R.E.: The Hydrolysis of Cations. Wiley-Interscience Publication, New York (1976)
Ågren, A.: The complex formation between iron(III) ion and some phenols. III. Salicylaldehyde, o-hydroxyacetophenone, salicylamide and methyl salicylate. Acta Chem. Scand. 9, 39–49 (1955)
Hernández-Gutiérrez, F., Pulido-Cuchi, F.: Identification et dosage de la salicylamide. Anal. Chim. Acta 5, 450–458 (1951)
Acknowledgements
I gratefully acknowledge the Dipartimento di Chimica e Tecnologie Chimiche, Università della Calabria for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Furia, E. Study of Complexation Equilibria Between the Iron(III) Ion and 2-Hydroxybenzamide in Aqueous Solution. J Solution Chem 46, 1596–1604 (2017). https://doi.org/10.1007/s10953-017-0665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0665-0