Abstract—
Polyethylene glycol (PEG 6000) was used to establish osmotic stress conditions during growth of the type strain Azospirillum brasilense Sp7 and its spontaneous variants Sp7.4 and Sp7.8, because it causes a stable decrease in the water potential and thus makes it possible to simulate the effect of drought on the bacterial population. While PEG suppressed the motility of azospirilla, it had no effect on the ability of strains Sp7 and Sp7.8 to form biofilms, as well as on the metabolic activity of the biofilms formed in the absence of stress. PEG 6000-caused osmotic stress promoted biofilm formation in Sp7.8. While the biofilms of the Sp7.4 variant were those most sensitive to the negative effect of the water stress, the growth variables of the planktonic culture of this variant under stress conditions exceeded the values for both Sp7 and Sp7.8. In biofilms, strains Sp7, Sp7.4, and Sp7.8 produced polysaccharides and the plant hormone IAA; desiccation-resistant cell forms emerged. The variants Sp7.4 and Sp7.8, similarly to Sp7, formed biofilms during plant root colonization and affected the morphology of the root system of wheat seedlings. Our results show that spontaneous variants of strain Sp7 may be of interest for further research directed at selection of promising Azospirillum strains to enhance the drought resistance of plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The rhizosphere-inhabiting alphaproteobacteria Azospirillum brasilense have a significant positive effect on plant growth and resistance to abiotic and biotic stresses. Owing to their ability to fix atmospheric nitrogen, to produce plant hormones, and to control plant pathogens, they improve the mineral nutrition of plants, increase their resistance to heavy metals, and neutralize toxic substances (Fibach-Paldi et al., 2012; Vurukonda et al., 2016; Fukami et al., 2018). Drought/water stress, together with soil salinization and climate changes, belongs to the main abiotic stresses encountered by plants and plant-associated bacteria (Vurukonda et al., 2016; Ansari et al., 2021). Consumption of growth factors increases under stress conditions, resulting in resource limitation, which causes deceleration of plant growth and development (Hsiao, 1973). Similarly to plants, rhizosphere bacteria also experience drought-caused stress, which affects their viability, as well as their physiological and biochemical functions (Ansari et al., 2021).
Establishing conditions for osmotic stress during cultivation of microorganisms and plants by introdu-cing various osmotic agents providing for the stable decrease in the water potential of the medium and blocking water absorption may simulate the effect of drought on bacterial populations and plants (Chutia and Borah, 2012; Ansari et al., 2021). This approach is required for investigation of the mechanisms and strategies used by microorganisms for protection against this stress in order to select drought-resistant rhizosphere bacteria promoting plant resistance to water deficiency.
The ability of bacteria to form biofilms on the surface of the plant host roots is of importance for the formation and successful functioning of a plant-microbial association, as well as for the ability of both plants and microorganisms to survive various abotic stresses (Shelud’ko et al., 2010, 2020; Fibach-Paldi et al., 2012; Bogino et al., 2013; Ansari et al., 2021). Bacteria in biofilms are embedded into the matrix, which, apart from its structural and protective roles, acts as a reservoir of water, enzymes, and nutrients (Flemming and Wingender, 2010). Drying of the biofilm matrix is slow, which protects the microorganisms from variations in the water potential. The composition, structure, and functions of the major biopolymers of the Azospirillum biofilm matrix, which are responsible for the fixation of mature biofilms on solid surfaces and act as a framework promoting the conditions for dinitrogen fixation, have been investigated (Wang et al., 2017; Shelud’ko et al., 2018, 2020). The morphological and functional polymorphism of the cell forms in Azospirillum biofilms was characterized (Wang et al., 2017; Shelud’ko et al., 2020). The presence of dormant forms in biofilm populations enhances their resistance to such negative impacts as frying and oxidative stress (Shelud’ko et al., 2020).
Application of rhizobacteria forming drought-resistant biofilms for countering the drought-related stress in plants may be expected (Ansari and Ahmad, 2019; Ansari et al., 2021). However, the information concerning the role of such bacteria in alleviating drought stress in plants is fragmentary. The main goal of the present work was therefore to assess resistance to water stress and drying for the biofilms of the type strain A. brasilense Sp7 and of its spontaneous variants with modifications in the genome structure. The work was aimed at characterization and analysis of biofilm resistance to drying and investigation of the effect of water stress on biofilm formation and physiological activity. The previously described spontaneous variants of strain Sp7 with genomic variations involving plasmid rearrangements were used (Petrova et al., 2010; Katsy and Petrova, 2015). Because phenotypic variations affect a broad range of the properties of azospirilla (Lerner et al., 2010; Petrova et al., 2010; Volfson et al., 2013; Katsy and Petrova, 2015), their resistance to water stress/drought was expected to differ from that of the original strain A. brasilense Sp7.
MATERIALS AND METHODS
Bacterial strains and nutrient media. The strains used were: A. brasilense Sp7, isolated in Brazil from the rhizosphere of Digitaria decumbens (IBPPM 150) (Tarrand et al., 1978), and its derivatives Sp7.4 (IBPPM 573) and Sp7.8 (IBPPM 576) (Petrova et al., 2010; Katsy and Petrova, 2015). The numbers in parentheses are the registration numbers of the Collection of Rhizosphere Microorganisms, Institute of Biochemistry and Physiology of Plants and Microorganisms, Saratov Scientific Center, Russian Academy of Sciences (IBPPM RAS), Saratov, Russia (http://collection.ibppm.ru/). Bacteria were grown at 30°C on a malate-salt medium (MSM) (Döbereiner and Day, 1976) with 1 g/L NH4Cl. When required, NH4Cl was omitted, and agar (up to 0.3, 0.4, 0.5, and 2%), or L-tryptophan (up to 5 mM). To establish the conditions of osmotic (water) stress, the nonpermeating osmotic agent polyethylene glycol (PEG) with an average molecular weight of 6000 was added (Chutia and Borah, 2012). PEG 6000 was added at concentrations of 5, 10, 15, 20, and 25%, which corresponded to –0.05, –0.15, –0.30, –0.49, or –0.73 MPa (Ansari et al., 2021).
Determination of bacterial motility and growth rate of planktonic cultures under stirring. Overnight (18 h) cultures were diluted with a sterile MSM to А590 = 0.05–0.10 (l = 0.5 cm) and incubated at 140 rpm and 30°С in an Excella E24 shaker incubator (New Brunswick Scientific, United States). The optical density of the culture at А590 (l = 0.5 cm) was measured every 2 h.
Bacterial motility was determined in hanging drop preparations from 24-h cultures. Examination under a Leica DM6000 B microscope (Leica Microsystems, Germany) was accompanied by video recording. The motility of all cells in the field of view was assessed. The video images were analyzed with the program developed in IBPPM. For investigation of bacterial movement in semisolid media, MSM plates with 0.4% agar were stab-inoculated with a loop. The size of macrocolonies formed by motile cells was determined after 48–72 h.
Analysis of biofilm formation and biofilm respiratory activity. Azospirillum cultures (24 h) grown in a liquid MSM with NH4Cl under aeration were washed with 50 mM phosphate buffer (PB), pH 7.0, and diluted with a fresh MSM with or without NH4Cl to А590 = 0.05–0.10 (l = 0.5 cm). The inoculated media were dispensed into glass test tubes (2 mL) or into the wells of polystyrene plates with 96 flat-bottom wells (0.2 mL) and incubated under stationary conditions at 30°С. Sterile media were used as the controls. To assess the relative amount of biomass in mature biofilms, they were stained with Crystal violet (O’Toole and Kolter, 1998). The biofilms were gently washed with distilled water and supplemented with a relevant volume (2 or 0.2 mL) of a 1% aqueous Crystal violet solution. After 10-min incubation at room temperature, the biofilms were washed with water. The stain bound to the biomass was extracted with ethanol (2 or 0.2 mL), and the OD590 of the solution was measured on a KFK-2 photocolorimeter (Zagorsk Optical Mechanical Plant, Russia) or on a Multiskan Ascent plate scanner (ThermoLabsystems, Finland). The rel-ative respiratory activity of the biofilm cells was determined by the fluorometric resazurin test with modifications. The planktonic culture was removed from the wells, and 0.2 mL AlamarBlue (Sigma, United States) dissolved in PB (pH 7.0) (0.01 g/L) was added. The plates were incubated at 30°С for 24 h. Fluorescence was recorded on a Cary Eclipse spectrofluorimeter (Agilent, United States) with the following parameters: excitation wavelength, 530 nm; emission wavelength, 600 nm; slit width, 10 nm. The same manipulations were carried out with the controls.
Phase contrast and transmission electron microscopy of the biofilms, suspensions of washed-off biofilms, and free-living cells from planktonic cultures was carried out with the equipment of the IBPPM Simbioz Center for the Collective Use of Research Equipment in the Field of Physical–Chemical Biology and Nanobiotechnology (Saratov, Russia): Leica DM6000 B (Leica Microsystems, Germany) and Libra 120 (Carl Zeiss, Germany).
Determination of the content of carbohydrate components in the biofilm matrix. The matrix components were isolated according to Wang et al. (2017). After removal of planktonic bacteria, the biomass of mature biofilms formed on glass surfaces was washed with 50 mM PB (pH 7.0), detached by pipetting with this buffer, and collected by centrifugation. The pellet was washed twice with PB (pH 7.0). The matrix components were extracted by boiling for 10 min in 0.5 M EDTA at 100°C. The content of carbohydrate components was determined in the extracts by the phenol-sulfuric acid method (Wang et al., 2017).
Determination of the content of indole-3-acetic acid (IAA). Biofilms were grown for 7 days under MSM with 5 mM L-tryptophan. Prior to staining, the culture liquid was collected and centrifuged, and IAA was determined in the supernatant. The analysis was carried out by reversed-phase HPLC on a Dionex Ultimate 3000 chromatograph (Thermo Scientific, United States) by using a Macherey-Nagel Nucleodur HTec С18 column with an average particle diameter of 5 µm, pores of 100 Å, and dimensions of 150 × 3.0 mm.
Chromatography was carried out under gradient elution conditions, with solvent A, HPLC-grade acetonitrile (Panreac, Spain); solvent B, phosphoric acid solution in MilliQ water (pH 2.5); solvent C, MilliQ water. The composition of the mobile phase was changed as follows: 0–1 min, 40% A, 30% B, 30% C; 1–4 min, 70% A, 15% B, 15% C; 4–7 min, 70% A, 15% B, 15% C; 7–8 min, 40% A, 30% B, 30% C; and 8–12 min, 40% A, 30% B, 30% C. Analysis duration was 12 min. The flow rate was 0.25 mL/min. The injected sample volume was 5 µL. Detection was carried out at 280 nm. Control of the chromatograph and data analysis was carried out by the Chromeleon v. 7.1.2.1478 software package (Thermo Scientific, Dionex, United States). IAA was identified by comparison of the retention time and absorption spectra with those of the standard sample. Its quantitative content was determined from the calibration curve with the correlation coefficient R2 = 0.9997 for linear approximation of the points within the concentration range of 0.78‒50.00 µg/mL.
Enumeration of the colony-forming units (CFU) in biofilms. Mature biofilms were gently washed with 50 mM PB (pH 7.0). The numbers of colony-forming units (CFU) were determined in the washed native (untreated) and dry biofilms. In the latter case, washed biofilms were dried at 30°С and stored at 37°С (Shelud’ko et al., 2020). For CFU determination, 2 mL of 50 mM PB (pH 7.0) was added to the test tube, and the biomass was washed off after 1-h incubation. The biomass was resuspended and vortexed for 1 min. The suspensions were examined by light microscopy in order to determine the presence of cell aggregates; tenfold dilutions of the suspension were then prepared and plated (100 µL) on MSM agar. The results were calculated for 2 mL (one biofilm), accor-ding to the relevant dilutions. The test tubes with a sterile medium incubated for 7 days were used as the controls. The results of staining the glass of the experimental test tubes after biofilm removal were the same as those for the control and did not exceed 0.04 U А590 (l = 0.5 cm), indicating complete biofilm detachment.
Analysis of ability of bacteria to colonize wheat seedling roots. Seeds of soft spring wheat (cultivar Saratovskaya 29), obtained from the Federal Center of Agriculture Research of the South-East Region (Saratov, Russia), were sterilized, grown for 3 days, and inoculated by incubating the seedlings in a bacterial suspension in 50 mM PB, pH 7.0 (OD590 = 0.5; l = 0.5 cm), under shaking (25 rpm), as described previously (Shelud’ko et al., 2010). The inoculated wheat seedlings were washed once with sterile 50 mM PB (pH 7.0) and placed above the liquid in test tubes with 10 mL of the sterile medium for plants containing the following (g/L): KH2PO4, 4; CaCl2, 1.25; H3BO3, 0.0016; CuSO4·5H2O, 0.00025; MgSO4, 0.09; Na2MoO4·2H2O, 0.0025; KJ, 0.008; ZnSO4, 0.015; FeSO4·7H2O, 0.028; disodium EDTA, 0.037; рН 6.0. The inoculated plants were grown in this liquid medium for 7 days at 22°С with illumination in a 16/8 h period of light/darkness. Bacterial distribution and biofilm formation on the root surface were investigated under a Leica DM6000 B microscope (Leica Microsystems, Germany) (for microscopy, the roots were immersed in 50% glycerol in 50 mM PB, pH 7.0).
Weighed samples of aseptically washed roots were homogenized, and CFU numbers were determined by plating tenfold serial dilutions on MSM agar. The zeroth and first dilutions were centrifuged; the pellet was dried at 30°С and stored at 37°С. These samples were used to detect the cells resistant to drying, as was described above. The numbers of bacteria revealed on the roots were recalculated for one plant. The presence of foreign bacteria in the root homogenates was controlled. The samples yielding the colonies morpholo-gically different from those of azospirilla were discarded.
Morphometric and morphophysiological variables of wheat. The following variables were determined for each plant: root system length, mm; percentage of branched roots (roots with lateral rootlets); and root hair deformation (the share of curved ones determined microscopically on 50–90 root sites). To determine wet and dry biomass (in mg), the root system was removed from the aerial part, weighed, and dried to constant weight at 70°С.
Statistical treatment of the results. For all quantitative measurements, at least three independent experiments were carried out in at least two replicates. The biofilm biomass of each strain was determined at least six times. Each time, the biofilms formed in five glass tubes were stained. The results were processed with Microsoft Office Excel 2010; confidence intervals were determined for a 95% significance level. The statistical significance of the differences between the average values was determined by a one-way analysis of variance (ANOVA) at a significance level of 0.05.
RESULTS AND DISCUSSION
Investigation of biofilm formation. Comparison of growth of A. brasilense strains Sp7, Sp7.4, and Sp7.8 in liquid media revealed that well-stirred planktonic cultures reached the stationary growth phase after 24 h of incubation. The cells possessed a polar flagellum, and on average 85.5 ± 2.2% of bacteria swam at the rate of 28–30 µm/s (Table 1). Planktonic cells of strain Sp7.4 were longer than those of strains Sp7 and Sp7.8 (Fig. 1).
The 24-h planktonic cultures described above were used to inoculate MSM with and without NH4Cl, which were incubated in glass test tubes under static conditions. Transition of planktonic cells of all studied strains (Fig. 1; day 0) to the biofilm mode resulted in increased cell length as early as the stage of adsorption/adhesion. In 7-day biofilms of strains Sp7 and Sp7.8, cell length varied depending on the presence of nitrogen in the medium (it decreased in the presence of NH4Cl for Sp7.8 and insignificantly decreased under nitrogen-free conditions for Sp7). In the case of strain Sp7.4, cell length increased at every stage of biofilm formation in the presence of NH4Cl (Fig. 1). On day 7 of cultivation, biofilm formation at the solid-li-quid interface by all three strains was complete, i.e., the biomass amount in the biofilms stabilized. Comparison of the biomass of mature biofilms revealed that while for strains Sp7 and Sp7.8 its amount depended on the presence of NH4Cl in the medium, it was not lower than the biomass of strain Sp7.4 (Table 2, column a). The biofilms of strains Sp7, Sp7.4, and Sp7.8 contained similar amounts of bacteria capable of CFU formation on agar media. In the material washed off from the tubes with biofilms, CFU numbers varied insignificantly within the order of magnitude of 1010 (CFU in the biofilm formed in one tube) and did not depend on the biofilm biomass and/or on the presence of a bound nitrogen source in the medium (Table 2, columns a and b).
The number of desiccation-resistant cell forms in the biofilms was assessed. The biofilms grown in MSM with or without NH4Cl (containing 1010 CFU; Table 2, column b) were dried at 30°C and stored at 37°C. After 10 days of storage, CFUs were revealed in the biofilms of all three strains. The highest number of 104 CFU per biofilm was found in Sp7 biofilms grown in the medium without bound nitrogen sources. The biofilms of strain Sp7.8 contained 103 CFU in the nitrogen-free medium and 104 in the presence of NH4Cl (Table 2, column c). For strain Sp7, the CFU number in the medium with NH4Cl decreased by more than an order of magnitude (Table 2, column c). In the case of strain Sp7.4, the CFU number in biofilms was the smallest (101) and did not depend on the presence of nitrogen in the medium. It should be noted that desiccation-resistant bacterial forms are involved in the mechanisms facilitating survival of microbial populations under conditions of drought/water stress (Berg et al., 1980; Sadasivan et al., 1987; Malinich and Bauer, 2018; Shelud’ko et al., 2020).
Analysis of the effect of osmotic stress on biofilm formation. Osmotic/water stress was simulated by using the nonpenetrating osmotic agent polyethylene glycol (PEG) with an average molecular weight of 6000. The presence of PEG decreases the water potential of the medium and prevents water absorption by bacteria and plant roots (Chutia and Borah, 2012; Ansari et al., 2021). This approach makes it possible to achieve a stable decrease in the water potential during a desired time interval and to simulate the effect of drought on bacterial populations and plants (Chutia and Borah, 2012; Ansari et al., 2021).
At the initial stage, we examined the effect of different PEG 6000 concentrations on the growth of planktonic cultures of strains Sp7, Sp7.4, and Sp7.8 in liquid MSM under stirring for 24 h. Growth of planktonic cultures (monitored as the A590 of the culture) was suppressed by 50% and more, as compared to the variables characterizing growth in the absence of PEG (the A590 of the culture without PEG), in the presence of 10% PEG for strain Sp7, and in the presence of 20% PEG for strains Sp7.4 and Sp7.8 (Table 1). The values of the growth variables of planktonic cultures of strain Sp7.4 in the presence of all tested PEG concentrations were higher than those for the Sp7 and Sp7.8 cultures (Table 1). Probably, the resistance of Sp7.4 planktonic cultures to osmotic stress resulted from the cell morphology of this strain (Fig. 1).
PEG affected the motility of azospirilla. Thus, the swimming rate of all three strains decreased significantly in the presence of 5% PEG (Table 1). Further increase in the PEG concentration (10–25%) blocked the motility of planktonic cells. PEG had also a negative effect on the formation of macrocolonies of all studied strains on semisolid agar media (Table 1). On the semisolid medium with 0.3% agar, PEG (5%) prevented motility of the swimming cells (they remained at the point of inoculation into the medium) (Table 1). When agar density was increased to 0.4–0.5%, azospirilla moved in the presence of 5% PEG, forming colonies of swarming bacteria, albeit of smaller diameter than in the unstressed control (Table 1).
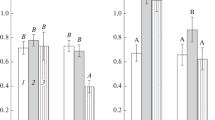
Analysis of biofilm formation under conditions of water stress (in the presence of PEG) revealed that the PEG concentration had different effects on biomass accumulation in the biofilms of the studied strains (Fig. 2a). Under MSM with NH4Cl, strains Sp7 and Sp7.4 formed biofilms with minimal amounts of biomass in the presence of PEG concentrations not lower than 15 and 10%, respectively. In the case of the nitrogen-free medium, both strains accumulated minimal amounts of biomass at 10% PEG or higher (Fig. 2a). In the case of strain Sp7.8, the lowest amount of biomass was accumulated in the biofilms formed under MSM with PEG concentrations 20% and higher, independently of the presence of NH4Cl. In the pre-sence of 5% PEG, the biofilms of this strain contained even more biomass than the unstressed control. The biofilms of Sp7.8 formed in the presence of 5–15% PEG contained more biomass than did those of strains Sp7 and Sp7.4 (Fig. 2a).
Effect of PEG 6000 on biofilm formation by A. brasilense strains Sp7 (1), Sp7.4 (2) and Sp7.8 (3) under the liquid medium (a) and respiratory activity of the biofilms formed without stress (b). MSM with NH4Cl (1) or without bound nitrogen (2) was used. The relative amount of biomass in biofilms formed after 7 days was estimated by measuring the A590 of Crystal violet desorbed after staining. The respiratory activity of 7-day biofilms formed without stress was determined after 10-day cultivation with PEG 6000. The results of one-way analysis of variance (ANOVA) in column (d) are represented by lowercase letters; different letters indicate statistically significant differences between the averages. The effect of PEG on the average values of the studied variables was analyzed; a indicates the lowest average values.
Analysis of the effect of osmotic stress on the respiratory activity of Azospirillum biofilms. The effect of PEG on the already formed biofilms (after 7 days of cultivation) was studied in another series of experiments. Mature biofilms were incubated for 10 days with different PEG concentrations, and the respiratory activity (an indicator of the metabolic activity of the cells) was measured (Petrova et al., 2021). In Sp7 biofilms formed under the medium with NH4Cl, respiratory activity decreased by more than 70%, as compared to the unstressed control at 15–25% (Fig. 2b). In mature Sp7.8 biofilms from the medium with ammonium, a significant decrease in the respiratory activity occurred after incubation with 25% PEG in the medium (Fig. 2b). In the films of Sp7 and Sp7.8 formed in the nitrogen-free MSM, a similar decrease in the respiratory activity occurred at PEG concentrations of at least 10%. In the case of Sp7.4, respiratory activity decreased by more than 70% in the presence of 5% PEG, independently of the presence of nitrogen in the medium (Fig. 2b).
It should be noted that the level of metabolic acti-vity in Sp7.8 biofilms formed without PEG stress under the nitrogen-containing medium was higher than those of the parent strain and Sp7.4 in the presence of all tested PEG concentrations (Fig. 2b). A similar response to the presence of PEG was observed in the Sp7 biofilms formed under the nitrogen-free medium (Fig. 2b).
The carbohydrate components of the matrix, which are part of the multicomponent system media-ting affinity of the biofilms to various substrates and their structural integrity (Wang et al., 2017; Shelud’ko et al., 2018), may contribute to the resistance of Azospirillum to water stress (Vurukonda et al., 2016; Shelud’ko et al., 2018; Gannesen et al., 2019). Estimation of the content of the carbohydrate components in the matrix obtained from the biofilms washed off the glass surface revealed that the content of the carbohydrate components extracted from Sp7.4 biofilms was less than those for Sp7 and Sp7.8, independently of the presence of nitrogen sources in the medium (Table 2). Bacteria from the native biofilms were the ones most sensitive to the negative effect of PEG (Fig. 2b).
Analysis of the adaptation of A. brasilense strain Sp7 and its derivatives to existence in the root system of wheat seedlings. The number or A. brasilense attached to the roots stabilized during 3 h of incubation of the bacterial suspension with 3-day-old wheat seedlings. The numbers of adsorbed cells of strains Sp7, Sp7.4, and Sp7.8 varied in the order of 107 CFU/plant (Table 3).
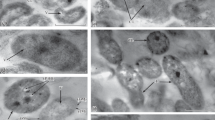
Six to seven days after inoculation with strains Sp7, Sp7.4, and Sp7.8, mono- and multilayer biofilms were formed on the surface of the root epidermis cells. In the suction zone, root hairs completely covered with the biofilms were observed. The biofilms were located at the place of contact between root hairs and at the root hair tips (Fig. 3).
The abundance of desiccation-resistant cells of strains Sp7, Sp7.4, and Sp7.8, colonizing wheat roots and forming biofilms, was determined (homogenates of inoculated roots were dried at 30°C and stored at 37°C). After 10 days of storage, dry roots contained 105 CFU of Sp7 or Sp7.8 and 104 CFU of Sp7.4 (the native roots prior to drying contained 109 CFU) (Table 3). A more pronounced tendency to decreased cell abundance in dried samples was observed for the biofilms formed on an abiotic surface under MSM (Table 2). Azospirilla adapted to the plant root system probably formed more desiccation-resistant cell forms than did bacteria from the biofilms formed under abiotic conditions (on glass under MSM). Alternatively, the potential resistance of root-associated cells to this stress was enhanced.
The presence of any of the studied strains in the seedling root system resulted in its decreased length and in larger numbers of branched roots and deformed root hairs (Table 3, Fig. 3). These effects on the root system were probably caused by the polysaccharides, IAA, or other plant hormones produced by the biofilms of strains Sp7, Sp7.4, and Sp7.8; the modulating effect of these compounds has been well characterized in the case of azospirilla (Fedonenko et al., 2004; Fibach-Paldi et al., 2012; Vurukonda et al., 2016; Fukami et al., 2018). The biofilms of all three strains produced similar amounts of IAA (Table 2, columns d, f).
A. brasilense has a multicomponent genome, which often undergoes spontaneous rearrangements, including those affecting their plasmid profiles. DNA rearrangements, including alterations in the plasmid profile of azospirilla, may affect root morphology; cell aggregation and motility; synthesis of capsules and pigments; production of exopolysaccharides (EPSs), lipopolysaccharides (LPSs), and other metabolites; resistance to salt stress, ampicillin, and surfactants; and ability to utilize carbohydrates (Lerner et al., 2010; Petrova et al., 2010; Volfson et al., 2013; Katsy and Petrova, 2015). A. brasilense type strain Sp7 possesses plasmids with molecular masses of 90 (pRhico), 115 (p115), and over 300 MDa. The spontaneous variants used in the present work, A. brasilense Sp7.4 and Sp7.8, lacked the 115-MDa plasmid, while the pRhico molecular mass was changed to ~131 and 124 MDa, respectively (Petrova et al., 2010; Katsy and Petrova, 2015). In the present work, spontaneous A. brasilense variants Sp7.4 and Sp7.8, similar to the parent strain, were shown to colonize roots, form biofilms, and affect the root system morphology. Biofilms of the variants, similar to those of strain Sp7, produced polysaccharides and the plant hormone IAA. In the course of adaptation to the seedling root system, all three strains developed desiccation-resistant cell forms. Such forms are part of the system of mechanisms promoting microbial survival under conditions of water stress. Such Azospirillum cells have been characterized as cystlike cells (CLCs) (Sadasivan et al., 1987; Malinich and Bauer, 2018; Shelud’ko et al., 2020). The CLCs of azospirilla have been compared to Azotobacter cysts (Berg et al., 1980). The absence of clear differentiation of the outer layer into the exine and intine is a noticeable difference between the Azospirillum CLCs and Azotobacter cysts (Berg et al., 1980). In the biofilms of A. brasilense Sp7, CLCs were characterized by their morphological and functional polymorphism (Wang et al., 2017; Shelud’ko et al., 2020). In dry biofilms after the death of the vegetative cells (Sadasivan et al., 1987; Malinich and Bauer, 2018; Shelud’ko, 2020), only cystlike forms probably retain viability.
The motility of planktonic and swarming cells of strains Sp7, Sp7.4, and Sp7.8 was suppressed under conditions simulating osmotic/water stress. At 10% PEG, the nonpermeating osmotic agent used to establish osmotic stress conditions, suppressed motility of the cells moving with a polar flagellum (Fla) in planktonic liquid culture. At 5%, PEG decreased the swimming rate of bacteria in planktonic culture, while on the semisolid medium with 0.3% agar, motility was blocked. At higher agar concentrations (0.4–0.5%) azospirilla produce numerous additional lateral flagella (Laf) (Petrova et al., 2020). Bacteria moved on these media in the presence of 5% PEG, forming co-lonies of swarming cells, albeit of smaller diameter than in the unstressed control.
At sufficient humidity the rhizosphere microorganisms are poorly adsorbed on the surface of soil particles and therefore may move in the direction of plant roots (Oliveira et al., 2004). Thus, at 20% soil humi-dity, azospirilla migrate from plant to plant (Bashan, 1986). The experimental model using an agar medium with PEG infusion to obtain a stable and reproducible decrease in the water potential is similar to the argonomical drought/water stress in soil (Frolov et al., 2017).
PEG at concentrations suppressing/blocking the motility of swimming/swarming bacteria (5–10%) had no significant effect on the ability of strains Sp7 and Sp7.8 to form biofilms and did not inhibit the metabolic activity of their cells in the biofilms formed without stress. The parameters characterizing the resistance of the biofilms of the Sp7.8 spontaneous variant to osmotic stress/desiccation exceeded those for the parent strain and the Sp7.4 variant. While the biofilms of Sp7.4 were the most sensitive to the negative effect of osmotic stress, at the same PEG concentrations planktonic cultures of this strain grew better than those of strains Sp7 and Sp7.8.
The type strain A. brasilense Sp7 is used as a model in the studies on the selection of rhizosphere bacteria able to enhance the resistance of cereals to the negative effect of drought/water stress (Notununu et al., 2022). Our data indicate that bacteria of this strain form biofilms under unfavorable effects of osmotic/water stress. In these biofilms, the cells of A. brasilense Sp7 adapted to the conditions simulating drought/modifications of the water regime. Thus, our results for the resistance of A. brasilense Sp7 biofilms to osmotic stress, together with the available literature data (Ansari and Ahmad, 2019; Ansari et al., 2021), support the application of this property in the selection of rhizobacteria promising for use to combat drought-induced stress in plants. The variants A. brasilense Sp7.4 and Sp7.8, derivatives of A. brasilense Sp7, are of interest for further research on the selection of promising strains enhancing drought resistance in plants.
REFERENCES
Ansari, F.A. and Ahmad, I., Fluorescent Pseudomonas FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes, Sci. Rep., 2019, vol. 9, p. 4547.
Ansari, F.A., Jabeen, M., and Ahmad, I., Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant, Int. J. Environ. Sci. Technol., 2021, vol. 18, pp. 3855–3870.
Bashan, Y., Migration of the rhizosphere bacteria Azospirillum brasilense and Pseudomonas fluorescens towards wheat roots in the soil, J. Gen. Microbiol., 1986, vol. 132, pp. 3407‒3414.
Berg, R.H., Tyler, M.E., Novick, N.J., Vasil, V., and Vasil, I.K., Biology of Azospirillum-sugarcane association: enhancement of nitrogenase activity, Appl. Environ. Microbiol., 1980, vol. 39, pp. 642–649.
Bogino, P.C., Oliva, M.M., Sorroche, F.G., and Giordano, W., The role of bacterial biofilms and surface components in plant-bacterial associations, Int. J. Mol. Sci., 2013, vol. 14, pp. 15838–15859.
Chutia, J. and Borah, S.P., Water stress effects on leaf growth and chlorophyll content but not the grain yield in traditional rice (Oryza sativa Linn.) genotypes of Assam, India: II. Protein and proline status in seedlings under PEG induced water stress, Am. J. Plant Sci., 2012, vol. 3, pp. 971–980.
Döbereiner, J. and Day, J.M., Associative symbiosis in tropical grass: Characterization of microorganisms and dinitrogen fixing sites, in Symposium on Nitrogen Fixation, Newton, W.E. and Nijmans, C.J., Eds., Pullman: Washington State Univ., 1976, pp. 518–538.
Fedonenko, Yu.P., Zdorovenko, E.L., Konnova, S.A., Ignatov, V.V., and Shlyakhtin, G.V., A comparison of the lipopolysaccharides and O-specific polysaccharides of Azospirillum brasilense Sp245 and its omegon-Km mutants KM018 and KM252, Microbiology (Moscow), 2004, vol. 73, pp. 143–149.
Fibach-Paldi, S., Burdman, S., and Okon, Y., Key physiological properties contributing to rhizosphere adaptation and plant growth promoting abilities of Azospirillum brasilense, FEMS Microbiol. Lett., 2012, vol. 326, pp. 99–108.
Flemming, H.-C. and Wingender, J., The biofilm matrix, Nat. Rev. Microbiol., 2010, vol. 8, pp. 623–633.
Frolov, A., Bilova, T., Paudel, G., Berger, R., Balcke, G.U., Birkemeyer, C., and Wessjohann, L.A., Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model, J. Plant Physiol., 2017, vol. 208, pp. 70–83.
Fukami, J., Cerezini, P., and Hungria, M., Azospirillum: benefits that go far beyond biological nitrogen fixation, AMB Expr., 2018, vol. 8, pp. 73–85.
Gannesen, A.V., Zdorovenko, E.L., Botchkova, E.A., Hardouin, J., Massier, S., Kopitsyn, D.S., Gorbachev-skii, M.V., Kadykova, A.A., Shashkov, A.S., Zhu-ina, M.V., Netrusov, A.I., Knirel, Y.A., Plakunov, V.K., and Feuilloley, M.G.J., Composition of the biofilm matrix of Cutibacterium acnes acneic strain RT5, Front. Microbiol., 2019, vol. 10, p. 1284. https://doi.org/10.3389/fmicb.2019.01284
Hsiao, T.C., Plant responses to water stress, Ann. Rev. Plant Physiol., 1973, vol. 24, pp. 519–570.
Katsy, E.I. and Petrova, L.P., Genome rearrangements in Azospirillum brasilense Sp7 with the involvement of the plasmid pRhico and the prophage ΦAb-Cd, Russ. J. Genet., 2015, vol. 51, pp. 1165–1171.
Lerner, A., Valverde, A., Castro-Sowinski, S., Lerner, H., Okon, Y., and Burdman, S., Phenotypic variation in Azospirillum brasilense exposed to starvation, Environ. Microbiol. Rep., 2010, vol. 2, pp. 577‒586.
Malinich, E.A. and Bauer, C.E., The plant growth promoting bacterium Azospirillum brasilense is vertically transmitted in Phaseolus vulgaris (common bean), Symbiosis, 2018, vol. 76, pp. 97–108.
Notununu, I., Moleleki, L., Roopnarain, A., and Adeleke, R., Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: a review, Pedosphere, 2022, vol. 32, pp. 90–106.
Oliveira, A.L.M., Canuto, E., Silva, E.E., Veronica, M.R., and Baldani, J.I., Survival of endophytic diazotrophic bacteria in soil under different moisture intensities, Braz. J. Microbiol., 2004, vol. 35, pp. 295–299.
O’Toole, G.A. and Kolter, R., Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis, Mol. Microbiol., 1998, vol. 28, pp. 449–461.
Petrova, L.P., Filip’echeva, Yu.A., Telesheva, E.M., Pylaev, T.E., and Shelud’ko, A.V., Variations in lipopolysaccharide synthesis affect formation of Azospirillum baldaniorum biofilms in planta at elevated copper content, Microbiology (Moscow), 2021, vol. 90, pp. 470–480.
Petrova, L.P., Shelud’ko, A.V., and Katsy, E.I., Plasmid rearrangements and alterations in Azospirillum brasilense biofilm formation, Microbiology (Moscow), 2010, vol. 79, pp. 121–124.
Petrova, L.P., Yevstigneeva, S.S., Borisov, I.V., Shelud’ko, A.V., Burygin, G.L, and Katsy, E.I., Plasmid gene AZOBR_p60126 impacts biosynthesis of lipopolysaccharide II and swarming motility in Azospirillum brasilense Sp245, J. Basic Microbiol., 2020, vol. 60, pp. 613–623.
Sadasivan, L. and Neyra, C.A., Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145, J. Bacteriol., 1987, vol. 169, pp. 1670–1677.
Shelud’ko, A.V., Filip’echeva, Y.A., Telesheva, E.M., Burov, A.M., Evstigneeva, S.S., Burygin, G.L., and Petrova, L.P., Characterization of carbohydrate-containing components of Azospirillum brasilense Sp245 biofilms, Microbiology (Moscow), 2018, vol. 87, pp. 610–620.
Shelud’ko, A.V., Mokeev, D.I., Evstigneeva, S.S., Fi-lip’echeva, Yu.A., Burov, A.M., Petrova, L.P., Ponomareva, E.G., and Katsy, E.I., Cell ultrastructure in biofilms of Azospirillum brasilense, Microbiology (Moscow), 2020, vol. 89, pp. 50–63.
Shelud’ko, A.V., Shirokov, A.A., Sokolova, M.K., Sokolov, O.I., Petrova, L.P., Matora, L.Yu., and Katsy, E.I., Wheat root colonization by Azospirillum brasilense strains with different motility, Microbiology (Moscow), 2010, vol. 79, pp. 688–695.
Tarrand, J.J., Krieg, N.R., and Döbereiner, J., A taxonomic study of the Spirillum lipoferum group with description of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum braslense sp. nov., Can. J. Microbiol., 1978, vol. 24, pp. 967–980.
Volfson, V., Fibach-Paldi, Sh., Paulucci, N.S., Dardanelli, M., Matan, O., Burdman, S., and Okon, Y., Phenotypic variation in Azospirillum brasilense Sp7 does not influence plant growth promotion effects, Soil Biol. Biochem., 2013, vol. 67, pp. 255–262.
Vurukonda, S.S.K.P., Sandhya, V., Shrivastava, M., and Ali, S.K.Z., Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria, Microbiol. Res., 2016, vol. 184, pp. 13–24.
Wang, D., Xu, A., Elmerich, C., and Ma, L.Z., Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions, ISME J., 2017, vol. 11, pp. 1602–1613.
ACKNOWLEDGMENTS
The authors are grateful to the IBPPM Simbioz Center for the Collective Use of Research Equipment in the Field of Physical–Chemical Biology and Nanobiotechnology, IBPPM RAS (Saratov, Russia).
Funding
The work was partially supported by the Russian Foundation for Basic Research, project no. 20-04-00006-a. The work on the assessment of the respiratory activity of the cells was partially supported by Saratov State Medical University, project no. SSMU-2021-001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of inte-rest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by P. Sigalevich
Rights and permissions
About this article
Cite this article
Mokeev, D.I., Volokhina, I.V., Telesheva, E.M. et al. Resistance of Biofilms Formed by the Soil Bacterium Azospirillum brasilense to Osmotic Stress. Microbiology 91, 682–692 (2022). https://doi.org/10.1134/S0026261722601567
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722601567