Abstract
A new dicationic ionic liquid, 1-methyl-3-(4-(tributylphosphonio)butyl)-1H-imidazol-3-ium di[bis(trifluoromethanesulfonyl)imide] [ImP][Tf2N]2, showing high hydrophobicity (solubility in water is 9.2 × 10–4 mol/L) has been synthesized. The extraction of U(VI), Th(IV), and lanthanides(III) from nitric acid solutions with mixtures of 1,5-N,N'-bis[(diphenylphosphoryl)acetyl(hexyl)amino]pentane (L) containing two bidentate Ph2P(O)CH2C(O)N(Hex)- fragments connected by pentamethylene spacer through amide nitrogen atoms and [ImP][Tf2N]2 in 1,2-dichloroethane (DCE) has been studied. During the extraction of metal ions in this system, a significant synergistic effect has been observed. The influence of the composition of aqueous and organic phases on the efficiency of metal ions extraction into organic phase has been considered and the stoichiometry of extracted complexes has been determined. The synergistic effect in the extraction of Ln(III) from 3 M HNO3 solutions with a mixture of L and [ImP][Tf2N]2 in DCE is an order of magnitude higher than in the L–[C8mim][Tf2N]–DCE system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, ionic liquids (ILs) obtain are widely used in different fields of science and technology because they have a complex of unique physicochemical properties such as excellent solvation properties, high dielectric constant, low volatility, non-flammability, low solubility in water, etc., which is not typical for common organic solvents. These properties make ILs to be extremely necessary in the synthesis of unique monomeric and polymeric materials [1, 2], catalytic processes [3], electrochemistry [4], liquid and gas chromatography [5], in processes of recovery and separation of organic compounds [6] and metal ions [7–10], and in reprocessing of spent nuclear fuel including [11]. It was shown that extraction of U(VI), Pu(IV) and Th(IV) with carbamoylmethylphosphine oxides (CMPO) increases by dozens of times as compared with common n-dodecane, when 1-butyl-3-methylimidazolium hexafluorophosphate was used as diluent [9], the use of pure IL is unessential because addition of even small amount of IL in common organic solvent leads to the same growth of extraction efficiency. This effect was revealed on the use of different extractants such as CMPO [12, 13], diglycoldiamides [14], calixarene derivatives [11, 15], etc., it is explained by the high hydrophobicity of IL anions which are involved in the formation of extracted complexes as counterions, thus increasing their hydrophobicity and facilitating transition into used diluent. At present, tens of different ILs are synthesized and certain features of the effect of their structure on metal ion extraction are established [11, 16, 17]. So, increase in the hydrophobicity of IL anion and decrease of cation hydrophobicity result in the growth of extraction ability of IL [16]. However, decrease of IL cation hydrophobicity, for example on the shortening of alkyl substituent at the nitrogen atom in the cationic portion of 1-alkyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imides [Cnmim][Tf2N], although leads to increase in extraction of Am(III) and Eu(III) with CMPO solutions [17], however is accompanied by marked transfer of IL into aqueous phase, which results in marked loss of IL and causes additional environmental problems on their application in extraction processes [18].
ILs with two cationic centers are considered to be more safe, their properties are intensely studied at present time [19–23]. However, there are no data in the literature on the influence of such ILs on extraction of metal ions.
In the present work, we studied effect of new IL with two cationic centers [ImP][Tf2N]2 on the extraction of U(VI), Th(IV), and lanthanide(III) cations from nitric acid solutions using 1,5-N,N'-bis[(diphenylphosphoryl)acetyl(hexyl)amino]pentane (L), whose molecule contains two bidentate Ph2P(O)CH2C(O)N(Hex)-fragments connected to each other by pentamethylene bridge via amide nitrogen atoms. We showed previously that distribution ratios for Ln(III), U(VI), and Th(IV) on extraction with solution of this compound in molecular solvents are considerably higher than on extraction with its mono analog CMPO Ph2Bu2 [24].
Extraction properties of this IL is compared with behavior of 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide [C8mim][Tf2N].

EXPERIMENTAL
Initial chemicals for the synthesis of extractant L and ionic liquids were of reagent or analytical grade (Sigma-Aldrich, USA) and were used as received.
1H, 13C, and 31P NMR spectra were recorded on a Bruker AV-400 spectrometer (operating at 400.13 MHz for 1H, 100.61 MHz for 13C, and 161.97 MHz for 31P) in CDCl3 solution using residual proton and carbon signals of the deuterated solvent as internal references (1H, 13C) and 85% H3PO4 (31P) as an external reference. 13C NMR spectra were registered in JMODECHO mode, signals of carbon atoms with even and odd number of protons have opposite polarity. IR spectra were registered on a Nicolet Magna IR750 spectrometer. Elemental analysis was performed in the Laboratory of Microanalysis, Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences.
1,5-N,N'-bis[(diphenylphosphoryl)acetyl(hexyl)-amino]pentane (L) [24], diphenyl(dibutylcarbamoylmethyl)phosphine oxide CMPO Ph2Bu2 [25], and ionic liquid 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide [C8mim][Tf2N] [26] were synthesized and purified by previously described procedures.
(4-Bromobutyl)(tributyl)phosphonium bromide was obtained by modified procedure [27]. Tributylphosphine (1.0 g, 0.005 mol) was added over 1 h to a solution of 5.3 g (0.025 mol) of dibromobutane in 5 mL of absolute EtOH at ambient temperature in argon flow. The resultant solution was stirred at 50°C for 1 h and at 80°C for 2 h, the solvent was removed. The residue was kept at 0.1 mmHg and 70°C until constant weight and washed with Et2O (4 × 15 mL). The resultant light yellow oil was kept at 0.1 mmHg until constant weight. Yield 1.8 g (87%).
31P NMR spectrum (δP, ppm) 33.24. 1H NMR spectrum (δH, ppm): 3.50 (t, 2H, CH2Br, 3JHH = 6.1 Hz); 2.66–2.57 (m, 2H, PCH2CH2CH2CH2Br); 2.44–2.36 (m, 6H, P(CH2CH2CH2CH3)3); 2.07 (quin, 2H, PCH2CH2CH2CH2Br, 3JHH = 6.4 Hz); 1.80–1.72 (m, 2H, PCH2CH2CH2CH2Br); 1.59–1.43 (m, 12H, P(CH2CH2CH2CH3)3); 0.95 (t, 9H, P(CH2CH2CH2CH3)3, 3JHH = 7.0 Hz).
1-Methyl-3-(4-(tributylphosphonio)butyl)-1H-imidazol-3-ium dibromide was obtained by modified procedure [22]. (4-Bromobutyl)(tributyl)phosphonium bromide (1.8 g, 0.0043 mol) was added over 30 min to a solution of 0.4 g (0.0052 mol) of N-methylimidazole in 2 mL of anhydrous CHCl3 at 0°C in argon flow. The resultant solution was stirred at 0°C for 3 days and at 40–45°C for 6 days. The solvent was removed, the residue was kept at 0.1 mm Hg and 40°C until constant weight. The residue was washed with Et2O (4 × 10 mL) and the resultant light yellow viscous oil was kept at 0.1 mm Hg and 30°C over P2O5 until constant weight. Yield 1.6 g (76%).
Found, %: C, 48.06; H 8.44; N, 5.59; P, 7.02.
For C20H41Br2N2P anal. calcd., %: C, 48.01; H, 8.26; N, 5.60; P, 6.19.
IR spectrum (ν, cm–1): 3429, 3143, 3074, 2961, 2933, 2874, 2189, 1622, 1573, 1465, 1412, 1383, 1283, 1232, 1171, 1099, 969, 923, 821, 730, 641, 624. 31P NMR spectrum (δP, ppm) 33.50. 1H NMR spectrum (δH, ppm): 10.21 (s, 1H, in Meim); 8.16 (s, 1H, in Meim); 7.31 (s, 1H, in Meim); 4.57 (br t, 2H, NCH2, 3JHH = 6.0 Hz); 4.02 (s, 3H, NCH3); 2.78–2.59 (m, 2H, NCH2CH2CH2CH2P); 2.41–2.26 (m, 8H, ‒CH2P(CH2CH2CH2CH3)3); 1.87 (br s, 2H, NCH2CH2CH2CH2P); 1.64–1.46 (m, 12H, P(CH2CH2CH2CH3)3); 0.94 (t, 9H, P(CH2CH2CH2CH3)3, 3JHH = 6.4 Hz). 13C NMR spectrum (δC, ppm): 136.33 (s, NCH=N); 122.92 and 122.75 (both s, NCH=CHN); 48.06 (s, NCH2–); 36.19 (s, NCH3); 30.36 (d, NCH2CH2CH2CH2P, 2JPC = 16.0 Hz); 23.32 (d, PCH2CH2CH2CH3, 2JPC = 15.3 Hz); 23.10 (d, PCH2CH2CH2CH3, 3JPC = 4.6 Hz); 18.42 (d, PCH2CH2CH2CH2N, 1JPC = 48.4 Hz); 18.23 (d, PCH2CH2CH2CH3, 1JPC = 47.0 Hz); 17.88 (s, NCH2CH2CH2CH2P); 12.94 (s, CH3).
1-Methyl-3-(4-(tributylphosphonio)butyl)-1H-imidazol-3-ium di[bis(trifluoromethanesulfonyl)imide] ([ImP][Tf2N]2). A solution of 2.1 g (7.3 mmol) of lithium bis(trifluoromethanesulfonyl)imide in 10 mL of water was added to a solution of 1.6 g (3.2 mmol) of 1‑methyl-3-(4-(tributylphosphonio)butyl)-1H-imidazol-3-ium dibromide in 15 mL of water and stirred for 2 h at 40°C. Next, 30 mL of 1,2-dichloroethane was added, stirred, and the organic phase was separated. The resultant solution was used in extraction experiments.
1,2-Dichloroethane (DCE) of reagent grade (Vekton) without additional purification was used as a solvent in experiments on extraction of metal ions. Extractant solutions in DCE were prepared using strictly weighed samples. Aqueous solutions of lanthanides(III), U(VI), and Th(IV) with concentration of 0.01 mol/L were prepared by dissolution of the appropriate nitrates in water. These solutions after dilution with subsequent addition of HNO3 were used for preparing initial aqueous solutions for extraction experiments. Nitric acid concentration in these solutions varied in the range 0.01–5 mol/L, metal ions concentration was 4 × 10–6 mol/L. On extraction of lanthanides(III), aqueous phase contained all Ln(III) except for Pm. All experiments on metal ions extraction were conducted in plastic test tubes at ambient temperature (22 ± 2°C) and 1 : 1 volume ratio of organic and aqueous phases. Phases were contacted in a rotor mixer at a rate of 60 rpm for 1 h. It was found preliminary, that this time is sufficient to reach constant values of distribution ratios (D).
Ln(III), U(VI), and Th(IV) concentration in initial and equilibrium aqueous solutions was determined by inductively coupled plasma mass spectrometry on a ThermoScientific XSeries 2 mass spectrometer (USA) by procedure [28]. Metal concentration in organic phase was found from the difference between their concentration in aqueous phase before and after extraction. Distribution ratio of metal ions was calculated as the ratio of metal ion concentrations in equilibrium phases. Error of D determination was ~10%. Concentrations [ImP2+] and [Tf2N–] in equilibrium aqueous solutions were found by determination of phosphorus and sulfur content by inductively coupled plasma–atomic emission spectroscopy on a Thermo Jarrel Ash ICAP-61 spectrometer. HNO3 concentration in equilibrium aqueous phase was determined by potentiometric titration with standard NaOH solution.
RESULTS AND DISCUSSION
Important characteristic of ionic liquids used in extraction of metal ions is their solubility in aqueous solutions. The solubility of [ImP][Tf2N]2 in water is 9.2 × 10–4 mol/L. For comparison, Table 1 shows the values of solubility of imidazolium ILs with Tf2N– anion. These data indicate that the hydrophobicity of [ImP][Tf2N]2 is much higher than that of [C4mim][Tf2N], which is most frequently used in extraction. This is a considerable advantage of [ImP][Tf2N]2.
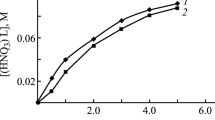
We considered the effect of HNO3 concentration in equilibrium aqueous phase on the extraction of U(VI), Th(IV), and Ln(III) ions with solutions of compound L in DCE containing 0.002 M of [ImP][Tf2N]2. For comparison, Fig. 1 displays the data on the extraction of these ions with solutions of compound L in DCE in the absence of IL. Increase in HNO3 concentration in aqueous phase is accompanied by the growth of distribution ratios of Ln(III) on the use of solution of L in DCE without IL. At [HNO3] > 3 mol/L, the growth of DU, DTh, and DEu is slightly lower (Fig. 1), which is due to the marked co-extraction of HNO3 [24]. Such a character of logD–log[HNO3] dependence was observed on extraction of U(VI), Th(IV), and Ln(III) ions with CMPO solutions in molecular solvents by solvate mechanism as coordination-solvated nitrates [30].
In the presence of IL in organic phase, the character of dependence logD–log[HNO3] sharply changes. On extraction of U(VI), Th(IV), and Eu(III) with mixtures of L and [ImP][Tf2N]2 in DCE, D values decrease when HNO3 concentration in aqueous phase increases (Fig. 1). Similar dependence of D on aqueous phase acidity was observed previously for the extraction of Ln(III) and actinides with solutions of neutral organophosphorus extractants and diglycolamides [12–14] in common organic diluents in the presence of IL [C4mim][Tf2N]. We assume the character of D–[HNO3] dependence for all noted extraction systems can be explained by decrease of free extractant concentration in organic phase on increase of aqueous phase acidity die to co-extraction of both HNO3 and HTf2N, which is present in aqueous phase because of transition of anionic component of IL into aqueous phase.
As shown by Fig. 1, at the equal concentration of HNO3 in aqueous phase and extractant L, addition of IL into organic phase leads to the considerable growth of distribution ratios for U(VI), Th(IV), and Eu(III). IL [ImP][Tf2N]2 itself does not extract U(VI), Th(IV), and Eu(III) from nitric acid solutions (D < 10–2). This fact indicates the emergence of considerable synergistic effect in L–[ImP][Tf2N]2–DCE system. The most probable reason of this effect is the participation of Tf2N– anions in the formation of extractable complexes as counterions. This leads to increase in the hydrophobicity of such complexes as compared with those produced with nitrate ions in the absence of IL in organic phase. We suppose that Tf2N– anions showing weak coordination ability [31] occupy outer coordination sphere of extracted complexes. Large Tf2N– anions are incompatible with strongly hydrogen-bonded structure of water in aqueous phase, which makes their transition into organic phase more energetically favorable than the transition of nitrate ions.
The value of synergetic effect on extraction with mixtures of L and IL can be assessed using synergy coefficient (SC) calculated as
where DL, DIL, and DL+IL are distribution ratios of metal ion on extraction with L and IL separately and their mixtures, respectively.
The comparison of dependences D–[HNO3] in L–DCE and L–[ImP][Tf2N]2–DCE systems (Fig. 1) exhibits that synergistic effect decreases when HNO3 concentration in aqueous phase rises. For example, on extraction of Eu(III), increase in HNO3 concentration from 0.5 to 5 mol/L leads to decrease of SC values from 1120 to 3.2.
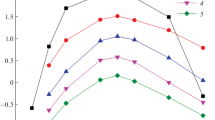
We compared effect of ionic liquids [ImP][Tf2N]2 and [C8mim][Tf2N] on extraction of Ln(III) with compound L and its mono analog (CMPO Ph2Bu2) from nitric acid solutions. Figure 2 shows that [ImP][Tf2N]2 displays much higher synergistic effect in extraction of Ln(III) than [C8mim][Tf2N], although the latter exhibits lower hydrophobicity (Table 1). In the presence of IL in organic phase, the efficiency of Ln(III) extraction from nitric acid solutions with compound L is considerably higher than that with its analog, CMPO Ph2Bu2. Consequently, the effect of preorganization of extractant L molecule [32] also appears in systems with IL.
Distribution ratios of lanthanides(III) on extraction from 3 M HNO3 solutions of compounds (1, 2, 5) L and (3, 4, 6) CMPO Ph2Bu2 in (5, 6) dichloroethane and (1, 3) dichloroethane containing 0.025 M of [ImP][Tf2N]2 and (2, 4) [C8mim][Tf2N]. Concentration of compounds L and CMPO Ph2Bu2 is 0.01 and 0.02 mol/L, respectively.
At moderate HNO3 concentration in equilibrium aqueous phase, extraction of Ln(III) decreases as atomic number (Z) of Ln(III) increases (Fig. 2). As a whole, this order of extractability in the series of lanthanides is typical for CMPO [33] and their bis-derivatives [24] in nitric acid media. The noted character of logDLn–Z dependence was explained by increase in the hydration energy of Ln(III) ions as their ionic radii decreases with increase in Z [33]. One can notice that the value of separation factor for La and Lu (βLa/Lu = DLa/DLu) in L–[ImP][Tf2N]2 system is larger than in L–[C8mim][Tf2N] system (Fig. 2).
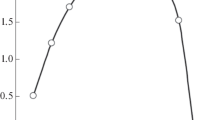
Stoichiometric metal : L ratio in complexes extracted in the presence of [ImP][Tf2N]2 was determined by equilibrium shift method. The slope of log DTh–log[L] dependence is close to 2 (Fig. 3), which indicates the extraction of Th(IV) ions from nitric acid solutions as disolvates. On extraction of U(VI) and Ln(III) with compound L, we observed non-integral slope for log D–log[L] dependence (Fig. 3). This feature may result from the formation of a mixture of mono- and disolvates in organic phase.
At constant HNO3 concentration (0.3 M), we studied effect of \({\text{NO}}_{{\text{3}}}^{ - }\) ions concentration in aqueous phase on extraction of Ln(III) with mixtures of L and [ImP][Tf2N]2 in DCE. The change in NH4NO3 concentration from 0.5 to 4 mol/L does not lead to marked variation in DLn value. Consequently, \({\text{NO}}_{{\text{3}}}^{ - }\) ions do not form extractable complexes in L–[ImP][Tf2N]2–DCE system under these conditions. It should be noted that Ln(III) ions are efficiently extracted with L and [ImP][Tf2N]2 mixtures in DCE also from HCl and H3PO4 solutions. For example, on extraction of Eu(III) with mixtures of 0.002 M L and 0.002 M [ImP][Tf2N]2 in DCE from 1 M HCl and H3PO4 solutions, the values of DEu were 63 and 35.5, respectively, although in the absence of IL Ln(III) ions do not transfer into organic phase (DEu values are not higher than 10–2). This fact indicates that on the extraction of Ln(III) from HCl and H3PO4 solutions, synergistic effect in the presence of [ImP][Tf2N]2 in organic phase appears much larger than on extraction from HNO3 solutions.
Taking into account found stoichiometric coefficients, extraction of Ln(III) ions into organic phase containing neutral ligand L and ionic liquid [ImP][Tf2N]2 by cation-exchange mechanism can be described by the following equilibriums:
These equations indicate that increase in [ImP][Tf2N]2 concentration in organic phase should result in increase in Ln(III) extraction efficiency, while increase in ImP2+ concentration in aqueous phase should decrease efficiency. Experimental data confirm this feature: the slope of log DLn–log[ImP(Tf2N)2] dependence is close to 1.5 (Fig. 4), while the slope of log DLn– log[ImP2+] dependence is close to –1.5 (Fig. 5). This fact indicates that Ln(III) ions are extracted with mixtures of ligand L and [ImP][Tf2N]2 by cation exchange mechanism.
CONCLUSIONS
The presented data have shown that extraction of U(VI), Th(IV), and lanthanides(III) from aqueous solutions of nitric acid with mixtures of bis[(diphenylphosphoryl)acetyl(hexyl)amino]pentane (L) and ionic liquid [ImP][Tf2N]2 in DCE display considerable synergistic effect. This effect is caused by the high hydrophobicity of IL anions involved in formation of extracted complexes as counterions. The process of metal ions extraction occurs via cation exchange mechanism. The value of synergistic effect on extraction of Ln(III) from 3 M HNO3 solutions with a mixture of L and [ImP][Tf2N]2 in DCE was shown to be an order of magnitude higher than in L–[C8mim][Tf2N]–DCE system.
REFERENCES
T. Welton, Chem. Rev. 99, 2071 (1999). https://doi.org/10.1021/cr980032t
D. Nosov, B. Ronnasi, E. I. Lozinskaya, et al., ACS Appl. Polym. Mater. 5, 2639 (2023). https://doi.org/10.1021/acsapm.2c02223
D. O. Ponkratov, A. S. Shaplov, and Ya. S. Vygodskii, Polym. Sci. Ser. C 61, 2 (2019). https://doi.org/10.1134/S1811238219010144
W. Wang and R. W. Murray, Anal. Chem. 79, 1213 (2007). https://doi.org/10.1021/ac0615697
A. Berthod, M. J. Ruiz-Angel, and S. Carda-Broch, J. Chromatogr., A 1184, 6 (2008). https://doi.org/10.1016/j.chroma.2007.11.109
M. Kamaz, R. J. Vogler, M. Jebur, et al., Sep. Purif. Technol. 236, 116237 (2020). https://doi.org/10.1016/j.seppur.2019.116237
M. Atanassova, J. Mol. Liq. 343, 117530 (2021). https://doi.org/10.1016/j.molliq.2021.117530
M. Iqbal, K. Waheed, S. B. Rahat, et al., J. Radioanal. Nucl. Chem. 325, 1 (2020). https://doi.org/10.1007/s10967-020-07199-1
G. Arrachart, J. Couturier, S. Dourdain, et al., Processes 9, 1202 (2021). https://doi.org/10.3390/pr9071202
V. V. Belova, Radiochemistry 63, 1 (2021). https://doi.org/10.1134/S106636222101001X
X. Sun, H. Luo, and S. Dai, Chem. Rev. 112, 2100 (2012). https://doi.org/10.1021/cr200193x
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Solvent Extr. Ion Exch. 30, 244 (2012). https://doi.org/10.1080/07366299.2011.639248
A. N. Turanov, V. K. Karandashev, E. V. Sharova, et al., Radiochim. Acta 106, 355 (2018). https://doi.org/10.1515/ract-2017-2851
A. N. Turanov, V. K. Karandashev, M. Boltoeva, et al., Sep. Purif. Technol. 164, 97 (2016). https://doi.org/10.1016/j.seppur.2016.03.004
Q. Gan, Y. Cai, K. Fu, et al., Radiochim. Acta 108, 239 (2020). https://doi.org/10.1515/ract-2019-3147
H. Luo, S. Dai, P. V. Bonnesen, et al., Solvent Extr. Ion Exch. 24, 19 (2006). https://doi.org/10.1080/07366290500388624
T. Sun, Y. Zhang, Q. Wu, et al., Solvent Extr. Ion Exch. 35, 408 (2017). https://doi.org/10.1080/07366299.2017.1379142
C.-W. Cho, T. P. T. Phan, Y. Zhao, et al., Sci. Total Environ. 786, 147309 (2021). https://doi.org/10.1016/j.scitotenv.2021.147309
M. G. Montalban, G. Villora, and P. Licence, Ecotoxicol. Environ. Saf. 150, 129 (2018). https://doi.org/10.1016/j.ecoenv.2017.11.073
J. I. Anderson, R. Ding, A. Ellern, and D. W. Armstrong, J. Am. Chem. Soc. 127, 593 (2005). https://doi.org/10.1021/ja046521u
H. Shirota, T. Mandai, H. Fukazawa, and T. Kato, J. Chem. Eng. Data 56, 2453 (2011). https://doi.org/10.1021/je2000183
R. R. Hawker, R. S. Haines, and J. B. Harper, Chem. Commun. 54, 2296 (2018). https://doi.org/10.1039/c8cc00241
E. A. Arkhipova, A. S. Ivanov, M. M. Levin, et al., J. Mol. Liq. 346, 117095 (2022). https://doi.org/10.1016/j.molliq.2021.117095
A. N. Turanov, V. K. Karandashev, E. V. Sharova, Solvent Extr. Ion Exch. 30, 604 (2012). https://doi.org/10.1080/07366299.2012.671117
A. N. Turanov, V. K. Karandashev, A. N. Kharitonov, et al., Russ. J. Gen. Chem. 69, 1068 (1999).
P. Bonhote, A. P. Dias, N. Papageorgiou, et al., Inorg. Chem. 35, 1168 (1996). https://doi.org/10.1021/ic951325x
E. Rothstein, R. W. Saville, and P. E. Horn, J. Chem. Soc. 3994 (1953). https://doi.org/10.1039/JR9530003994
V. K. Karandashev, A. Yu. Leikin, V. A. Khvostikov, et al., Inorg. Mater. 52, 1391 (2016). https://doi.org/10.1134/S0020168516140053
S. L. I. Toh, J. McFarlane, C. Tsouris, et al., Solvent Extr. Ion Exch. 24, 33 (2006). https://doi.org/10.1080/07366290500388400
A. M. Rozen and B. V. Krupnov, Russ. Chem. Rev. 65, 973 (1996). https://doi.org/10.1070/RC1996v065n11ABEH000241
K. Binnemans, Chem. Rev. 107, 2592 (2007). https://doi.org/10.1021/cr050979c
H. H. Dam, D. N. Reinhoudt, and W. Verboom, Chem. Soc. Rev. 36, 367 (2007). https://doi.org/10.1039/b603847f
E. P. Horwitz, K. A. Martin, H. Diamond, and L. Kaplan, Solvent Extr. Ion Exch. 4, 449 (1986). https://doi.org/10.1080/07366298608917877
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation under the State assignment for the Osipyan Institute of Solid State Physics, Russian Academy of Sciences, the Institute of Microelectronics Technology and High-Purity Materials, Russian Academy of Sciences, and the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, (no. 075-03-2023-642) for 2023 using equipment of the Center for molecular structure studies, Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences. Mass spectral measurements were performed in the Shared Facility Center, Institute of Microelectronics Technology and High-Purity Materials, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by I. Kudryavtsev
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turanov, A.N., Karandashev, V.K., Sharova, E.V. et al. Extraction of Actinides and Lanthanides from Nitric Acid Solutions with Mixtures of 1,5-N,N'-Bis[(Diphenylphosphoryl)Acetyl(Hexyl)Amino]pentane and New Asymmetrical Phosphonium- and Imidazolium-Based Ionic Liquid. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023623602933
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023623602933