Abstract
Bacteriocins produced by a number of lactic acid bacteria (LAB) comprise a variety of antimicrobial proteins that may be used as antibiotic alternative in food preservation. The present study aimed to isolate and characterize LAB-derived bacteriocin-like substances (BLS) from local fermented foods. A total of twenty-one bacterial strains were isolated and subjected to an antibacterial assay. Five isolates designated as AB1, AB2, AK1, AK2, AnK1 showed inhibitory activity against selected indicator organisms and were subjected to further characterization. Upon BLAST analysis of the 16S rRNA gene sequences, the isolates showed close relationship to Lactobacillus plantarum with similarity ranging from 91 to 97%. Moreover, the antibacterial metabolite production of the isolates was observed to peak on the 5th to 7th day of incubation. Since AnK1 showed the highest zone of inhibition among 5 isolates, it was selected for characterization of the BLS isolated by ammonium sulfate precipitation and anion exchange chromatography. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) showed that the AnK1-BLS had a single band and a molecular weight around 15 kDa, and a total protein concentration of 7.06 µg/mL. Interestingly, AnK1-BLS had a broad antimicrobial spectrum on both gram-negative and gram-positive food-borne pathogens, including Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, and Bacillus subtilis. Moreover, the purified AnK1-BLS exhibited good thermal stability when heated at 80‒121°C. Likewise, its inhibitory activity was not affected by pH in the range of 3.0 to 6.5, with maximum activity recorded at pH 3.0. The results suggest that AnK1-BLS may have application prospects in the food industry. Further studies on the complete protein characterization of AnK1-BLS is recommended to fully harness its capabilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Food-borne pathogenic bacteria are responsible for numerous illnesses and mortalities worldwide. Food spoilage caused by microorganisms results in substantial economic losses (Bintsis, 2017; Gram et al., 2002). Currently, antibiotics and chemical preservatives are commonly used to inhibit or kill these pathogens. However, some chemically synthesized preservatives may have toxicity and can accumulate in the human body, causing severe harm to human health. Furthermore, overuse of these antibiotics leads to the development of drug resistance among pathogenic bacteria. Therefore, there is a continuous requirement to find natural, safe, and efficient antimicrobial agents for the food and medical industry. In recent years, the potential of bacteriocins has attracted significant attention in food preservation because of its GRAS (Generally Recognized as Safe) status. Bacteriocins are ribosomally synthesized peptides or proteins with antibacterial activity toward strains within the same species or across different genera (De Vuyst and Leroy, 2007; Silva et al., 2018).
In the Philippines, fermentation processes employed in producing indigenous fermented foods often rely on the natural microflora of the raw material and the surrounding environment. The procedure is hand down from one generation to the next. Because traditional food fermentation industries are commonly home-based and highly reliant on indigenous materials without the benefit of using commercial starter cultures, microbial assemblages are unique and highly variable. Hence, discovering elite and novel organisms, products, and interactions is likely.
Various microorganisms are involved in standard food fermentation processes, including lactic acid bacteria (LAB). LAB is a group of gram-positive, non-spore-forming bacteria, either microaerophilic or anaerobic, which lack the cytochrome system and do not produce catalase (Cintas et al., 2001; Mokoena et al., 2021). LAB-derived bacteriocins may be used as food preservatives due to such features as their protein nature, with proteases inactivating them in the gastrointestinal tract. They are generally non-immunogenic and heat-resistant, maintaining antimicrobial activity even after pasteurization and sterilization. These elite characteristics make the studied LAB-derived bacteriocin a promising candidate to replace chemical preservatives (Gao et al., 2010; Todorov et al., 2022). The goal of the present work was to screen and partially identify the bacteriocin-like producing LAB isolated from fermented foods. as well as, to purify and partially characterize the bacteriocin-like substance (BLS), which would provide insights into its potential use as a preservative in the food industry.
MATERIALS AND METHODS
Collection of samples and isolation of LAB-like strains. The samples of Fermented food (buro, kimchi, soya milk, tofu, bagoong, and fermented shrimp or “alamang”) were collected from the local market and brought to the laboratory. To isolate LAB, 10 g of each sample was mixed thoroughly in 90 mL distilled water, and 100 μL was spread plated on MRS agar. The plates were incubated for 24 h at room temperature in both oxic and anoxic conditions. After incubation, the colonies were picked out from the plates according to their morphology. Gram-positive and catalase-negative isolates were then streaked an MRS agar slant and stored. Glycerol stocks were also prepared for long-term storage of the bacteria. Test pathogenic bacteria (E. coli BIOTECH 1634, P. aeruginosa BIOTECH 1335, and B. subtilis BIOTECH 1056) were provided by the Philippine National Collection of Microorganisms (PNCM) based at University of the Philippines. Meanwhile, S. enterica JCM 1651 was obtained from the Japan Collection of Microorganisms.
Preliminary screening of antibacterial activity using the agar plug method. The antimicrobial activity of putative LAB isolates was determined by the agar plug diffusion method (Elleuch et al., 2010; Ortlieb et al., 2021) with some modifications. Briefly, LAB isolates were streaked on MRS agar plates and incubated for seven days. After incubation, an agar plot was cut using one-mL sterile tips and placed on the agar surface of an MHA plate previously inoculated with the test pathogenic bacteria. The plates were incubated at 37°C for 12 h. The zone of inhibition around the wells indicated antibacterial activity of the putative LAB isolates.
Antibacterial metabolite production. To determine the highest peak of metabolite production, the LAB isolates were subjected to a prolonged incubation period via liquid-state fermentation. Thereafter, their antibacterial activity was assessed using an agar-well assay (Reeves, 1989). Initially, the isolates were grown in MRS broth at 37°C for 3, 5, 7, and 10 days. The cell-free culture supernatant (CFS) was obtained by centrifugation at 14 000 g for 10 min. The test pathogenic bacteria were cultivated in nutrient broth to the logarithmic phase and were used as indicator strains. Plates were prepared by spreading the indicator bacterium (1.5 × 108 CFU/mL) onto MHA agar, slotting wells using 1 mL sterile tips, and then 100 μL of LAB CFS was added to the well and subsequently incubated for 12 h at 37°C. The zones of clearance around wells were measured to evaluate the antibacterial activity of the putative LAB isolates.
Partial identification using 16S rRNA gene sequencing. The genomic DNA of putative LAB isolates was extracted using the Wizard(R) Genomic DNA Purification Kit (Promega, United States). PCR was performed using the universal primers 27F (5‑AGAGTTTGATCMTGGCTCAG-3) and 149R (5-TACGGCTACCTTGTTACGAC-3). The PCR program used was as follows: initial denaturation of 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing of 55°C for 30 s, and a primer extension step at 68°C for 1 min and 30 s, and a final extension at 68°C for 5 min. To visualize and assess the quality of the extracted DNA, 2 µL of each reaction was subjected to gel electrophoresis on a 1% agarose gel in 0.5× Tris-Acetate-EDTA (TAE) buffer at 60 V for 30 min, and DNA bands were visualized in Chemidoc.
The amplified DNA samples were sent to Macrogen Inc. (Seoul, South Korea) for double-pass sequencing using the forward and reverse primers used for amplification. The raw sequences were obtained from the sequencing facility. The evaluation of the sequences and elimination of the poor-quality base calls were done using BioEdit Software (www.bioedit.software.informer.com). After quality control, the cleaned sequences were subjected to nucleotide BLAST (BLASTn) analysis using default parameters at http://blast.ncbi.nlm.nih.gov/Blast.cgi to determine the putative identity of the isolates.
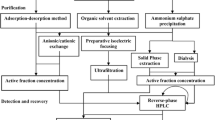
Extraction of BLS by ammonium sulfate precipitation. The isolate with the greatest zone of clearance diameter was selected for further BLS extraction and characterization. The crude extract of BLS was obtained using ammonium sulfate precipitation (Guangfu Technology Development Co., Ltd., Tianjin, China). The culture of the LAB isolate was inoculated into MRS broth and cultivated anaerobically for 7 days. Cells were removed by centrifugation at 12 000 g for 15 min at 4°C to obtain the supernatant. Ammonium sulfate was added to the supernatant to a final concentration of 80%, and then mixed overnight at 4°C. The precipitate was collected by centrifugation at 12 000 g at 4°C for 15 min. The precipitate was then resuspended in 0.01 M phosphate buffer saline (PBS), and dialyzed using a membrane with a molecular weight cut off of 12 000 Da to remove the excess salt from the protein solution (Ahmad and Rasool, 2003; Saleem et al., 2009).
Purification and SDS-PAGE. The dialyzed ammonium sulfate precipitate was purified via anion exchange chromatography using the AKTA purifier system.
Bradford assay. Total protein concentration was determined using the method described by Bradford (1976). About 20 μL of the sample was added to 1 mL of Coomassie blue reagent and was mixed thoroughly. The absorbance of the mixture was read at 595 nm. The bovine serum albumin was used as standard.
Thermal and pH stability of the activity of BLS. To evaluate the thermal stability, 1 mL aliquot of BLS was exposed to the temperatures of 80, 100, and 121°C, respectively, for 15 min (Wang et al., 2018). The antibacterial activity was then tested using the agar-well diffusion method against E. coli BIOTECH 1634 and compared to non-exposed BLS as a control. The sensitivity of the BLS activity at different pH values was estimated by adjusting the pH from 2.0 to 9.0 with 1.0 M HCl or 1.0 M NaOH. After 1 h of incubation at 37°C, the antibacterial activity was tested as described earlier and compared to pH 6.4 as a control.
Statistical analysis. All experiments were performed in triplicate. The data were analyzed using ANOVA and post-Hoc test with Star 2.0 (International Rice Research Institute, IRRI). All results were presented as mean ± standard deviations (SD), and p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Isolation of LAB-like strains and preliminary screening for antibacterial activity. A total of twenty-one (21) LAB-like strains were isolated from fermented foods such as kimchi, “buro,” soya milk, tofu, and “alamang.” Ten (10) bacterial isolates came from the aerobic set-up, and eleven (11) from the anaerobic set-up. To initially characterize the isolated bacteria, morphological and cultural characteristics were determined. Cell shape, cell and colony morphology, catalase and gram-stain reaction were used to identify and isolate strains of LAB (El-shenawy et al., 2017). Two dominant morphologies were observed: (i) rod-shaped, gram-positive, entire margin, round white colony, catalase-negative and (ii) short rod, gram-positive, round off-white colony, entire margin, negative catalase reaction.
Preliminary screening revealed that among the 21 bacterial isolates, only 5, namely, AK1, AK2, AnK1, AB1, AB2, exhibited antibacterial activities against such pathogenic bacteria as E. coli BIOTECH 1634, S. enterica JCM 1651, and P. aeruginosa BIOTECH 1335. This variation on morphology and antibacterial activity evidences the diverse ecology of LABs isolated in this study. Further investigations on microbial diversity is required to fully characterize the LAB community in local fermented foods.
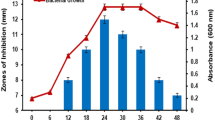
Antibacterial metabolite production of the isolates. An increasing trend in the production of antibacterial metabolites was also observed for the five putative LAB isolates starting from the fifth day and peaking on the seventh day of incubation (Fig. 1). A slight decrease in the activity of the antimicrobial metabolites was observed in all isolates on the tenth day. Among the 5 LAB isolates, isolate AnK1 from kimchi showed the highest zones of inhibition against the four indicator organisms.
Assessment of metabolite production by bacterial isolates during long-term cultivation using the agar-well assay against E. coli BIOTECH 1634 (a), S. enterica JCM 1651 (b), P. aeruginosa BIOTECH 1335 (c), and B. subtilis (d). The BLS are represented by numbers, AnK1 (1), AB1 (2), AB2 (3), AK2 (4), AK1 (5), and AnT (6).
In contrast to the findings of this investigation, Hassan et al. (2021) observed inhibitory effects of L. helviticus against S. aureus within 24 h of incubation. Similarly, another study showed that L. sakei displayed the largest inhibition zone against E. coli after 48 h of incubation. Moreover, Wang et al. (2018) found that L. plantarum LPL-1 exhibited heightened efficacy against Listeria monocytogenes 54002 at the 32-h mark of incubation. Likewise, Lei et al. (2019) reported that L. plantarum strain zrx03 demonstrated a zone of inhibition measuring 13.83 ± 0.47 mm against E. coli JM109 after just 18 h of incubation. Thus, this study represents the first instance of an extended incubation period lasting up to 10 days.
Partial identity of isolates using 16S rRNA gene sequencing. Our results indicated that AB1 and AB2 isolated from buro were similar to L. plantarum (Accession no. 115605.1) with >96% similarity. Meanwhile, the isolates AK1, AK2, and AnK1 obtained from kimchi exhibited >91% homology with L. plantarum (Accession no. 04573.1).
Purification and molecular characteristics of AnK1-BLS. Since isolate AnK1 formed the largest zone of inhibition, it was selected for further BLS extraction and characterization. The results showed a significant difference in the inhibitory activity of precipitated and cell-free supernatant against all indicator organisms (Table 2). The precipitated AnK1-BLS displayed the strongest antibacterial activity against E. coli BIOTECH 1634 with an inhibition zone diameter of 25.08 ± 1.37 mm. This illustrates that 80% ammonium sulfate could be used as a crude method for BLS purification, consistent with the results from other research groups (Lu et al., 2014b).
As shown on Fig. 2, an elution volume of 58 to 80 shows proteins with a low affinity with DEAE column resin. Meanwhile, the absorbance peak between 140 and 180 nm exhibited the protein successively eluted with NaCl attached to DEAE column showing high affinity. Further assessment of the collected fractions was conducted using the agar well method to validate the antibacterial efficacy of AnK1-BLS following extraction and purification. As depicted in Table 3, the eluate containing AnK1-BLS maintained its inhibitory activity against indicator organisms even after extraction and purification. The largest inhibition zone with a mean diameter of 13.27 mm was observed for E. coli BIOTECH 1634.
Nonetheless, despite achieving a high degree of purification, the inhibitory potency of purified AnK1-BLS somewhat declined. This phenomenon aligns with findings by Wen et al. (2016), who observed a decrease in the inhibitory effects of purified bacteriocins, namely plantaricin K25 and lactocin MM4, possibly due to suboptimal culture media optimization. Enhanced comprehension of metabolite production in these bacteria necessitates optimization of the culture medium and growth conditions. Furthermore, genetic engineering involvement in bacteriocin-producing strains could lead to heightened bacteriocin expression (Garsa et al., 2014).
Interestingly, this study indicates that the decreased antibacterial activity of AnK1-BLS did not compromise its purity. Likewise, the purified AnK1-BLS was active against gram-negative bacteria, including E. coli, S. enterica, and P. aeruginosa. Today, the only known bacteriocin approved for food usage is nisin which only inhibits gram-positive bacteria (Alvarez-Sieiro et al., 2016). Hence, the purified AnK1-BLS can be a potential source of natural and high-efficacy preservatives, which can be exploited by the food industry.
Figure 3 shows a single band, indicating that the resultant BLS is a single active peptide responsible for the antibacterial activity. Approximately, the molecular weight of the BLS was found to be around 15 kDa which conformed to molecular mass characteristics of class II bacteriocin. However, the detected molecular size for BLS was slightly larger than for most bacteriocins previously described for the genus Lactobacillus (De Vuyst and Vandamme, 1994). Nevertheless, Martinez et al. (2013) reported a similar molecular weight of a bacteriocin produced by L. plantarum ST71KM. Bacteriocin produced by L. plantarum ST23LD was estimated to be within approximate sizes of 3.0 and 15.0 kDa (Todorov and Dicks, 2015). The total amount of pure protein was also estimated by Bradford assay. After the purification, the final protein content was 7.06 µg/mL.
Thermal and pH stability of the activity of AnK1-BLS. The influence of heat treatment on the antibacterial activity of AnK1-BLS is shown in Table 4. The results revealed that the inhibitory activity of BLS, when incubated at 80°C, was not significantly different from that of untreated BLS. However, the activity was significantly reduced when heated at 100 to 121°C. Nevertheless, the inhibitory activity was not completely lost. This indicates that AnK1 is a heat-stable protein, possibly due to its amino acid composition. According to Van et al. (1999), bacteriocins from lactic acid bacteria have high glycine content and high hydrophobicity, which possibly explains their heat resistance. Abo-Amer (2017) and Fatima and Mebrouk (2013) have reported similar results in their studies, highlighting the functionality of L. plantarum bacteriocin in food preservation procedures due to its high temperature tolerance.
Similar studies pointed out that bacteriocins produced by Lactobacillus have high-temperature resistance. For example, the good thermal stability was observed for the bacteriocins produced by L. casei (Park et al., 2003) and L. paracasei (Lie et al., 2011). These characteristics support the previous claim that the purified BLS belongs to class II bacteriocin. Class II bacteriocins are heat-stable and non-lantibiotic peptides commonly stabilized by disulfide bridges. N‑terminal modifications are also known in some class II bacteriocins (Kleerebezem et al., 1997). Hence, the BLS produced by L. plantarum may constitute an advantage for potential use as a biopreservative in combination with thermal processing in order to preserve food products.
Furthermore, its inhibitory activity was not affected by pH ranges from 3 up to 6.5, with maximum activity recorded at pH 3 (Table 5). This corresponds with the findings of Fatima and Mebrouk (2013), where the bacteriocin synthesized by L. plantarum had maximum activity at acidic pH. Additionally, studies reported that neutral and alkaline conditions readily inactivate most bacteriocins, including nisin, plantaricin JLA-9 produced by L. plantarum JLA-9 (Zhao et al., 2016), and bacteriocin R1333 (Todorov et al., 2011). Following exposure to alkaline conditions at pH 8‒9, the AnK1-BLS did not show any activity. Sankar et al., (2012) reported the same results, where bacteriocin maintained only partial antibacterial activity when the pH was changed from basic to acid.
Additionally, Zapata et al. (2009) reported that L. plantarum LPBM10 bacteriocin had a maximum activity at pH 4.0, which decreased sharply at pH values higher than 5.0. Nevertheless, these results differ from those for the L. plantarum ST23LD, ST341LD, bacST202Ch, bacST216Ch, and ST71KS bacteriocins, which have shown stability between pH 2.0 up to 12.0 (Todorov et al., 2010; Martinez et al., 2013). Different results were also indicated by ST28MS and ST26MS from L. plantarum that showed a 50% decrease in the antimicrobial activity at pH values lower than 4.0 (Todorov et al., 2005b).
To conclude, this study provides information on the antimicrobial activity of LAB-BLS from local fermented foods. Based on the results, five isolates coded as AB1, AB2, AK1, AK2, AnK1 have exhibited antibacterial activity against prominent food-borne pathogens. Analysis of the 16S rRNA gene sequences revealed that the isolates were homologous to Lactobacillus plantarum. The highest metabolite production of the isolates was recorded on the 7th day of the incubation where AnK1 showed the highest zone of inhibition.
The bacteriocin-like substance produced by isolate AnK1 possesses a single band with a molecular weight of 15 kDa and total protein content of 7.06 μg/mL. At 80°C, the antibacterial activity of the purified AnK1-BLS is comparable to the results for 30°C. Increasing the temperature to 100 and 121°C decreases its antibacterial activity. At acidic pH levels ranging from 2 to 6.5, it shows high antibacterial activities, while none at alkaline condition ranging from pH 8 to 9. These results suggest that AnK1-BLS may have application prospects in the food industry. The conduct of studies on the complete protein characterization of AnK1-BLS may be conducted to comprehensively describe its antimicrobial spectrum and fully harness its capabilities.
REFERENCES
Abo-Amer, A.E., Characterization of a bacteriocin-like inhibitory substance 459 produced by Lactobacillus plantarum isolated from Egyptian home-made yogurt, Science Asia, 2007, vol. 33, no. 3, pp. 313–319. https://doi.org/10.2306/scienceasia1513-1874.2007.33.313
Ahmad, S. and Rasool, S.A., Isolation and biochemical characterization of mutacin VSM43 isolated from human oral Streptococcus mutants VSM43, Pak. J. Pharm. Sci., 2003, vol. 16, no. 2, pp. 43–50. PMID: 16414575.
Alvarez-Sieiro, P., Montalban-López, M., Mu, D., and Kuipers, O.P., Bacteriocins of lactic acid bacteria, Appl. Microbiol. Biotechnol., 2016, vol. 100, no. 7, pp. 2939–2951. https://doi.org/10.1007/s00253-016-7343-9
Bintsis, T., Foodborne pathogens, AIMS Microbiol., 2017, vol. 3, no. 3, pp. 529–563. https://doi.org/10.3934/microbiol.10.3934/microbiol.2017.3.529
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, vol. 7, no. 72, pp. 248–254. https://doi.org/10.1006/abio.1976.9999
Cintas, L.M., Casaus, M.P., Herranz, C., Nes, I.F., and Hernandez, P.E., Review: bacteriocins of lactic acid bacteria, Food Sci. Tech. Int., 2001, vol. 7, pp. 281–305. https://doi.org/10.1106/R8DE-P6HU-CLXP-5RYT
De Vuyst, L. and Leroy, F., Bacteriocins from lactic acid bacteria: production, purification, and food applications, J. Mol. Microbiol. Biotechnol., 2007, vol. 13, no. 4, pp. 194–199. https://doi.org/10.1159/000104752
De Vuyst, L. and Vandamme, E., Lactic acid bacteria and bacteriocins: their practical importance, in Bacteriocins of Lactic Acid Bacteria: Microbiology, Genetics and Applications, New Dehli: Springer, 1994, pp. 1–11.
Elleuch, L. Shaaban, M. Smaoui, S. Mellouli, L. Karray-Rebai, I. Fourati-Ben Fguira, L., Shaaban, K.A., and Laatsch, H., Bioactive secondary metabolites from a new terrestrial Streptomyces sp. TN262, Appl. Biochem. Biotechnol., 2010, vol. 162, no. 2, pp. 579–593. https://doi.org/10.1007/s12010-009-8808-4
El-Shenawy, M., Dawoud, E, I., Amin, G. A., El, S. K., Fouad, M. T., and Soriano, J. M., Antimicrobial activity of some lactic acid bacteria isolated from local environment in Egypt, Afric. J. Microbiol. Res., 2017, vol. 11, no. 8, pp. 327–334. https://doi.org/10.5897/AJMR2016.8191
Fatima, D. and Mebrouk, K., Characterization and determination of the factors affecting anti-listerial bacteriocins from Lactobacillus plantarum and Pediococcus pentosaceus isolated from dairy milk products, Afric. J. Food Sci., 2013, vol. 7, no. 3, pp. 35–44.
Gao, Y., Jia, S., Gao, Q., and Tan, Z., A novel bacteriocin with a broad inhibitory spectrum produced by Lactobacillus sake C2, isolated from traditional Chinese fermented cabbage, Food Cont., 2009, vol. 21, no. 1, pp. 76–81. https://doi.org/10.1016/j.foodcont.2009.04.003
Garsa, A.K., Kumariya, R., Kumar, A., Lather, P., Kapila, S., and Sood, S., Industrial cheese whey utilization for enhanced production of purified pediocin PA-1, LWT-Food Sci. Technol., 2014, vol. 59, no. 2, pp. 656–665.
Gram, L., Ravn, L., Rasch, M., Bruhn, J.B., Christensen, A.B., and Givskov, M., Food spoilage interactions between food spoilage bacteria, Int. J. Food Microbiol., 2002, vol. 15, no. 78, pp. 79−97.
Hassan, M., Nayab, H., Rehman, T., Williamson, M., Haq, K., Shafi, N., and Shafique, F., Characterisation of bacteriocins produced by Lactobacillus spp. isolated from the traditional Pakistani yoghurt and their antimicrobial activity against common foodborne pathogens, BioMed Res. Int., 2020, e-collection 2020.
Kleerebezem, M., Boekhorst, J., van Kranenburg, R., Molenaar, D., Kuipers, O.P., Leer, R., Tarchini, R., Peters, S.A., Sandbrink, H.M., Fiers, M.W.E.J., Stiekema, W., Lankhorst, R.M.K., Bron, P.A., Hof-fer, S.M., Groot, M.N.N., Kerkhoven, R., de Vries, M., Ursing, B., de Vos, W.M., and Siezen, R.J., Complete genome sequence of Lactobacillus plantarum WCFS1, Proc. Natl. Acad. Sci. U. S. A., 2003, vol. 100, no. 4, pp. 1990–1995.
Kleerebezem, M., Quadri, L.E., Kuipers, O.P., and De Vos, W.M., Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria, Mol. Microbiol., 1997, vol. 24, no. 5, pp. 895–904.
Laemmli, U., Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, vol. 227, pp. 680–685. https://doi.org/10.1038/227680a0
Lei, S., Zhao, R., Sun, J., Ran, J., Ruan, X., and Zhu, Y., Partial purification and characterization of a broad-spectrum bacteriocin produced by a Lactobacillus plantarum zrx03 isolated from infant’s feces, Food Sci. Nutr., 2020, vol. 8, no. 5, pp. 2214–2222. https://doi.org/10.1002/fsn3.1428
Lu, X., Yi, L., Dang, J., Dang, Y., and Liu, B., Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms, Food Control, 2014, vol. 46, pp. 264–271.
Martinez, R.C., Wachsman, M., Torres, N.I., LeBlanc, J.G., Todorov, S.D., and Franco, B.D., Biochemical, antimicrobial and molecular characterization of a noncytotoxic bacteriocin produced by Lactobacillus plantarum ST71KS, Food Microbiol., 2013, vol. 34, no. 2, pp. 376–381. Mokoena, M.P., Omatola, C.A., and Olaniran, A.O., Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens, Molecules, 2021, vol. 26, no. 22.https://doi.org/10.3390/molecules26227055
Ortlieb, N., Klenk, E., Kulik, A., and Niedermeyer, T.H.J., Development of an agar-plug cultivation system for bioactivity assays of actinomycete strain collections, PLoS One, 2021, vol. 16, no. 11.
Oyedeji, O., Ogunbanwo, S.T., and Onilude, A.A., Predominant lactic acid bacteria involved in the traditional fermentation of fufu and ogi, two Nigerian fermented food products, Food Nutr. Sci., 2013, vol. 4, no. 11, pp. 40–46. https://doi.org/10.4236/fns.2013.411A006
Park, S.H., Itoh K., and Fujisawa, T., Characteristics and identification of enterocins produced by Enterococcus faecium JCM 5804T, J. Appl. Microbiol., 2003, vol. 95, pp. 294–300.
Reeves, D.S., Antibiotic assays, in Medical Bacteriology, a Practical Approach, Hawkey, P.M. and Lewis, D.A., Eds., Oxford: IRL, 1989, pp. 195–221.
Saleem, F., Samia, A., Umair, Z., and Rasool, S., Comparative study of two bacteriocins produced by representative indigenous soil bacteria, Pak. J. Pharm. Sci., 2009, vol. 22, no. 8, pp. 252–258. PMID: 19553169.
Sankar, N.R., Priyanka, V.D., Reddy, P.S., Rajanikanth, P., Kumar, V.K., and Indira M., Purification and characterization of bacteriocin produced by Lactobacillus plantarum isolated from cow milk, Int. J. Microbiol. Res., 2012, vol. 3, no. 2, pp. 133–137. https://doi.org/10.5829/idosi.ijmr.2012.3.2.62182
Silva, C.C.G., Silva, S.P.M., and Ribeiro, S.C., Application of bacteriocins and protective cultures in dairy food preservation, Front. Microbiol., 2018, vol. 9. https://doi.org/10.3389/fmicb.2018.00594
Todorov, S.D., Popov, I., Weeks, R., and Chikindas, M.L., Use of bacteriocins and bacteriocinogenic beneficial organisms in food products: benefits, challenges, concerns, Foods, 2022, vol. 11, no. 19. https://doi.org/10.3390/foods11193145
Todorov, S.D. and Franco, B.D., Lactobacillus plantarum: characterization of the species and application in food production, Food Rev. Int., 2010, vol. 26, no. 3, pp. 205–229. https://doi.org/10.1080/87559129.2010.484113
Todorov, S.D., Ho, P., Vaz-Velho, M., and Dicks, L.M., Characterization of bacteriocins produced by two strains of Lactobacillus plantarum isolated from Beloura and Chouriço, traditional pork products from Portugal, Meat Sci., 2010, vol. 84, no. 3, pp. 334–343. https://doi.org/10.1016/j.meatsci.2009.08.053
Todorov, S.D. and Dicks, L.M., Characterization of bacteriocins produced by lactic acid bacteria isolated from spoiled black olives, J. Bas. Microbiol., 2005a, vol. 45, no. 4, pp. 312–322. https://doi.org/10.1002/jobm.200410532
Todorov, S.D. and Dicks, L.M.T., Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria, Enz. Micro. Technol., 2005b, vol. 36, no. 2, pp. 318–326.
Todorov, S.D., Prévost, H., Lebois, M., Dousset, X., Le-Blanc, J.G., and Franco, B.D.G.M., Bacteriocinogenic Lactobacillus plantarum ST16Pa isolated from papaya (Carica papaya), Food Res. Int., 2011, vol. 44, pp. 1351–1363.
Wang, Y., Shang, N., Qin, Y., Zhang, Y., Zhang, J., and Li, P., The complete genome sequence of Lactobacillus plantarum LPL-1, a novel antibacterial probiotic producing class IIa bacteriocin, J. Biotechnol., 2018, vol. 266, pp. 84–88.
Wang, Y., Wu, J., Lv, M., Shao, Z., Hungwe, M., Wang, J., Bai, X., Xie, J., Wang, Y., and Geng, W., Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry, Front. Bioeng. Biotechnol., 2021, vol. 9. https://doi.org/10.3389/fbioe.2021.612285
Wen, L.S., Philip, K., and Ajam, N., Purification, characterization and mode of action of plantaricin K25 produced by Lactobacillus plantarum, Food Cont., 2016, vol. 60, pp. 430–439. https://doi.org/10.1016/j.foodcont.2015.08.010
Zapata, S., Munoz, J., Ruiz, O., Montoya, and O., and Gutieerez, P., Isolation of Lactobacillus plantarum LPBM10 and partial characterization of its bacteriocin, Vitae Biotechnol., 2009, vol. 16, no. 1. https://doi.org/10.17533/udea.vitae.1428
Zhao, S., Han, J., Bie, X., Lu, Z., Zhang, C., and Lv, F., Purification and characterization of plantaricin JLA-9: a novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage, J. Agric. Food Chem., 2016, vol. 64, no. 13, pp. 2754–2764.
ACKNOWLEDGMENTS
The authors would like to express their deepest gratitude to DOST-SEI for providing the budgetary requirement for this research.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inovejas, R.C., Divina, C.C., Jacob, J.K. et al. Antimicrobial Activities and Characterization of Bacteriocin-Like Substances Isolated from Lactic Acid Bacteria of Local Fermented Foods. Microbiology 93, 450–458 (2024). https://doi.org/10.1134/S0026261723601999
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261723601999