Abstract
The interphase distribution of La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu between aqueous HNO3 solutions and solutions of tetraphenylmethylenediphosphine dioxide in 1,2-dichloroethane was studied. The latter solutions also contained ionic liquids with an anion of bis[(trifluoromethyl)sulfonyl]imide and cations of quaternary ammonium bases. It was detected that the extraction of metal ions significantly increases in the presence of ionic liquids in the organic phase. The stoichiometry of the extracted complexes was determined, and analysis was made of the effect of the HNO3 concentration in the aqueous phase and the nature of the cationic part of an ionic liquid on the efficiency of the extraction of metal ions into the organic phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The concept of closed-loop nuclear fuel cycle with reprocessing of spent nuclear fuel is based on the selective extraction of its contained f elements necessary for nuclear medicine and some other industries [1]. Extraction methods are widely used to recover, concentrate, and separate actinides and lanthanides. High ability to extract these elements is characteristic of polydentate neutral organophosphorus compounds [2–4], including substituted methylenediphosphine dioxides [5, 6]. Substitution of aryls for alkyl substituents at phosphorus atoms in the molecules of these compounds leads to an abrupt increase in the ability to extract actinides and lanthanides in nitric acid media, which is due to the anomalous aryl strengthening of complexes being extracted [7].
Interest has recently increased in using ionic liquids (IL) in extraction methods of concentration and separation of organic and inorganic substances as a phase immiscible with water [8–11]. It was shown that the extraction of actinides and lanthanides(III) with solutions of diaryl(dialkylcarbamoylmethyl)phosphinoxides (CMPO) in such IL as hexafluorophosphates and bis[(trifluoromethyl)sulfonyl]imides of 1-alkyl-3-methylimidazolia significantly exceeds the extraction with CMPO solutions in conventional organic solutions [12, 13]. It was noted that, in such systems, to extract metal ions with a theoretically calculated yield, a significant excess of \({\text{NO}}_{3}^{ - }\) ions in the aqueous phase is not required, whereas such an excess is a necessary condition for extraction with this extractant in conventional organic solvents. Examples of using IL in extraction and concentration of lanthanides(III) and actinides were given in reviews [14–20]. It was determined that, for efficient extraction of lanthanides(III) from solutions of HNO3 and HCl, it is sufficient to ensure even a relatively low IL concentration in an organic solvent containing neutral donor-active extractants [21–24]. Owing to this, IL can be considered as an active component of the synergistic mixture.
The efficiency of bis[(trifluoromethyl)sulfonyl]imide as an anionic component of IL is due to its higher hydrophobicity and hydrolytic stability in nitric acid media in comparison with hexafluorophosphate anion [14]. In most of the published studies of metal ion extraction, IL based on 1-alkyl-3-methylimidazolium cations were mainly used. Recently, in extraction practice, IL with cations of quaternary ammonium bases have been more popular, which is owing to their higher availability and lower toxicity [25].
The purpose of this work was to investigate the effect of the nature of the cationic part of IL on the efficiency of the extraction of lanthanides(III) from nitric acid solutions with solutions of tetraphenylmethylenediphosphine dioxide in organic solvents. For this purpose, we considered the interphase distribution of Ln(III) ions between HNO3 solutions and the organic phase containing tetraphenylmethylenediphosphine dioxide Ph2P(O)CH2P(O)Ph2 and bis[(trifluoromethyl)sulfonyl]imides of tetraethylammonium ([Et4N][Tf2N]), triethylbenzylammonium ([Et3BnN][Tf2N]), tetrabutylammonium ([Bu4N][Tf2N]), and trioctylmethylammonium ([Oct3MeN][Tf2N]).
EXPERIMENTAL
Tetraphenylmethylenediphosphine dioxide (TPMDPO) was produced according to a published procedure [26]. Ionic liquids [Et4N][Tf2N], [Et3BnN][Tf2N], [Bu4N][Tf2N], and [Oct3MeN][Tf2N] were obtained by the method [27] of the metathesis reaction of bis[(trifluoromethyl)sulfonyl]imide lithium salt (Sigma-Aldrich) with bromides of tetraethylammonium, triethylbenzylammonium, and tetrabutylammonium and trioctylmethylammonium chloride (Aliquat 336, Sigma-Aldrich), respectively. The organic solvent was chemically pure 1,2-dichloroethane without additional purification. Solutions of TPMDPO and IL in the organic solvent were prepared using accurately weighed samples. The TPMDPO and IL concentrations were varied in the ranges 3 × 10–4–3 × 10–3 and 0.001–0.1 mol/L, respectively.
The Ln(III) ion distribution in the extraction systems was studied using model solutions containing 0.03–7.0 mol/L HNO3. The initial aqueous solutions containing 2 × 10–6 mol/L each of Ln(III) were prepared by dissolution of the corresponding nitrates in water with subsequent addition of HNO3 to a required concentration. The reagents used in the experiments were chemically pure.
Extraction experiments were carried out in glass-stoppered test tubes with at a temperature of 22 ± 1°C and a ratio between the volumes of the organic and aqueous phases of 1 : 1. The phases were brought into contact in a rotary apparatus while stirring at 60 rpm for 1 h. It was preliminarily established that this time is enough for the distribution ratios D to reach constant values.
The contents of lanthanides(III) in the initial and equilibrium aqueous solutions were found by inductively coupled plasma mass spectrometry with an X-7 mass spectrometer (Thermo Electron, USA). The contents of elements in the organic phase were determined from the difference of the initial and equilibrium concentrations in the aqueous phase. The distribution ratios DLn of lanthanides were calculated as the ratio of their concentrations in the equilibrium phases. The error of determining the distribution ratios did not exceed 5%. The Tf2N– ion concentration in the equilibrium aqueous concentrations was found by inductively coupled plasma mass spectrometry with an ICAP-61 spectrometer (Thermo Jarrel Ash, USA) from the sulfur content. The HNO3 concentrations in the equilibrium aqueous phases were measured by potentiometric titration with a NaOH solution.
RESULTS AND DISCUSSION
It was determined that the solutions of the studied IL in dichloroethane does not extract Ln(III) from nitric acid solutions (GLn do not exceed 10–2). However, the extraction of Ln(III) with TPMDPO–IL mixtures in dichloroethane is characterized by a significant increase in the Ln(III) recovery into the organic phase (Fig. 1). The observed synergistic effect can be related to the incorporation of hydrophobic Tf2N– anions into complexes being extracted as counterions of solvated Ln(III) cations, which leads to an increase in the hydrophobicity of the latter in comparison with coordination-solvated Ln(III) nitrates. Such a process explains the considerable increase in the extraction of Ln(III) and Am(III) with solutions of neutral extractants in the presence of hydrophobic picrate anions [28], tetrakis[3,5-bis(trifluoromethyl)phenyl]borate anions [29], or chlorinated cobalt dicarbollide anions [30].
The synergistic effect S is defined as S = D/(D1 + D2), where D is the distribution ratio of Ln(III) in extraction with a TPMDPO–IL mixture; and D1 and D2 are the distribution ratios in the extraction with individual components of the mixture under the same conditions. In the studied systems, S increases in the order of IL [Oct3MeN][Tf2N] < [Bu4N][Tf2N] < [Et3BnN][Tf2N] < [Et4N][Tf2N] (Figs. 2, 3) with decreasing hydrophobicity of the IL cation. It was previously detected that the efficiency of the extraction of metal ions with solutions of neutral donor-active extractants in IL based on 1-alkyl-3-methylimidazolium cations decreases with increasing their hydrophobicity as the length of the alkyl chain in the IL cation increases [31].
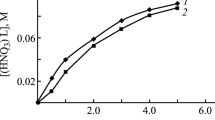
Dependence of the distribution ratios of Eu(III) on the HNO3 concentration in the equilibrium aqueous phase in the extraction with 0.001 mol/L TPMDPO solutions in dichloroethane containing (1) 0.002 mol/L [Et4N][Tf2N], (2) 0.01 mol/L [Et3BnN][Tf2N], (3) 0.01 mol/L [Bu4N][Tf2N], and (4) 0.01 mol/L [Oct3MeN][Tf2N] and (5) in pure dichloroethane.
In the extraction from the 1 mol/L HNO3, S increases in the order in the lanthanide(III) series from La(III) to Lu(III) (Fig. 2) with decreasing ionic radii of Ln3+ ions and increasing their hydration energy [32]. Probably, the substitution of more hydrophobic Tf2N– ions for \({\text{NO}}_{3}^{ - }\) anions in the complex being extracted has a stronger effect on the extraction of more hydrated rare-earth-metal(III) ions. This leads to a decrease in the La/Lu separation factor βLa/Lu = DLa/DLu from 60.3 in the extraction with the TPMDPO solution to 20.4 in the extraction with the same extractant in the presence of [Oct3MeN][Tf2N] in the organic phase (Fig. 2).
The presence of IL in the organic phase significantly changes the behavior of the dependence of the efficiency of the extraction of Ln(III) with the TPMDPO solutions on the HNO3 concentration in the aqueous phase (Fig. 3). In the presence of less hydrophobic IL [Et3BnN][Tf2N] and [Et4N][Tf2N], DEu decreases with increasing [HNO3], which was also previously detected in the extraction with TPMDPO and CMPO solutions in the presence of with a cation of 1-butyl-3-methylimidazolium (bmimTf2N) [21–24]. The causes of such a behavior of the dependence D([HNO3]) in systems with ILK were discussed before [33, 34]. Another behavior of the dependence DEu([HNO3]) is observed in the systems with more hydrophobic IL [Oct3MeN][Tf2N] and [Bu4N][Tf2N] (Fig. 3). In this case, DEu increases with increasing [HNO3] to 1 mol/L, which can be related to the incomplete substitution of hydrophobic Tf2N– ions for \({\text{NO}}_{3}^{ - }\) anions in complexes being extracted of Ln(III), i.e., to the participation of NO3– ions in the formation of the complexes being extracted in such systems. The decrease in DEu with a further increase in [HNO3] is due to the decrease in the concentration of the free extractant in the organic phase because of the coextraction of HNO3 and HTf2N. The decrease in DEu with increasing [HNO3] is most noticeable in the case of less hydrophobic IL. Figure 3 shows that an increase in [HNO3] from 2 to 5 mol/L is accompanied by a decrease in DEu by a factor of 85, 26, and 16 in the systems with [Et3BnN][Tf2N], [Bu4N][Tf2N], and [Oct3MeN][Tf2N], respectively. This is caused by the fact that the Tf2N– ion concentration in the aqueous phase increases with decreasing hydrophobicity of the IL cation; i.e., the coextraction of HTf2N exerts a less marked effect on the metal ion extraction in the presence of more hydrophobic IL. Note that, in the absence of IL, an increase in [HNO3] from 2 to 5 mol/L in the extraction with a TPMDPO solution in dichloroethane is accompanied by a decrease in DEu by a factor of only 3.7.
It is known that extraction of metal ions with neutral extractants in the presence of IL is accompanied by a noticeable transfer of IL ions into the aqueous phase [35]. The distribution ratios of the Tf2N– ion between 0.1 mol/L IL solutions in dichloroethane and a 1 mol/L HNO3 solution increase in the order Et4NTf2N (5.0) < Et3BnNTf2N (19.8) < Bu4NTf2N (302) < Oct3MeNTf2N (>1000) with increasing hydrophobicity of the cationic part of these IL. The IL loss in the course of the extraction decreases in the same order.
Although the synergistic effect in the extraction of lanthanides(III) with a TPMDPO solution from nitric acid solutions in the system with [Oct3MeN][Tf2N] is weaker than that in the systems with less hydrophobic [Et4N][Tf2N], [Et3BnN][Tf2N], and also bis[(trifluoromethyl)sulfonyl]imides of 1-butyl-3-methylimidazolium (bmimTf2N) [21, 22], using [Oct3MeN][Tf2N] significantly decreases the IL loss during extraction. Moreover, the decrease in the distribution ratios of metal ions with decreasing [HNO3] (Fig. 3) allows one to considerably simplify their stripping process. The extraction of Ln(III) from a 3 mol/L HNO3 solution with a solution of 0.05 mol/L TPMDPO and 0.01 mol/L [Oct3MeN][Tf2N] or [Et3BnN][Tf2N] in dichloroethane was followed by stripping of Ln(III) with water. In the system with [Oct3MeN][Tf2N], the Ln(III) stripping efficiency exceeds 90%, whereas in the system with [Et3BnN][Tf2N], Ln(III) has not been virtually transferred to the aqueous phase. Therefore, below, we consider equilibria in the interphase distribution of Ln(III) between HNO3 solutions and the organic phase containing TPMDPO and [Oct3MeN][Tf2N].
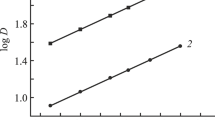
The synergistic extraction of lanthanides(III) from nitric acid solutions with TPMDPO solutions (L) in the presence of hydrophobic IL [Oct3MeN][Tf2N] (RA) can be described by the equation
where the subscripts (a) and (o) refer to components of the aqueous and organic phases, respectively. The equation shows that the extraction of metal ions with the TPMDPO–[Oct3MeN][Tf2N] mixture from nitric solutions is accompanied by the formation of trioctylmethylammonium nitrate (RNO3) in the organic phase. Therefore, the addition of RNO3 to the organic phase containing the TPMDPO–[Oct3MeN][Tf2N] mixture should lead to a shift of equilibrium (1) to the left, i.e., should to a decrease in the metal ion extraction. Indeed, at constant concentrations of TPMDPO and [Oct3MeN][Tf2N] in the organic phase, the slope of the logDLn(log[RNO3]) plot is close to –1 (Fig. 4). At constant TPMDPO concentration in the organic phase, the slope of the logDLu(log[RA]) is close to 1 (Fig. 5), which corresponds to the transfer of Ln(III) to the organic phase as complexes with a Ln3+ : Tf2N– ratio of 1 : 1.
The stoichiometric ratio Ln(III) : L in complexes being extracted in the presence of IL is close to 1 : 3 (Fig. 6), whereas, in the absence of IL, Ln(III) ions are extracted with TPMDPO solutions from nitric acid solutions mainly as complexes with a Ln(III) : L ratio of 1 : 2 [36]. The increase in the solvation number in the system with IL is due to the weak coordinating ability of Tf2N– ions [37], which are likely to occur in the outer coordination sphere of the complex LnL3(NO3)2(Tf2N) being extracted. The increase in the number of extractant molecules participating in the formation of complexes being extracted leads to an increase in their hydrophobicity, which favors an increase in the efficiency of the extraction of metal ions in to the organic phase.
CONCLUSION
It was shown that the efficiency of the extraction of lanthanides(III) from nitric acid solutions with TPMDPO solutions significantly increases in the presence of bis[(trifluoromethyl)sulfonyl]imides of quaternary ammonium bases. The synergistic effect decreases with increasing hydrophobicity of the IL cation. However, using hydrophobic IL [Oct3MeN][Tf2N] substantially decreases the IL loss in the course of the extraction and considerably simplifies the stripping of the extracted metal ions.
REFERENCES
B. F. Myasoedov, S. N. Kalmykov, Yu. M. Kulyako, et al., Geochem. Int. 54, 1156 (2016). https://doi.org/10.1134/S0016702916130115
M. Yu. Alyapychev, V. A. Babain, and Yu. A. Ustynyuk, Russ. Chem. Rev. 85, 943 (2016). https://doi.org/10.1070/RCR4588
A. Leoncini, J. Huskens, and W. Verboom, Chem. Soc. Rev. 46, 7229 (2017). https://doi.org/10.1039/C7CS00574A
A. M. Wilson, P. J. Bailey, and P. A. Tasker, Chem. Soc. Rev. 43, 123 (2014). https://doi.org/10.1039/C3CS60275C
A. M. Rozen, V. I. Volk, A. Yu. Vakhrushin, Radiochemistry 41, 215 (1999).
A. M. Rozen and B. V. Krupnov, Russ. Chem. Rev. 65, 973 (1996). https://doi.org/10.1070/RC1996v065n11ABEH000241
A. M. Rozen, Z. I. Nikolotova, N. A. Kartasheva, and K. S. Yudina, Dokl. Akad. Nauk SSSR 222, 1151 (1975).
A. E. Visser, R. P. Swatloski, W. M. Reichert, et al., Ind. Eng. Chem. Res. 39, 3596 (2000).
M. Koel, CRC Crit. Rev. Anal. Chem. 35, 177 (2005). https://doi.org/10.1080/10408340500304016
H. Zhao, S. Xia, and P. Ma, J. Chem. Technol. Biotechnol. 80, 1089 (2005).
M. L. Dietz, Sep. Sci. Technol. 41, 2047 (2006).
K. Nakashima, F. Kubota, T. Maruyama, et al., Anal. Sci. 19, 1097 (2003). https://doi.org/10.2116/analsci.19.1097
A. E. Visser and R. D. Rogers, J. Solid State Chem. 171, 109 (2003).
Z. Kolarik, Solvent Extr. Ion Exch. 31, 24 (2013). https://doi.org/10.1080/07366299.2012.700589
F. Kubota, Y. Baba, and M. Goto, Solvent Extr. Res. Dev. Jpn. 19, 17 (2012).
I. Billard, in Handbook on the Physics and Chemistry of Rare Earths, Ed. by J.-C. G. Bünzli and V. Pecharsky (Elsevier, Amsterdam, 2013), Vol. 43, Ch. 256, p. 213.
I. A. Shkrob, T. W. Marin, and M. P. Jensen, Ind. Eng. Chem. Res. 53, 3641 (2014). https://doi.org/10.1021/ie4036719
P. K. Mohapatra, Chem. Prod. Proc. Model. 10, 135 (2015).
N. K. Gupta, J. Mol. Liq. 269, 72 (2018).
C. A. Hawkins, M. A. Momen, and M. L. Dietz, Sep. Sci. Technol. 53, 1820 (2018). https://doi.org/1080/01496395.2017.1302478
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Russ. J. Inorg. Chem. 53, 970 (2008). https://doi.org/10.1134/S0036023608060272
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Radiochemistry 50, 266 (2008). https://doi.org/10.1134/S1066362208030090
A. N. Turanov, V. K. Karandashev, and A. N. Yarkevich, Radiochemistry 55, 382 (2013).
A. N. Turanov, V. K. Karandashev, and A. N. Yarkevich, Russ. J. Inorg. Chem. 63, 406 (2018). https://doi.org/10.1134/S0036023618030221
T. J. Bell and Y. Ikeda, Dalton Trans. 40, 10125 (2011).
V. A. Chauzov, Yu. N. Studnev, M. G. Iznostkova, and A. V. Fokin, Zh. Obshch. Khim. 57, 54 (1987).
P. Bonhote, A. P. Dias, N. Papageorgiou, et al., Inorg. Chem. 35, 1168 (1996).
H. Naganawa, H. Suzuki, S. Tachimori, et al., Phys. Chem. Chem. Phys. 3, 2509 (2001).
H. Suzuki, H. Naganawa, and S. Tachimori, Phys. Chem. Chem. Phys. 5, 726 (2003).
J. Rais and S. Tachimori, J. Radioanal. Nucl. Chem. Lett. 188, 157 (1994).
S. Dai, Y. H. Ju, and C. E. Barnes, J. Chem. Soc., Dalton Trans. 1201 (1999).
K. B. Yatsimirskii, N. A. Kostromina, Z. A. Sheka, et al., Chemistry of Complex Compounds of Rare-Earth Elements (Nauk. Dumka, Kiev, 1966) [in Russian].
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Solvent Extr. Ion Exch. 26, 77 (2008). https://doi.org/10.1080/07366290801904871
A. N. Turanov, V. K. Karandashev, and V. A. Khvostikov, Solvent Extr. Ion Exch. 35, 461 (2017).
C. Gaillard, M. Boltoeva, I. Billard, et al., Chem. Phys. Chem. 16, 2653 (2015). https://doi.org/10.1002/cphc.201500283
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Radiochemistry 43, 72 (2001).
K. Binnemans, Chem. Rev. 107, 2593 (2007). https://doi.org/10.1021/cr050979c
Funding
This work was performed under the 2019 state assignment for the Institute of Solid-State Physics, Russian Academy of Sciences, and the Institute of Problems of Microelectronics Technology and High-Purity Materials, Russian Academy of Sciences (both in Chernogolovka, Moscow oblast, Russia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Turanov, A.N., Karandashev, V.K. Extraction of Lanthanides(III) from Nitric Acid Solutions with Tetraphenylmethylenediphosphine in the Presence of Bis[(trifluoromethyl)sulfonyl]imides of Quaternary Ammonium Bases. Russ. J. Inorg. Chem. 65, 113–118 (2020). https://doi.org/10.1134/S0036023620010180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620010180